The development of inhibitors targeting the programmed cell death 1/B7 homolog 1 (PD-1/B7-H1) (PD) pathway, a mechanism adaptively used by tumors to evade the immune response, has been ground breaking in the treatment of a broad spectrum of advanced cancers, especially solid tumors. Antibody blockade of the PD pathway (anti-PD therapy) repairs this immune deficit in the tumor microenvironment, producing previously unseen durable responses in many patients with advanced-stage cancers, but a large fraction of patients still fail to respond.1 As clinical responses to anti-PD therapy correlate with the presence of both (B7-H1) expression and immune responses in the tumor,2 we propose a way of categorizing patients based on Tumor Immunity in the MicroEnvironment (TIME) and discuss its implications in cancer treatment.
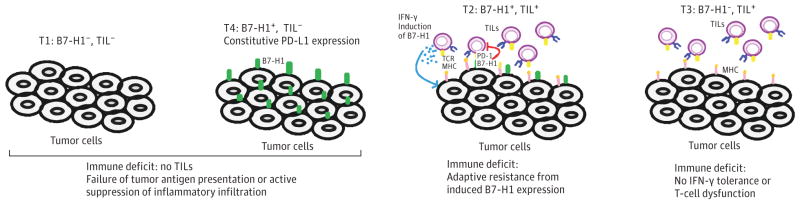
Based on B7-H1 expression and the presence of immunity (mainly the presence of tumor-infiltrating lymphocytes [TILs]) in tumor biopsies, 4 distinct TIME subtypes were previously described3,4: T1 (B7-H1−, TIL−), T2 (B7-H1+, TIL+), T3 (B7-H1−, TIL+), and T4 (B7-H1+, TIL−) (Figure). B7-H1 expression by tumors correlates with the presence of TILs,4 since interferon-γ (IFN-γ) production by TILs on antigen recognition can induce B7-H1 expression in virtually any nucleated cells tested thus far. Therefore, B7-H1–negative cells in the tumor microenvironment are likely those that (1) do not express tumor antigens capable of inducing T-cell recognition, (2) have no access to TILs, or (3) have access to TILs, but those TILs, unfortunately, are not proinflammatory and fail to produce IFN-γ. By using the TIME classification, we hypothesize about the underlying immune deficits and impairments in the tumor, and predict responses to anti-PD therapy, which help select which treatments and agents that will best unleash the power of the immune system.
Figure. Tumor Immunity in MicroEnvironment (TIME) Classification.
B7-H1 indicates B7 homolog 1; IFN, interferon; MHC indicates major histocompatibility complex; PD-1, programmed cell death 1; T1–T4, tumor subtypes; TIL, tumor-infiltrating lymphocyte.
T2 tumors are predicted to account for most responses to anti-PD therapy. In principle, these tumors contain TILs and other immune cells, but these tumors are adaptively resistant to elimination via TILs owing to B7-H1 expression. B7-H1 is rarely expressed in normal tissue but is induced during inflammation, where its physiological function is to prevent the spread of inflammation.5 However, in the setting of cancer, this becomes an inappropriate response, where it impairs the killing of tumor cells. By targeting the PD pathway, the ongoing antitumor immune response is rescued. This hypothesis is supported by clinical data: B7-H1–positive tumor tissues correlate with better responses to anti-PD therapy.2 The T2 tumors that fail to respond to anti-PD therapy point to the existence of additional dominant immune inhibitory pathway (s). These additional inhibitory pathways remain to be elucidated, and, until they are, treatment of these refractory tumors to anti-PD therapy remains difficult.
T1 and T4 tumors account for most tumors4 and likely account for most nonresponders to anti-PD therapy. While T1 and T4 tumors share a common problem, the lack of TILs, T4 differs from T1 because it has intrinsic B7-H1 expression, likely due to the activation of oncogenic pathways.3 The lack of TILs in these tumors may be caused by many factors, with possibilities spanning from a failure of tumor antigen presentation, to active suppression of inflammatory infiltration. Practically, focal radiation, locally administered oncolytic viruses, and cryotherapy can generate an inflammatory environment, and increase the availability of tumor antigens.6 Anticytotoxic T-lymphocyte-associated protein–4 antibody may also promote inflammatory infiltration. In addition, cancer vaccines and adoptive T-cell therapy, including chimeric antigen receptor T cells may increase TIL accumulation. Costimulatory targeting to 4-1BB or OX40 molecules has been shown to increase tumor infiltration.6,7 It is possible, however, that the tumor microenvironment actively prevents entry of inflammatory cells, and we are just starting to understand the mechanisms that impair accumulation of immune cells at the tumor site.
T3 tumors have TILs but lack B7-H1 expression, likely owing to the absence of IFN-γ production by T effector cells (Teff), a sign of cellular dysfunction.3 Molecular pathways that are responsible for the dysfunction of Teff have yet to be elucidated. These T cells can potentially be rescued by costimulation with OX-40 or 4-1BB agonists, which have been shown to break existing T-cell tolerance.7 Using this strategy, T3 tumors can be converted into the more treatable T2 tumors.
Owing to considerable intratumoral variation of B7-H1 expression and TIL distribution, characterization of a tumor as positive or negative for B7-H1 and TILs may be jeopardized by the collection of small biopsy specimens. This may be a major reason why some B7-H1–negative tumors still respond to anti-PD therapy. In the future, intratumoral variation may potentially be solved by image-based in vivo detection.
Conceptually, we propose this TIME classification model, because this model is based on the underlying mechanisms of immune deficits and/or impairments that are occurring in tumors and has important clinical implications for how to best harness the power of the immune system. Currently, biopsies used to classify TIME are done at the time of diagnosis but rarely are repeated at the time of progression. While the TIME classification is important in helping with the selection of therapy at the time of biopsy, defining the TIME will require repeated biopsies. Cancer immunology is a complex dynamic arena, in which a multitude of factors are at play in the same tumor, and at the same time, and can change with treatments or over time. Additional characteristics can be built into this model in the future to further help characterize tumor immune responses as we enter the golden age of cancer immunotherapy.
Acknowledgments
Funding/Support: This publication was made possible by Clinical and Translational Science Award grant No. UL1 TR000142 from the National Center for Advancing Translational Science, a component of the National Institutes of Health (NIH). This study is also supported partially by NIH grants Yale Specialized Programs of Research Excellence (SPORE grant in skin cancer P50 CA121974; SPORE grants in Lung Cancer P50 CA196530, P30 CA16359, and United Technologies Corporation Endowed Chair.
Additional Contributions: We thank our project specialist, Beth Cadugan, MS, for editing the manuscript; she received no additional compensation besides her salary.
Footnotes
Correction: This article was corrected on October 13, 2016, to fix an error in the Figure.
Conflict of Interest Disclosures: Dr Chen is a consultant and/or advisory board member of and receives consulting fees from MedImmune, NextCure, and Pfizer. Dr Chen also receives patent and/or licensing payments from Bristol-Myers Squibb and Ventana, and currently has sponsored research grants from Boehringer Ingelheim, Pfizer, and NextCure. No other disclosures are reported.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Yu Zhang, Department of Medicine (Medical Oncology), Yale Cancer Center, Yale University School of Medicine, New Haven, Connecticut.
Lieping Chen, Department of Medicine (Medical Oncology), Yale Cancer Center, Yale University School of Medicine, New Haven, Connecticut; and Department of Immunobiology, Yale Cancer Center, Yale University School of Medicine, New Haven, Connecticut.
References
- 1.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42(4):587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014;20(4):256–261. doi: 10.1097/PPO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]