Abstract
Rationale
Animal models with predictive and construct validity are necessary for developing novel and efficient therapeutics for psychiatric disorders.
Objectives
We have carried out a pharmacological characterization of the Roman high-(RHA-I) and low-avoidance (RLA-I) rat strains with different acutely administered propsychotic (DOI, MK-801) and antipsychotic drugs (haloperidol, clozapine), as well as apomorphine, on prepulse inhibition (PPI) of startle and locomotor activity (activity cages).
Results
RHA-I rats display a consistent deficit of PPI compared with RLA-I rats. The typical antipsychotic haloperidol (dopamine D2 receptor antagonist) reversed the PPI deficit characteristic of RHA-I rats (in particular at 65 and 70 dB prepulse intensities) and reduced locomotion in both strains. The atypical antipsychotic clozapine (serotonin/dopamine receptor antagonist) did not affect PPI in either strain, but decreased locomotion in a dose-dependent manner in both rat strains. The mixed dopamine D1/D2 agonist, apomorphine, at the dose of 0.05 mg/kg, decreased PPI in RHA-I, but not RLA-I rats. The hallucinogen drug DOI (5-HT2A agonist; 0.1–1.0 mg/kg) disrupted PPI in RLA-I rats in a dose-dependent manner at the 70 dB prepulse intensity, while in RHA-Irats, only the 0.5 mg/kg dose impaired PPI at the 80 dB prepulse intensity. DOI slightly decreased locomotion in both strains. Finally, clozapine attenuated the PPI impairment induced by the NMDA receptor antagonist MK-801 only in RLA-I rats.
Conclusions
These results add experimental evidence to the view that RHA-I rats represent a model with predictive and construct validity of some dopamine and 5-HT2A receptor-related features of schizophrenia.
Keywords: Prepulse inhibition, Activity, Propsychotic drugs, Antipsychotic drugs, Roman, high- and low-avoidance rats, Genetically based rat model, Predictive validity, Schizophrenia
Introduction
Some very commonly used rodent models of schizophrenia symptoms tend to replicate aspects of the positive symptoms of the disorder, such as hyperactivity or prepulse inhibition (sensorimotor gating) deficits. Prepulse inhibition (PPI; reduction of the startle response elicited by a preceding non-startling stimulus that is shorter and less intense than the startling stimulus) of the acoustic startle response is a measure of sensorimotor gating that is thought to be relevant in some mental disorders like schizophrenia (Kohl et al. 2013), as schizophrenic patients have PPI deficits. Some of the most popular rodent models derive from the similarity of the human effects of psychotomimetic or psychostimulant drugs to the symptoms of schizophrenia. Thus, the administration of psychostimulant or psychotomimetic (hallucinogenic) drugs to rats or mice generally leads to PPI deficits and, very often, induces hyperactivity (Sawa and Snyder 2002; Powell and Miyakawa 2006; Jones et al. 2011; Del Río et al. 2014). These drugs may also produce some of the negative and/or cognitive deficits/symptoms of schizophrenia, although their specific profile of effects depends on the main neurochemical mechanisms influenced by each drug class. In general, these symptoms can be reversed either by typical or atypical antipsychotic drugs, which gives pharmacological (predictive) validity to these models (Sawa and Snyder 2002; Powell and Miyakawa 2006; Jones et al. 2011; Del Río et al. 2014). The findings that psychotic-like states or symptoms are produced by dopaminergic psychostimulants (dopamine receptor agonists) and that such symptoms are reversed by typical antipsychotic drugs (i.e., D2 dopamine receptor antagonists; Geyer et al. 2001; Jones et al. 2011) support the dopaminergic hypothesis of schizophrenia. On the other hand, the finding that serotoninergic psychotomimetic (serotonin receptor—5-HT2A—agonists) drugs, as well as NMDA (ionotropic glutamate receptor), antagonists also induce psychotic-like symptoms which are reversed by (mostly) atypical antipsychotic drugs (Halberstadt and Geyer 2013 and Geyer et al. 2001) has provided support to the more modern serotonin and glutamate hypotheses of schizophrenia (Sawa and Snyder 2002; Powell and Miyakawa 2006; Jones et al. 2011; Del Río et al. 2014, Halberstadt and Geyer 2013).
On the other hand, using schizophrenia models in which symptoms (or deficits) are spontaneously present in naive (untreated) animals is important in order to elucidate whether typical or atypical antipsychotics can ameliorate symptoms in vulnerable subjects (e.g., Geyer et al. 2001). We have recently proposed that one such model, the Roman rat strains, displays genetically determined deficits/impairments in several schizophrenia-related features (Del Rio et al. 2014; Oliveras et al. 2015; Esnal et al. 2016). The Roman high- (RHA) and low-avoidance (RLA) rat lines/strains (lines refers to the outbred, and strains refers to the inbred animals) have been selectively and bidirectionally bred for their rapid (RHA) vs extremely poor (RLA) ability to acquire the two-way active avoidance task (Bignami 1965; Driscoll and Battig 1982; Steimer and Driscoll 2003; Escorihuela et al. 1999, Rio-Alamos et al. 2015). There is extensive evidence showing that RHA/RLA rats display differential schizophrenia-relevant features. For instance, the RHA strain/line displays impaired performance in several learning/memory tasks (Aguilar et al. 2002; Driscoll et al. 1995; Escorihuela et al. 1995b; Nil and Bättig 1981; Oliveras et al. 2015) and enhanced impulsive behavior in the 5-CSRTT and DRL-20 operant tasks (Klein et al. 2014; Moreno et al. 2010; Zeier et al. 1978). RHA rats exhibit deficits in latent inhibition (Fernandez-Teruel et al. 2006; Esnal et al. 2016), augmented mesocortical dopaminergic response to stress (Giorgi et al. 2003), and enhanced locomotor as well as mesolimbic dopaminergic sensitization upon the repeated administration of dopaminergic psychostimulants (Corda et al. 2005; Giorgi et al. 2007; Guitart-Masip et al. 2008a, b), a more robust functional tone of nigrostriatal and mesolimbic dopamine neurons (Tournier et al. 2013) and neurochemical and neuromorphological evidence of decreased hippocampal function (Garcia-Falgueras et al. 2012; Meyza et al. 2009; Salles et al. 2001). With regard to other schizophrenia-linked neurotransmitter systems, like serotonin and glutamate, Klein et al. (2014) have shown that RHA-I rats exhibit a dramatically reduced density of mGluR2 receptors in the prefrontal cortex and hippocampus and increased binding density of 5HT2A receptors in the prefrontal cortex (Klein et al. 2014; Wood et al. 2016), similarly to what has been observed post mortem in the brains of schizophrenic patients. Gonzalez-Maeso et al. (2007, 2008) proposed that 5-HT2A and mGlu2 receptors engage in a functional interaction that plays a crucial role in pre-attentive processes (sensorimotor gating) and in psychotic states. Thus, RHA rats display a series of neurobehavioral traits that resemble some schizophrenia—relevant symptoms or associated neural processes.
On the basis of that evidence, we would expect that the typical antipsychotic haloperidol (a preferential dopamine D2 receptor antagonist) would reverse the PPI deficit of RHA-I rats whereas the dopamine (D1/D2) agonist apomorphine would further impair such a deficit. Moreover, given the above mentioned deficit of mGlu2 receptors in RHA-I rats, and because these receptors are functionally linked to 5-HT2A receptors (Gonzalez-Maeso et al. 2008), we would also predict that the propsychotic agent DOI (5-HT2A receptor agonist) and the atypical antipsychotic clozapine (5-HT2A receptor antagonist, but also antagonist at many other receptors, including D2 receptors) would be less effective on PPI and locomotion in RHA-I than RLA-I rats. Accordingly, it would also be expected that clozapine would show greater ability to reverse the MK-801-induced impairment of PPI in RLA-I than RHA-I rats. Most of these hypotheses were confirmed by our experimental results.
Materials and methods
Animals
Animals used for the present studies were male rats of the inbred Roman high-(RHA-I, N = 283) and low-avoidance (RLA-I, N = 294) rat strains from the permanent colonies maintained at our laboratory (Medical Psychology Unit, Dept. Psychiatry and Forensic Medicine, School of Medicine, Autonomous University of Barcelona) since 1996. They were approximately 4 months old at the beginning of the experiments (weight range 320–420 g) and were housed in same-sexed pairs in standard (50 × 25 × 14 cm) macrolon cages. They were maintained under a 12:12 h light-dark cycle (lights on at 08:00 a.m.), with controlled temperature (22 ± 2 °C) and humidity (50–70%) and with free access to food and water. Naive rats were used for each of the ten experiments described below. Experimental procedures were approved by the Committee of Ethics of the Autonomous University of Barcelona and they were carried out in accordance with the European Council Directive (2010/63/EU) and Spanish legislation (RD 53/2013).
Drugs
Haloperidol, clozapine, 2,5-dimethoxy-4-iodoamphetamine (DOI), MK-801 (dizocilpine), and apomorphine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Haloperidol and clozapine were dissolved in a small amount of glacial acetic acid and then diluted in distilled water. DOI, apomorphine, and MK-801 were dissolved in saline (0.9% NaCl). All the solutions were freshly prepared each day.
Treatments
All the drug doses and their respective vehicles were administered subcutaneously (s.c.) in a volume of 1 ml/kg body weight. Haloperidol (0.1, 0.25, 0.50, or 1 mg/kg) or its vehicle were administered 30 min before testing. Apomorphine (0.05 mg/kg) or its vehicle was administered 5 min before testing. DOI (0.1, 0.25, 0.50, or 1 mg/kg) or its vehicle was administered s.c. 15 min before testing. Clozapine (2.5, 5, or 10 mg/kg) or its vehicle was administered 60 min before testing, and MK-801 (0.1 mg/kg) or its vehicle was subcutaneously administered 20 min before testing.
To assess the effects on locomotor activity of haloperidol, DOI, and clozapine, the two lowest doses of each drug used in the PPI experiments were chosen and injected at the same time interval before starting the registration of locomotion as in the PPI studies. The effective dose of apomorphine was selected according to a previous report (Sanna et al. 2014a, b), but the effects of apomorphine on locomotor activity were not addressed here because they have been well characterized in our previous study (Giménez-Llort et al. 2005). For the experiment with clozapine and MK-801, the doses chosen were based on previous studies (Bast et al. 2000; Bubeníková et al. 2005; Cilia et al. 2010; Hadamitzky et al. 2007) and also on our pilot experiments showing that at a dose 0.1 mg/kg, MK-801 reduced significantly %PPI in both Roman strains (data not shown).
Prepulse inhibition of the acoustic startle response
Four sound-attenuated boxes (SR-Lab Startle Response System, San Diego Inst., San Diego, USA) were used. Each box consists of a Plexiglas cylinder placed on top of a platform with a sensor that detects the intensity of the force made by the rat in each trial. Two speakers placed at 15 cm on each side of the cylinder deliver the acoustic stimuli, and a white noise generator provides the background noise (55 dB) throughout the whole session. Each box is lit by a 10 W lamp. The data are transduced by an accelerometer into a voltage which is saved into a computer for further analysis.
The startle session started with a 5-min habituation period in the startle chambers. Then, 10 pulse-alone trials (105 dB, 40 ms) were delivered in order to obtain a basal measure of the ASR (BASELINE 1). Next, each one of the 6 different types of trials was randomly administered 10 times (i.e., 60 trials in total):
Pulse-alone trials (105 dB 40 ms, BASELINE 2)
Prepulses of 65/70/75/80 dB (20 ms) followed by the startle stimulus (105 dB, 40 ms), with an inter-stimulus interval of 100 ms.
No stimulus trials (background noise 55 dB)
Finally, in order to measure the habituation to the startle stimulus, 5 pulse-alone trials were delivered (BASELINE 3).
The interval between trials was 10–20 s with a mean of 15 s. The startle magnitude was recorded for 200 ms after the onset of the pulse.
The degree of PPI (in percentage) was calculated according to the formula:
Locomotor activity assessment
Locomotor activity of RHA-I and RLA-I rats was evaluated with 3 identical plexiglas activity cages (40 × 40 × 40 cm). These cages are equipped with photocell beams that are interfaced with a PC to record the locomotor activity of the rats using program Acti-track (Panlab, Spain).
The procedure used was identical in the four experiments except for the intervals between the injection of each drug and the beginning of the testing session, which were the same as in the respective PPI experiments (see above). There was no previous habituation to the apparatus or the testing room. Locomotor activity was measured as the total photocell beam breaks every 5 min for a total of 30 min.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS, version 17).
To assess the percentage PPI data as the dependent variable, we performed repeated measures ANOVAs, with the 4 prepulse intensities as a within-subject factor, and the 2 strains and the administered doses as between-subject factors. To assess the differences in the startle response during pulse-alone trials, we performed an ANOVA with baseline 2 as the dependent variable and the doses and strain as between-subject factors. For locomotor activity experiments, we assessed the distance traveled data as the dependent variable using repeated measures multifactor ANOVAs with drugs doses and strains as between-subject factors and 6 time intervals (5 min each) as a within-subject factor. Post hoc pair wise contrasts with the LSD test were performed on PPI and activity measures after significant Strain × Treatment ANOVA effects.
To assess the pharmacological interaction between clozapine and MK-801, we conducted a repeated measures ANOVA with strain, clozapine, and MK-801 as between-subject factors and the prepulse intensities as a within-subject factor. Post hoc pair wise contrasts with the LSD test were performed after significant Strain × Treatment ANOVA effects. The threshold for statistical significance was set at p ≤ 0.05.
The number of animals in each experimental group is indicated in the legends to figures. Only the comparisons between the drug doses and the respective vehicle group are indicated in the figures except for the study with clozapine and MK-801 in which the significant comparisons with the veh/MK-801 group are also indicated.
Results
Effects of antipsychotics on PPI and locomotor activity
Haloperidol (Exps. 1–2) effects on PPI
In Exp. 1 (Fig. 1), the repeated measures factorial ANOVA (2 strains × 5 haloperidol doses × 4 prepulse intensities—as within-subject factor-) revealed significant prepulse intensity (Huynh-Feldt F (2.86, 182.86) = 373.91, p ≤ 0.001), prepulse intensity × strain, and prepulse intensity × treatment effects (Huynh-Feldt F (6.11, 182.86) = 6.11, p ≤ 0.001, and F(11.43,182.86) = 3.51, p ≤ 0.001, respectively). There were also strain and treatment effects (F (1, 64) = 13.00, p ≤ 0.001; F (1, 64) = 3.64, p ≤ 0.010, respectively) (Fig. 1a). The strain effect is due to the overall better PPI performance of the RLA-I rats, and the treatment effect indicates a global increase of PPI with haloperidol.
Fig. 1.
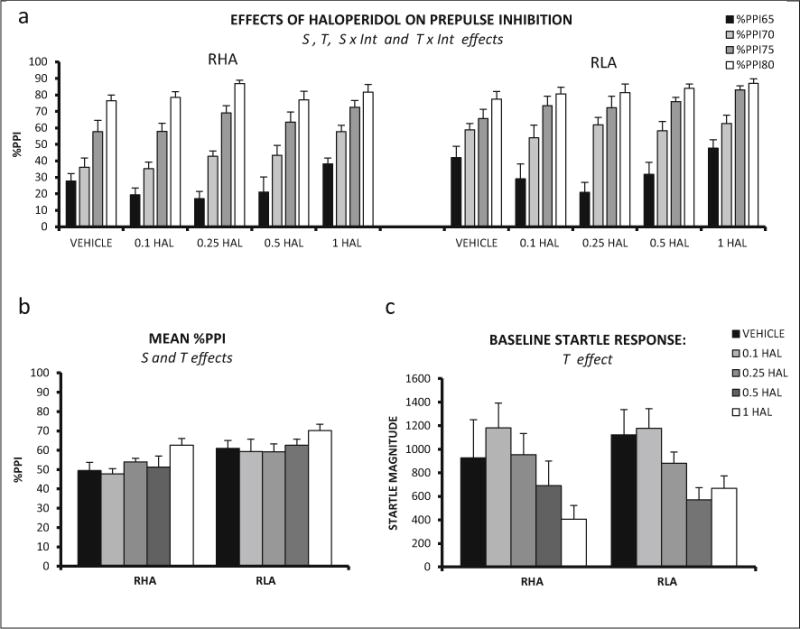
a Mean prepulse inhibition (±SEM) of RHA-I and RLA-I rats is shown for each prepulse intensity and haloperidol doses. b Mean prepulse inhibition (averaged for the four intensities ± SEM) is shown for all the treatments in RHA-I and RLA-I. c Mean ± SEM of the startle response magnitude in BASELINE 2 for each treatment administered to the RHA-Is and RLA-Is. S strain effect (ANOVA), T treatment effect (ANOVA), S × Int strain × prepulse intensity interaction (ANOVA), T × Int treatment × prepulse intensity interaction (ANOVA). The number of rats in each experimental group was as follows: RHA-I, vehicle, n = 6; 0.1 mg/kg, n = 6; 0.25 mg/kg, n = 6; 0.5 mg/kg, n = 6; 1 mg/kg, n = 10; RLA-I, vehicle, n = 8; 0.1 mg/kg, n = 7; 0.25 mg/kg, n = 7; 0.5 mg/kg, n = 8; 1 mg/kg, n =10
A two-way ANOVA showed a significant treatment effect on baseline startle response (F (4, 64) = 5.21, p ≤ 0.001), as haloperidol overall decreased this measure in both strains (Fig. 1c).
As a clear trend for an increase of PPI with haloperidol 1 mg/kg was observed in Exp. 1, Exp. 2 was carried out to test whether this dose had differential, strain-dependent effects. Indeed, the repeated measures ANOVA (2 strains × 2 haloperidol doses × 4 prepulse intensities—within subject factor-) showed that the second-order interaction (prepulse intensity × strain × treatment) was significant (Huynh-Feldt F(2.17,78.23) = 4.91, p ≤ 0.008) as well as the strain and treatment effects (F(1,36) = 24.40, p ≤ 0.001, and F(1,36) = 5.17, p ≤ 0.029, respectively, Fig. 2a). Further, post hoc pair wise comparisons, to determine the source of that interaction, revealed that %PPI was increased in RHA-I rats treated with 1 mg/kg haloperidol at 65 and 70 dB intensities whereas no effects were observed in RLA-I rats (Fig. 2a)
Fig. 2.
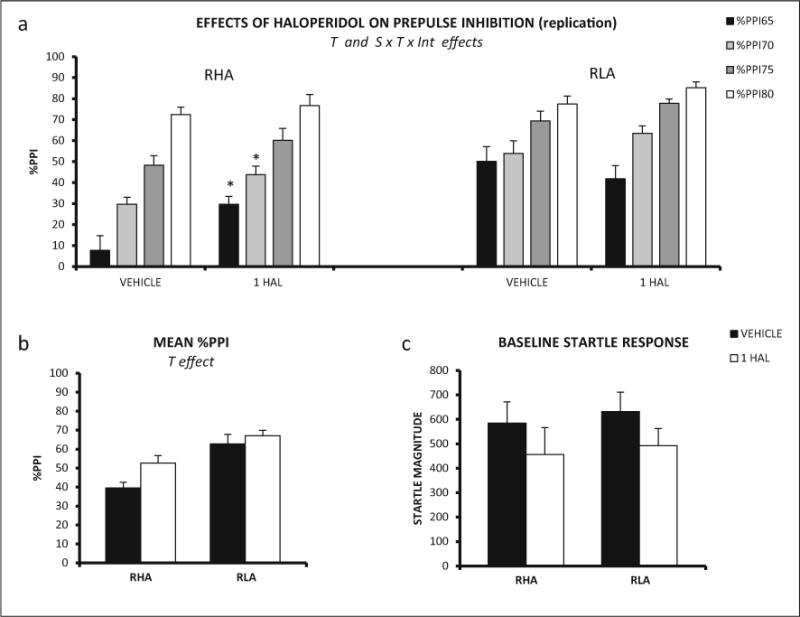
a Mean prepulse inhibition (±SEM) of RHA-I and RLA-I rats is shown for each prepulse intensity and haloperidol doses (n = 10/group in both strains). b Mean prepulse inhibition (averaged for the four intensities ± SEM) is shown for all the treatments of RHA-I and RLA-I rats. c Mean ± SEM of the baseline startle response for each treatment administered to the RHA-I and RLA-I rats. T treatment effect (ANOVA), S × T × Int strain × treatment × prepulse intensity second-order interaction (ANOVA). *p < 0.05 vs the prepulse intensity-matched and vehicle-treated RHA-I group (LSD test after significant S × T × Int effect in ANOVA)
Two-way ANOVA on baseline startle response showed no significant differences between strains or treatments in Exp. 2 (Fig. 2c).
In summary, both Exps. 1 and 2 show that RHA-I rats display lower %PPI than RLA-I rats and that haloperidol globally increases %PPI, whereas Exp. 2 shows that haloperidol 1 mg/kg improves %PPI in RHA-I rats without significantly affecting %PPI in the RLA-I strain.
Clozapine (Exp. 7) effects on PPI
In Exp. 7, the repeated measures factorial ANOVA (2 strains × 4 clozapine doses × 4 prepulse intensities—as within-subject factor-) revealed a significant interaction between strain and prepulse intensity (Huynh-Feldt F(2.93,161.37) = 13.19, p ≤ 0.001). The only main effect found was the strain effect (F(1,55) = 8.89, p ≤ 0.004) (Fig. 7a), overall indicating the better performance of the RLA-I rats (Fig. 7a, b).
Fig. 7.
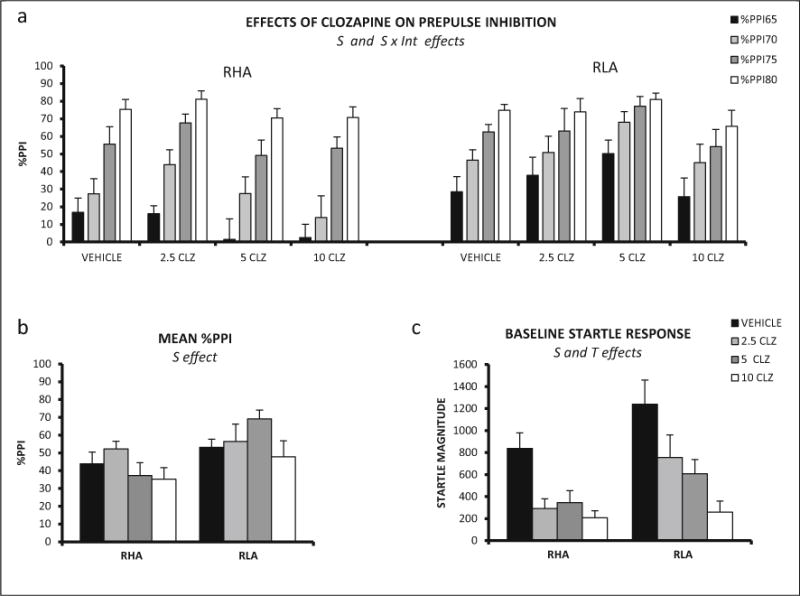
a Mean prepulse inhibition (±SEM) of RHA-I and RLA-I rats is shown for each prepulse intensity and clozapine dose. b Mean prepulse inhibition (averaged for the four intensities ± SEM) is shown for all the treatments in RHA-I and RLA-I rats. c Mean ± SEM baseline startle response for each treatment administered to the RHA-I and RLA-I rats. S strain effect (ANOVA), T treatment effect (ANOVA), S × Int strain × prepulse intensity interaction (ANOVA). The number of rats in each experimental group was as follows: RHA, vehicle, n = 9; 2.5 mg/kg, n = 7; 5 mg/kg, n = 8; 10 mg/kg, n = 8; RLA-I, vehicle, n = 8; 2.5 mg/kg, n = 7; 5 mg/kg, n = 8; 10 mg/kg, n = 8
Assessment by two-way ANOVA of the baseline startle yielded significant strain and treatment effects (F (1, 55) = 8.70, p ≤ 0.005, and F (3,55) = 12.19 p ≤ 0.001, respectively; Fig. 7c). The strain effect is due to the increased startle response of the RLA-I whereas the treatment effect reflects the overall reduction of startle response in clozapine-treated rats.
Haloperidol (Exp. 3) and clozapine (Exp. 8) effects on locomotor activity
In Exp. 3, the overall repeated measures ANOVA (2 strains × 3 haloperidol doses × 6 time intervals—within subject factor-) revealed a significant interval × strain × treatment interaction effect on locomotion (Huynh-Feldt F (8.85, 181.33) = 2.40, p ≤ 0.014, Fig. 3a, b). Moreover, there was a significant treatment effect (F(2,41) = 14.07, p ≤ 0.001, Fig. 3a, b). Post hoc pair wise comparisons performed to dissect that interaction indicated that both haloperidol doses reduced locomotor activity in the first three 5-min intervals in RLA-I rats, whereas the 0.1 mg/kg dose was clearly less effective in RHA-I vs RLA-I rats (Fig. 3a, b). These results show that haloperidol reduced locomotion in both strains, but the effect elicited by 0.1 mg/kg haloperidol was more marked in RLA-I rats.
Fig. 3.
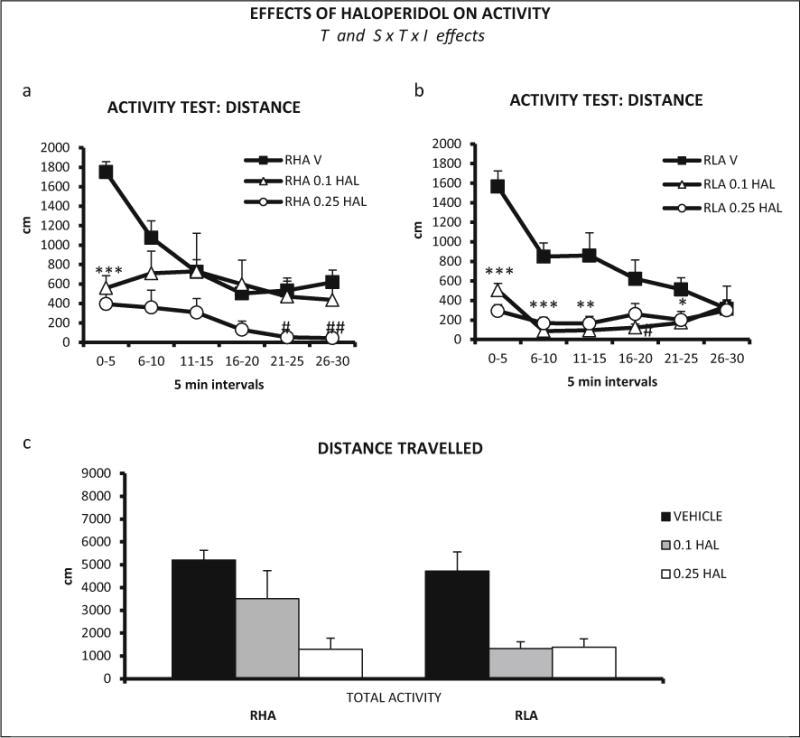
a Distance (±SEM) traveled in each 5-min interval of the locomotor activity test, by RHA-I rats and haloperidol doses (vehicle, n = 8; 0.1 mg/kg, n = 8; 0.25 mg/kg, n = 7). b Distance (±SEM) traveled in each 5-min interval of the locomotor activity test, by RLA-I rats and haloperidol doses (n = 8/group). c Total distance (±SEM) traveled by RHA-I and RLA-I rats in the 30-min activity session. a, b ***p < 0.001, **p < 0.01, *p < 0.05 both doses vs the vehicle-treated group (V); ##p < 0.01, #p < 0.05 vs the vehicle-treated group (dose indicated). T treatment effect (ANOVA), S × T × I strain × treatment × time interval second-order interaction (ANOVA). c ***p < 0.001, **p < 0.01 vs vehicle-treated group (post hoc LSD test following significant S × T × I effect in ANOVA)
Repeated measures ANOVA (2 strains × 3 clozapine doses × 6 time intervals—within-subject factor-) was performed on activity (distance traveled). A significant treatment × interval interaction was found (Huynh-Feldt F(8.98,188.57) = 5.57, p ≤ 0.001), as well as strain (F(1,42) = 4.24, p ≤ 0.046) and treatment (F(2,42) = 12.87, p ≤ 0.001) effects (Fig. 8a, b). The strain effect is due to the higher activity exhibited by the RHA-I rats, and the treatment effect is due to the global reduction of activity induced by clozapine.
Fig. 8.
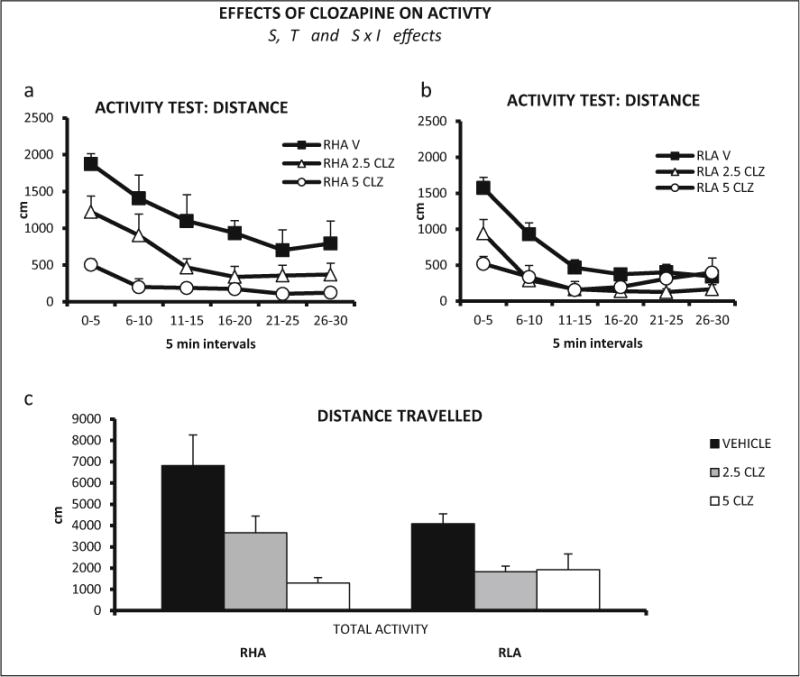
a Distance (±SEM) traveled in each 5-min interval of the locomotor activity test, by RHA-I rats and clozapine doses (n = 8 rats/group). b Distance (±SEM) traveled in each 5-min interval of the activity test, by RLA-I rats and clozapine doses (n = 8 rats/group). c Total distance (±SEM) traveled by RHA-I and RLA-I rats in the 30-min activity session. S strain effect (ANOVA), T treatment effect (ANOVA), S × I strain × time interval interaction (ANOVA)
Repeated measures ANOVA for the first 15 min of the sessions (3 intervals) for both drugs yielded similar results (data not shown).
Apomorphine and DOI effects on PPI and locomotor activity
Effects of apomorphine (Exp. 4) on PPI
The repeated measures factorial ANOVA (2 strains × 2 apomorphine doses × 4 prepulse intensities—within subject factor-) revealed a significant strain × prepulse intensity effect (Huynh-Feldt F(2.82,118.22) = 5.19, p ≤ 0.003). The interaction strain × treatment was also significant (F (1,42) = 4.46, p ≤ 0.042), as well as the strain and treatment effects (F (1, 42) = 17.28, p < 0.001, and F (1, 42) = 6.92, p ≤ 0.012, respectively) (Fig. 4a, b). Post hoc comparisons to determine the source of the strain × treatment interaction revealed that RHA-I rats treated with apomorphine showed a reduction in %PPI at all prepulse intensities, whereas PPI performance of RLA-I rats was not disrupted by apomorphine at any prepulse intensity (Fig. 4a, b).
Fig. 4.
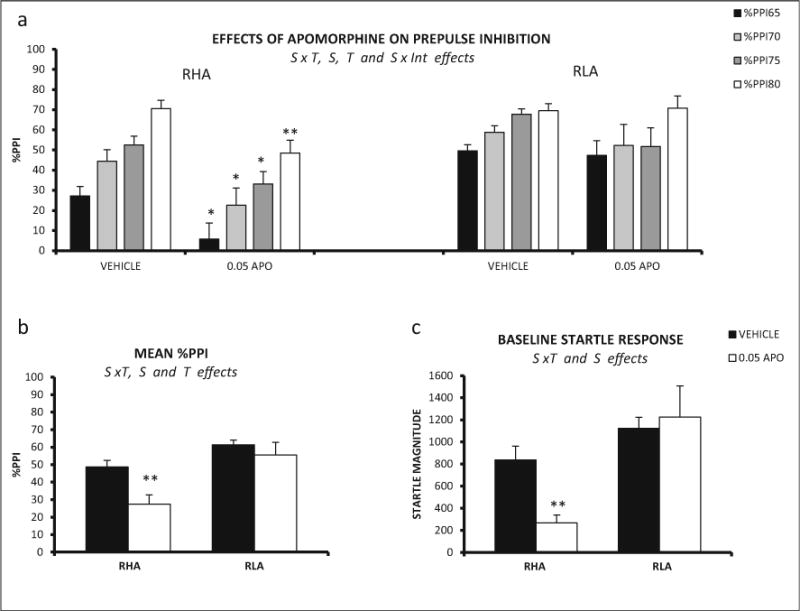
a Mean prepulse inhibition (±SEM) of RHA-I and RLA-I rats is shown for each prepulse intensity and apomorphine dose (RHA-I: vehicle n = 15; 0.05 mg/kg, n = 9; RLA-I: vehicle, n = 15; 0.05 mg/kg, n = 7). b Mean prepulse inhibition (averaged for the four intensities ± SEM) is shown for both treatments and both strains. c Mean ± SEM of the baseline startle response for each treatment administered to the RHA-I and RLA-I rats. S strain effect (ANOVA), T treatment effect (ANOVA), S × T strain × treatment interaction (ANOVA), S × Int strain × prepulse intensity interaction (ANOVA). **p < 0.01, *p < 0.05 (0.05 mg vs the respective vehicle group (post hoc pair wise contrasts with the LSD test following significant S × T effect from ANOVA)
Assessment by means of two-way ANOVA baseline startle revealed a significant strain effect (F(1,42) = 15.99, p ≤ 0.001) and a significant strain × treatment interaction (F(3,78) = 6.50, p ≤ 0.015) (Fig. 4c). Post hoc comparisons showed that baseline startle was reduced by apomorphine in RHA-I rats but not in their RLA-I counterparts (Fig. 4c).
Effects of DOI (Exp. 5) on PPI
Repeated measures ANOVA (2 strains × 5 DOI doses × 4 prepulse intensities—within subject factor-) revealed significant strain and treatment effects (F(1,78) = 5,70, p ≤ 0.019, and F(4, 78) = 4.04, p ≤ 0.005, respectively) and also a significant interaction between both factors (F(4,78) = 2.55, p ≤ 0.046) (Fig. 5a). The post hoc comparisons evidenced that 0.5 mg/kg DOI significantly reduced %PPI of RHA-I rats at 80 dB prepulse intensity (see post hoc tests in Fig. 5a, b). In RLA-I rats, all DOI doses significantly diminished %PPI at the 70 dB prepulse intensity in a dose-dependent manner (see post hoc comparisons in Fig. 5a, b).
Fig. 5.
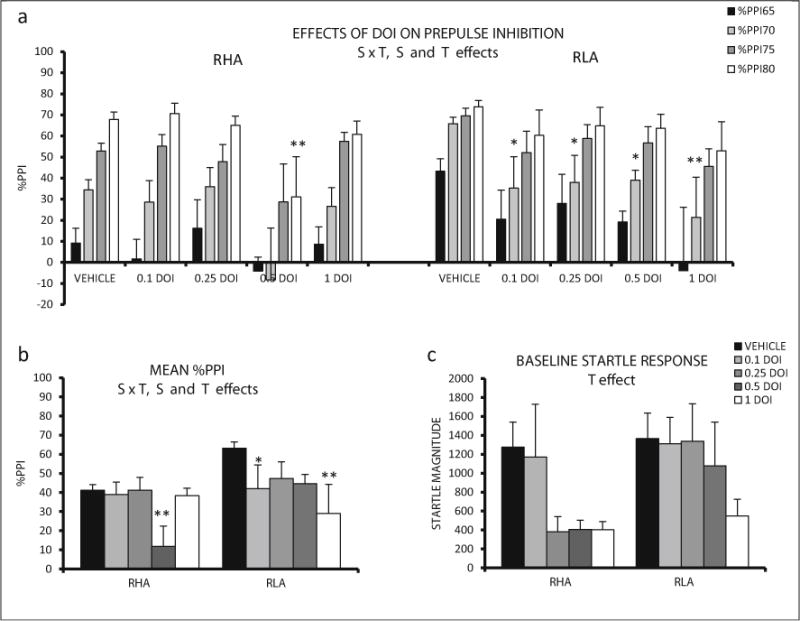
a Mean prepulse inhibition (±SEM) of RHA-I and RLA-I rats is shown for each prepulse intensity and DOI doses. b Mean prepulse inhibition (averaged for the four intensities ± SEM) is shown for all the treatments in RHA-I and RLA-I rats. c Mean ± SEM of baseline startle for each treatment administered to the RHA-Is and RLA-Is. S strain effect (ANOVA), T treatment effect (ANOVA), S × T strain × treatment interaction (ANOVA). **p < 0.01, *p < 0.05 vs the respective vehicle group (post hoc pair wise contrasts with the LSD test following significant S × T effect from ANOVA). The number of rats in each experimental group was as follows: RHA, vehicle, n = 14; 0.1 mg/kg, n = 8; 0.25 mg/kg, n = 8; 0.50 mg/kg, n = 6; 1 mg/kg, n = 8; RLA-I, vehicle, n = 16; 0.1 mg/kg, n = 8; 0.25 mg/kg, n = 6; 0.50 mg/kg, n = 8; 1 mg/kg, n = 6
Two-way ANOVA evaluation of the baseline startle revealed a significant treatment effect (F (4, 78) = 2.74, p ≤ 0.034), indicating an overall decrease of startle response magnitude induced by DOI in both strains (Fig. 5c).
Effects of DOI (Exp. 6) on locomotor activity
Repeated measures ANOVA (2 strains × 3 DOI doses × 6 time intervals—within-subject factor-) revealed a significant interval × treatment interaction (Huynh-Feldt F(9.45,189.07) = 4.12, p ≤ 0.001). There were also significant strain (F (1, 40) = 7.52, p ≤ 0.009) and treatment effects (F (2, 40) = 3.33, p ≤ 0.046) (Figs 6a, b). The strain effect is due to more intense locomotion of the RHA-I rats, while the treatment effect is apparently due to the reduction of activity induced by 0.25 mg/kg DOI in both rat strains.
Fig. 6.
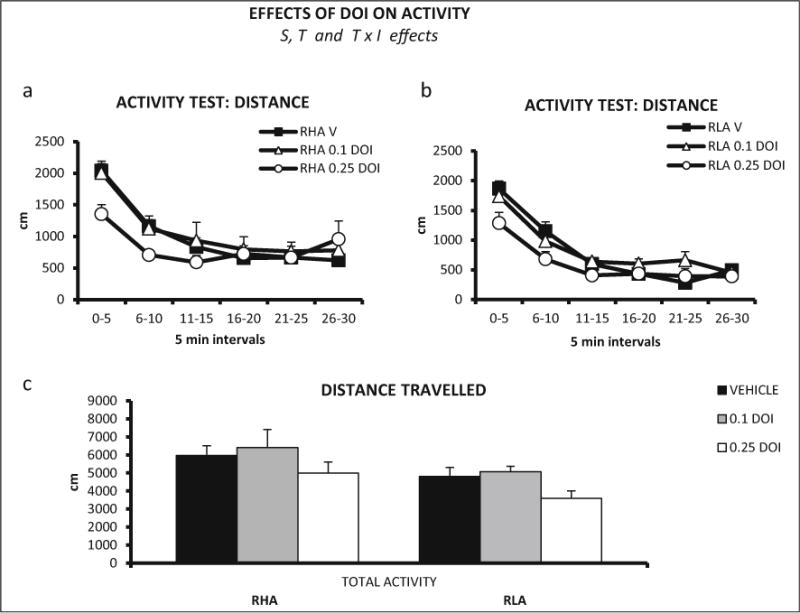
a Distance (±SEM) traveled in each 5-min interval of the activity test, by RHA-I rats and DOI doses (vehicle, n = 8; 0.1 mg/kg, n = 7; 0.25 mg/kg, n = 7). b Distance (±SEM) traveled in each 5-min interval of the activity test, by RLA-I rats and DOI doses (n = 8 rats/group). c Total distance (±SEM) traveled by RHA-I and RLA-I rats in the 30-min activity session. S strain effect (ANOVA), T treatment effect (ANOVA), T × I treatment × time interval interaction (ANOVA)
Repeated measures ANOVA for the first 15 min of the sessions (3 intervals) yielded similar results (data not shown).
Clozapine effects on MK-801-induced deficits on PPI (Exp. 9)
peated measures ANOVA (2 strains × 2 clozapine doses × 2 MK-801 doses × 4 prepulse intensities—within-subject factor-) revealed a significant prepulse intensity × strain interaction (F(3, 216) = 2.82, p ≤ 0.040), as well as significant effects of the three main factors (strain F(1,72) = 13.21, p ≤ 0.001; clozapine F(1, 72) = 4.60, p ≤ 0.035; MK-801 F(1, 72) = 41.93, p ≤ 0.001). There was also a significant strain × clozapine × MK-801 interaction (F(1,72) = 4.39, p ≤ 0.040) (Fig. 9a, b).
Fig. 9.
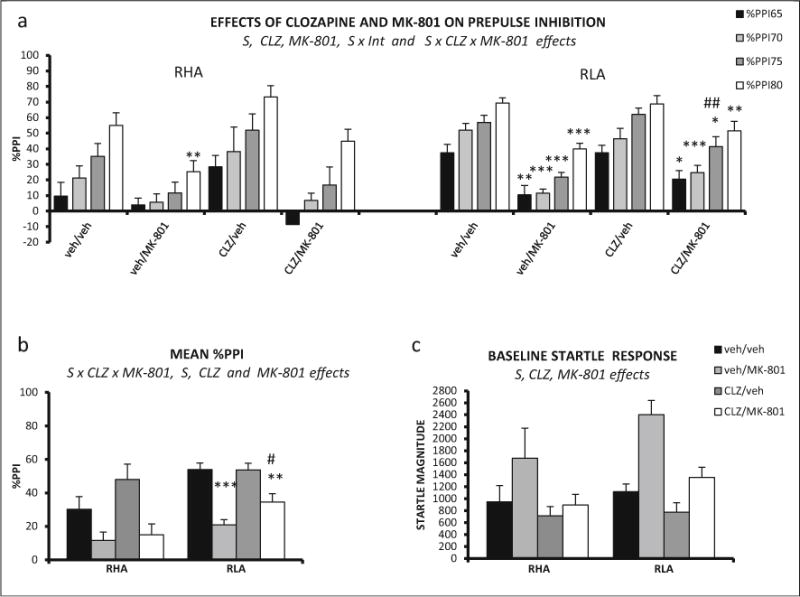
a Mean prepulse inhibition (±SEM) of RHA-I and RLA-I rats is shown for each prepulse intensity and each treatment combination. b Mean prepulse inhibition (averaged for the four intensities ± SEM) is shown for all the treatments in RHA-I and RLAI rats. c Mean ± SEM of baseline startle response for each treatment administered to the RHA-Is and RLA-Is. S strain effect (ANOVA), CLZ clozapine effect (ANOVA), MK-801 MK-801 effect (ANOVA), S × CLZ × MK-801 strain × clozapine × MK-801 (ANOVA). ***p < 0.001, **p < 0.01, *p < 0.05 vs vehicle-treated group; ##p < 0.01 vs veh/MK-801 (post hoc LSD test, following significant S × CLZ × MK-801 effect in ANOVA). The number of rats in each experimental group was as follows: RHA-I, veh/veh, n = 11; veh/MK-801, n = 9; CLZ/veh, n = 8; CLZ/MK-801, n = 8; RLA-I, veh/veh, n = 14; veh/MK-801, n = 10; CLZ/veh, n = 10; CLZ/MK-801, n = 10
Post hoc tests applied to clarify the source of this second-order interaction showed that MK-801 impaired PPI in RHA-I rats only at the 80 dB prepulse intensity and this effect was not attenuated by clozapine (see Fig. 9a), while the impairment in RLA-Is was observed at all prepulse intensities (Fig. 9a) and it was attenuated by clozapine at the 75 dB intensity (Fig. 9a). Likewise, post hoc comparisons on the total % PPI measure (Fig. 9b) showed that MK-801 did not significantly impair total %PPI in RHA-I rats, whereas this measure was impaired in RLA-I rats and clozapine significantly (though partially) attenuated such an impairment (Fig. 9b).
Three-way ANOVA on baseline startle revealed significant strain (F(1,72) = 4.13, p ≤ 0.046), clozapine (F(1,72) = 11.98, p ≤ 0.001), and MK-801 (F(1,72) = 15.90, p ≤ 0.001) effects, as baseline startle was overall higher in RLA-I vs RHA-I rats and was decreased by clozapine but enhanced by MK-801 (Fig. 9c).
Clozapine effects on MK-801-induced hyperactivity (Exp. 10)
Repeated measures ANOVA on activity results (the whole 30-min session) revealed a significant time interval × clozapine interaction (Huynh-Feldt F (3.50, 161.06) = 6.53, p ≤ 0.001). Significant effects of MK-801 and clozapine (F(1,46) = 21.38, p ≤ 0.001 and F(1, 46) = 20.25, p ≤ 0.001, respectively) were also found (Fig. 10a, b), indicating that in both strains, MK-801 induced a statistically significant hyperactivity while clozapine reduced locomotor activity.
Fig. 10.
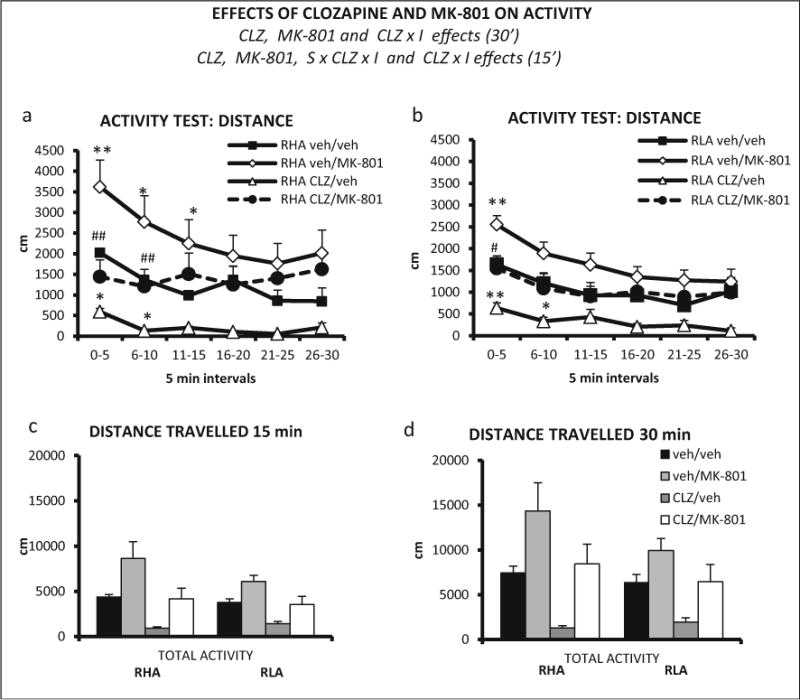
a Distance (±SEM) traveled in each 5-min interval of the activity test, by RHA-I rats and treatment combinations (n = 6 rats/group). b Distance (±SEM) traveled in each 5-min interval of the activity test, by RLA-I rats and treatment combinations (veh/veh, n = 7; veh/MK-801, n = 7; CLZ/veh, n = 7; CLZ/MK-801, n = 8). c Total distance (±SEM) traveled by RHA-I and RLA-I rats in the first 15 min of the activity session. Total distance (±SEM) traveled by RHA-I and RLA-I rats in the 30 min of the activity session. d CLZ clozapine effect (ANOVA), MK-801 MK-801 effect (ANOVA), CLZ × I clozapine × time interval interaction (ANOVA), S × CLZ × I strain × clozapine × time interval interaction. **p <0.01, *p <0.05 vs veh/veh group; ##p <0.01, #p <0.05 between CLZ/MK-801 and veh/MK-801 groups (post hoc pair wise contrasts with the LSD test following significant S × CLZ × I effect from ANOVA)
We also conducted a repeated measures ANOVA with the first three intervals (i.e., first 15 min) of the session as within-subject factor. Main effects of clozapine and MK-801 were observed (F(1,46) 28.04, p ≤ 0.001, and F(1, 46) = 24.22, p ≤ 0.001, respectively), as well as a clozapine × interval (F (1.80, 82.63) = 14.39, p ≤ 0.001) and a strain × clozapine × interval interaction (F (1.80, 82.63) = 3.27, p ≤ 0.048). Post hoc comparisons to dissect this second-order (i.e., strain × clozapine × interval) interaction on locomotor activity during the first three 5-min (0–5, 6–10, 11–15) intervals showed that MK-801 increased locomotor activity of RHA-I rats during the three 5-min intervals (Fig. 10a), but only during the first 5-min interval in RLA-I rats (Fig. 10b). Moreover, the clozapine + MK-801 treatment produced a more marked attenuation of MK-801-induced hyperactivity in RHA-I (see intervals 0–5 and 6–10 in Fig. 10a) than in RLA-I rats (see interval 0–5 in Fig. 10b).
Discussion
The present report provides the first pharmacological characterization of the effects of antipsychotics (haloperidol, a typical antipsychotic, and clozapine, an atypical antipsychotic), propsychotic drugs (DOI and MK-801), and the mixed dopamine D1/D2 receptor agonist apomorphine on PPI and locomotor activity in the Roman rat strains. The main findings are as follows: (1) haloperidol normalized the deficit in PPI showed by RHA-I rats, without affecting PPI in RLA-I rats; moreover, haloperidol reduced locomotor activity more markedly in the RLA-I strain; (2) clozapine (a 5-HT2A and dopamine D2 receptor antagonist) failed to affect PPI in either strain, but reduced locomotor activity in both strains; (3) apomorphine impaired PPI only in RHA-I rats; (4) the propsychotic drug DOI (5-HT2A receptor agonist) disrupted PPI in RLA-I rats in a dose-dependent manner while only the 0.5 mg/kg dose impaired PPI in RHA-I rats; (5) DOI moderately decreased locomotor activity in both strains; (6) clozapine significantly antagonized the MK-801-induced PPI deficit only in RLA-I rats; and (7) clozapine appeared to antagonize MK-801-induced hyperactivity more effectively in RHA-I vs RLA-I rats.
Remarkably, a general finding of all PPI experiments was that the total averaged %PPI was consistently lower in RHA-I than in RLA-I rats, which is in agreement with previous studies showing that the RHA-I strain displays a relative deficit in PPI (Oliveras et al. 2015; Esnal et al. 2016; Del Río et al. 2014).
Haloperidol and clozapine effects on PPI and locomotor activity
In order to assess the predictive validity of genetically based rat models of schizophrenia-related symptoms, it is crucial that propsychotic and antipsychotic drugs are tested alone in naive animals, to evaluate whether they are able to further impair or reverse PPI deficits, respectively (e.g., Geyer et al. 2001). For example, using this approach, Hadamitzky et al. (2007) found that the deficits displayed by rats bred for their low scores of PPI could be restored by haloperidol, but not by clozapine. Moreover, Cilia et al. (2010) reported that Brattleboro rats (which have a mutant gene for vasopressin and display impaired PPI) show a significant improvement of PPI upon treatment with haloperidol and atypical antipsychotics like clozapine and risperidone. In the present study, we have used this approach in order to gather evidence on the pharmacological validity of the Roman rat strains (which markedly differ in their basal PPI performance) as a model of differential schizophrenia-related features.
We report that haloperidol 1 mg/kg reverses the PPI deficit of RHA-I rats, without affecting RLA-I rats, whereas the atypical antipsychotic clozapine does not affect PPI in either strain. Moreover, haloperidol reduced baseline startle of both strains in Exp. 1 but did not affect it in Exp. 2, while clozapine (Exp. 7) also decreased startle in both strains. The fact that in Exp. 2, haloperidol increased PPI in RHA-I rats in the absence of changes of baseline startle, strengthens the idea that the drug genuinely improves sensorimotor gating in this strain (Swerdlow et al. 2000). Conversely, the lack of effects of clozapine might be related to its startle-decreasing effects (in pulse-alone trials) in both strains. Similar effects were observed by Hadamitzky et al. (2007) in their selectively bred low-PPI rat line, as they also found that haloperidol but not clozapine improved PPI. The authors attributed the lack of effects of clozapine to its (baseline) startle-inhibiting effects, although they also noted that when this drug has an effect, it is often in a narrow dose range (i.e., it is likely to miss an effective dose in a particular study) and that its effects (both on startle and on PPI) vary between different rat strains, with Wistar rats (which are the original strain from which the RHA/RLA lines were derived) being a particularly resistant strain (Hadamitzky et al. 2007). These results are in contrast with previous studies usually showing that typical antipsychotics do not ameliorate PPI deficits while atypical antipsychotics revert PPI deficits (among other negative and cognitive symptoms) in naive rats (see Geyer et al. 2001; but also see Cilia et al. 2010; Feifel et al. 2001; Hadamitzky et al. 2007).
Finally, haloperidol more markedly reduced locomotor activity in RLA-Is than RHA-I rats, while clozapine reduced activity of both rat strains to a similar extent. While there have been no previous studies with clozapine in these strains, the effects of haloperidol on locomotor activity are consistent with previous studies with the Roman rats. For example, Sanna et al. (2014b) reported that haloperidol impaired copulatory behavior in RLA, but not RHA rats. The authors interpreted that these effects may be due to the lowered dopaminergic tone and increased D2 receptor density (Tournier et al. 2013) in the brain of RLA rats, which might make this strain more sensitive than RHA rats to the inhibition of locomotion induced by haloperidol, thereby interfering with copulatory behavior. Moreover, in keeping with this hypothesis and with our present results, Eilam and Szechtman (1989) reported that RHA rats are less sensitive than their RLA counterparts to the inhibitory effect on locomotion of quinpirole, which is thought to be mediated by the activation of presynaptic inhibitory D2 autoreceptors.
Apomorphine and DOI effects on PPI and locomotor activity
The mixed D1/D2 receptor agonist apomorphine and the propsychotic (5-HT2A agonist) DOI globally impaired PPI, in a strain-dependent manner. Thus, the apomorphine-induced impairment was evident only in RHA-I rats, whereas the PPI-disrupting effects of DOI were more robust in RLA-I rats. Moreover, apomorphine decreased startle responses to pulse-alone stimuli in RHA-I rats, and a similar trend was observed with DOI. It might be argued that the disruption of PPI induced by apomorphine in RHA-I rats might be related to the observed reduction of startle responses in that strain; however, the PPI-disrupting apomorphine effect is a well-established phenomenon (e.g., Swerdlow et al. 2000), which is sometimes paralleled by a drug-induced reduction of startle responses to pulse-alone trials (e.g., Breier et al. 2010; Swerdlow et al. 2000). As the phenomenon has been replicated in several rat strains and has been shown to be heritable, regardless of whether or not it is sometimes associated to a reduction of startle responses, it is generally considered as solid evidence that apomorphine induces an impairment of sensorimotor gating (e.g., Breier et al. 2010; Swerdlow et al. 2000). Similarly, the PPI-disruptive effect of DOI, also a well-characterized phenomenon (e.g., Farid et al. 2000; Wischhof et al. 2012, see below), is present in RLA-I rats at doses devoid of effects on baseline startle response (see Fig. 5b, c). A moderate decrease of locomotor activity was also observed with DOI (as said in “Materials and methods” section, the effects of apomorphine on locomotor activity in both strains were not addressed here because they have been well characterized in our previous study; Gimenez-Llort et al. 2005).
The above results give support to our hypotheses as, based on neurochemical and neuropharmacological evidence of differential central dopaminergic function between both Roman rat lines/strains (see “Introduction”), we predicted that RHA-I rats would be more sensitive to the haloperidol-improving and apomorphine-impairing effects on PPI. In fact, there is mounting evidence supporting the view that the functional tone of nigrostriatal and mesolimbic dopamine neurons is more robust in RHA than RLA rats, and it has been suggested that the lower availability of inhibitory D2 autoreceptors in the cell bodies of nigrostriatal and mesolimbic dopaminergic neurons of RHA rats (Tournier et al. 2013) may account (at least in part) for the behavioral differences between the two rat lines/strains (e.g., Driscoll et al. 2009; Giorgi et al. 2007; Guitart-Masip et al. 2008a, b; Sanna et al. 2013, 2014a, b; Tournier et al. 2013). Together with the finding that RHA rats show increased sensitivity to dopaminergic psychostimulants (Corda et al. 2005; Giorgi et al. 2007; Gimenez-Llort el al. 2005) and to the behavioral and neurochemical sensitization effects of these drugs (Corda et al. 2005; Giorgi et al. 2007; Guitart-Masip et al. 2008a), the results of the present PPI experiments using haloperidol and apomorphine lend further support to the validity and usefulness of RHA-I vs RLA-I rats as a model of some dopamine-related schizophrenia-relevant symptoms.
On the other hand, in keeping with previous reports, DOI significantly disrupted PPI in both strains (e.g., Sipes and Geyer 1997; Johansson et al. 1995; Padich et al. 1996; Sipes and Geyer 1995b), and consistent with our hypothesis, this effect was more pronounced in RLA-I than in RHA-I rats (see Fig. 5). A differential strain-related sensitivity to the PPI-disrupting effect of DOI has also been reported by Wischhof et al. (2012), who observed DOI-induced PPI deficits in Wistar, but not Lister Hooded, rats. Our results also show that RLA-I rats are more sensitive to DOI than their RHA-I counterparts. Several mechanisms may explain these strain-related differences in the effects of DOI on PPI, including drug sensitivity, receptor densities or affinities, as well as the coupling to second messengers (Wischhof et al. 2012). In this context, the recently discovered relationship between 5-HT2A (which are the molecular target of DOI) and mGlu2 receptors (Delille et al. 2012; Gonzalez-Maeso et al. 2008) may also be involved in the contrasting effects mentioned above. Thus, Gonzalez-Maeso et al. (2008) and Delille et al. (2012), among others, have proposed that a receptor complex containing 5-HT2A and mGluR2 integrates serotoninergic and glutamatergic signaling in order to convey sensorimotor information in a proper manner. Furthermore, the finding that these receptors are dysregulated in schizophrenic patients supports the idea that the 5-HT2A/mGluR2 receptor complex plays a major role in the neurochemistry of schizophrenia. Accordingly, the expression of psychotic states by 5-HT receptor agonists and antipsychotic effects by atypical antipsychotic drugs (i.e., 5-HT receptor antagonists) requires the presence of both 5-HT2A and mGluR2 receptors (Fribourg et al. 2011; Gonzalez-Maeso et al. 2008; Klein et al. 2014). Remarkably, as reported by Klein et al. (2014), RHA-I rats showed no detectable protein levels of mGlu2 receptor protein in the frontal cortex, hippocampus, and the striatum, a deficit that is apparently due to a mutation in the grm2 gene (Wood et al. 2016). Thus, in line with the above evidence, it seems plausible that the reduced PPI-impairing effects of DOI in RHA-I rats (relative to RLA-Is) may be due to their low cerebral density of mGluR2 receptors. On the other hand, although there were overall decreasing effects of DOI on baseline startle, taking both rat strains together, the impairment of PPI in RLA-I rats was independent of DOI effects on the basal startle responses (see Fig. 5b, c).
Finally, it is noteworthy that both DOI (i.e., 5-HT2A and 5-HT2C agonist) and clozapine (5-HT2A antagonist and antagonist of various 5-HT, DA, and other receptors), despite having partially opposite neurochemical mechanisms of action (i.e., agonist and antagonist of 5-HT2A receptors, respectively), induced decreases of locomotor activity that were similar in both rat strains. At least three possibilities may be proposed to explain this discrepancy: (i) the locomotor activity test is not specific enough to distinguish between opposite drug-receptor interactions, (ii) the neural mechanism(s) of action that lead to the decrease of locomotion may be different from those involved in PPI effects, and (iii) given that RHA-I and RLA-I rats present the above mentioned differences in central 5-HT2A receptor levels, the mechanisms underlying clozapine- and DOI-induced reduction of activity may not involve (at least not preferentially) 5-HT2A receptors.
Impairment of PPI and hyperactivity induced by MK-801: reversal by clozapine
It is well established that NMDA receptor antagonists (PCP, MK-801, ketamine) impair PPI in rodents (for review, see Geyer et al. 2001). Most studies indicate that the disruption of PPI induced by PCP can be reversed/attenuated by clozapine in different species (Andreasen et al. 2006; Ballmaier et al. 2001; Linn et al. 2003; Lipina et al. 2005; Swerdlow et al. 1996), but there is comparatively more controversial evidence on the effects of atypical antipsychotics on MK-801-impaired PPI (Bakshi et al. 1994; Bast et al. 2000; Bubenikova et al. 2005; Fijal et al. 2014; Levin et al. 2005, 2007; Zangrando et al. 2013). However, the experimental evidence seems to suggest that the PPI deficits induced by the administration of NMDA antagonists in rodents can be reversed/attenuated by some atypical antipsychotics but not by the typical antipsychotic haloperidol (e.g., Adell et al. 2012; Bubenikova et al. 2005; Jentsch and Roth 1999). Thus, following the recent finding that RHA-I rats exhibit increased 5-HT2A receptor density (related to their mGluR2 deficit) and to assess whether this characteristic may lead to different pharmaco-behavioral profiles as compared to RLA-I rats, we chose to antagonize MK-801 effects on PPI and locomotion with clozapine.
The results show that MK-801 produced PPI deficits in both rat strains. Interestingly, the MK-801-induced deficit in PPI was more clearly attenuated by clozapine in RLA-I rats. Also, in agreement with previous reports, MK-801 induced a global increase of startle responses in both rat strains (Bakshi et al. 1994; Bast et al. 2000; Bubenikova et al. 2005; Fijal et al. 2014; Levin et al. 2005). As discussed for the effects of DOI on PPI (see above), the finding that clozapine antagonizes the PPI deficit elicited by MK-801 more effectively in RLA-I than RHA-I rats is consistent with the putative important role of the 5-HT2A/mGluR2 receptorial complex in sensorimotor gating and preattentive processes (Gonzalez-Maeso et al. 2008). Thus, because of their dramatic deficit of mGlu2 receptors (Klein et al. 2014), RHA-I rats would be expected to display weaker effects of 5-HT2A-receptor ligands on PPI as compared with their RLA-I counterparts (Gonzalez-Maeso et al. 2008; Fribourg et al. 2011). This prediction is confirmed by the effects on PPI of DOI (Exp. 4) and clozapine + MK-801 (Exp. 9) reported herein.
The finding that MK-801 increases the baseline startle response suggests that this effect may have contributed to the impairment of PPI induced by MK-801 observed in the present study. However, several lines of evidence argue against this hypothesis. First, the PPI-disrupting effect of MK-801 in rats is a well-established phenomenon that has been observed either in the presence (Bakshi et al. 1994; Bast et al. 2000; Bubenikova et al. 2005; Fijal et al. 2014; Levin et al. 2005) or in the absence of increases of the baseline startle response (Bast et al. 2000; Levin et al. 2005, 2007; Zangrando et al. 2013). Second, it is generally assumed that calculating individual PPI as a percentage of each animal’s startle response diminishes the influence that changes in startle reactivity have on PPI (e.g., Feifel et al. 2001; Swerdlow et al. 1994; but see also Swerdlow et al. 2000). Third, baseline startle response and %PPI appear to be dissociable, as suggested by manipulations that change baseline startle but not %PPI (e.g., Feifel et al. 2001; Rigdon 1990; Swerdlow et al. 1986), treatments that alter %PPI but not startle (e.g., Feifel et al. 2001; Furuya et al. 1999; Rigdon 1990; Swerdlow et al. 1990b), or interventions that lead to changes of baseline startle and %PPI either in the same or opposite directions (e.g., Acri et al. 1995; al-Amin and Schwarzkopf 1996). Fourth, according to several studies, individual startle amplitude and %PPI are not necessarily correlated (Feifel 1999; Feifel et al. 2001; Oliveras et al. 2015; Paylor and Crawley 1997), although sometimes, they show low positive associations (Logue et al. 1997; Oliveras et al. 2015; Sanchez-Gonzalez et al. 2016). Fifth, although it has been reported that baseline startle tends to be negatively related with %PPI in some studies with rodents and humans (Csomor et al. 2008; Ellenbroek et al. 1995), other studies, using the Roman rat strains as well as the genetically heterogeneous NIH-HS rat stock, have demonstrated just the opposite, i.e., a positive association between baseline startle and %PPI (Del Rio et al. 2014; Esnal et al. 2016; Oliveras et al. 2015; Sanchez-Gonzalez et al. 2016). Sixth, evidence supporting the dissociation of baseline startle and %PPI comes also from the present study, as haloperidol increased %PPI and did not alter startle in RHA-I rats (Exp. 2), apomorphine and DOI reduced %PPI and also baseline startle in RHA-I rats, MK-801 reduced %PPI but increased the baseline startle, while DOI reduced %PPI at doses not affecting startle in RLA-I rats.
Finally, we observed that MK-801 induced a more robust increment in locomotion in RHA-I vs RLA-I rats, especially during the first 15 min of the activity test, which was also more effectively attenuated by clozapine in the RHA-I strain. One possible explanation for these result is that the larger increment in locomotion elicited by MK-801 in RHA-I vs RLA-I rats could depend on the stimulatory effect of MK-801 on mesolimbic dopaminergic neurons which, under basal conditions, display a more robust physiological tone in RHA vs RLA rats (see Giorgi et al. 2007, and references therein). In fact, there is evidence suggesting that NMDA antagonists mediate some symptoms, such as hyperactivity and sensorimotor deficits, through their actions on limbic dopaminergic pathways (Meltzer et al. 2011). Specifically, the NMDA antagonist-induced stimulation of DA release in the nucleus accumbens has been suggested to result from decreased mesocortical dopaminergic function, which in turn enhances (i.e., disinhibits) mesolimbic dopaminergic activity (Del Arco et al. 2007; Del Arco and Mora 2008, 2009; Lannes et al. 1991; Jentsch et al. 1998; Zangrando et al. 2013). The different physiological tone of the mesolimbic dopaminergic projections of RHA and RLA rats may also be involved in the more effective antagonism of the MK-801-elicited locomotor activation by clozapine in RHA-I vs RLA-I rats.
Conclusions
In conclusion, regarding sensorimotor gating, the main novel findings of the present study are that (see Table 1), compared to RLA-I, RHA-I rats show higher sensitivity to the improving effects of haloperidol and to the impairing effects of apomorphine on PPI. Conversely, RLA-I rats are more sensitive than their RHA-I counterparts to the PPI-impairing effects of DOI and MK-801, as well to the reversal of MK-801 effect by clozapine.
Table 1.
Qualitative summary of the main effects on %PPI and locomotor activity observed with the tested drugs
| RHA
|
RLA
|
|||
|---|---|---|---|---|
| %PPI | Locomotor activity | %PPI | Locomotor activity | |
| Exps. 1, 2–3 | ||||
| Haloperidol | ↑ | ↓ | = | ⇊ |
| Exps. 7–8 | ||||
| Clozapine | = | ↓ | = | ↓ |
| Exp. 4 | ||||
| Apomorphine | ↓ | NT | = | NT |
| Exps. 5–6 | ||||
| DOI | ↓ | ↓ | ⇊ | ↓ |
|
Exps. 9–10 (clozapine + MK801) |
||||
| Clozapine | = | ↓ | = | ↓ |
| MK-801 | ↓ | ⇈ | ⇊ | ↑ |
| Clozapine + MK-801 | = | ⇈ | ↑ | ↑ |
| (antagonism of MK-801 effects by CLZ)* | ||||
Symbols: = no effect; ↑, ⇈ increase;
antagonist effect; ↓, ⇊ decrease or impairment NT not tested, CLZ clozapine
With regard to locomotor activity, its reduction by haloperidol is more pronounced in RLA-I rats, whereas MK-801-induced hyperactivity and its reversal by clozapine appear to be more marked in RHA-I rats (see Table 1).
The well-known between-strain differences in central dopaminergic function (RHA > RLA) may underlie the observed strain-dependent effects of haloperidol and apomorphine on PPI and locomotor activity. As discussed above, these dopaminergic differences may also be involved in the more robust hyperactivity induced by MK-801 (and its reversal by clozapine) in the RHA-I strain.
Moreover, the results obtained with DOI and clozapine + MK-801 on PPI are consistent with the finding that the RHA-I strain has an alteration of the 5-HT2A/mGluR2 complex (i.e., a deficit of mGlu2 receptors and a relative increase of 5-HT2A receptors; Klein et al. 2014; Wood et al. 2016), similar to that observed in schizophrenic patients (Gonzalez-Maeso et al. 2008). Thus, RHA-I rats may represent a model of dopamine- and 5-HT2A/mGluR2-related schizophrenia-relevant features.
Acknowledgments
This work was supported by grants PSI2013-41872-P (MINECO), 2014SGR-1587 (DGR), “ICREA-Academia 2013” to AF-T and by a grant from the Regional Government of Sardinia (RAS) (L.R.7/2007, Project code no. CRP-59842) to OG. IO is recipient of a PhD FI fellowship (DGR 2014). AS-G is recipient of a PhD FPI fellowship. MAP is a recipient of a PhD fellowship from RAS.
Abbreviations
- PPI
Prepulse inhibition
- RLA
Roman high-avoidance
- RHA
Roman low-avoidance
References
- Acri JB, Brown KJ, Saah MI, Grunberg NE. Strain and age differences in acoustic startle responses and effects of nicotine in rats. Pharmacol Biochem Behav. 1995;50:191–198. doi: 10.1016/0091-3057(94)00285-Q. [DOI] [PubMed] [Google Scholar]
- Adell A, Jimenez-Sanchez L, Lopez-Gil X, Romon T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophrenia Bull. 2012;38:9–14. doi: 10.1093/schbul/sbr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar R, Escorihuela RM, Gil L, Tobeña A, Fernández-Teruel A. Differences between two psychogenetically selected lines of rats in a swimming pool matching-to-place task: long-term effects of infantile stimulation. Behav Genet. 2002;32:127–134. doi: 10.1023/A:1015253807488. [DOI] [PubMed] [Google Scholar]
- Al-Amin HA, Schwarzkopf SB. Effects of the PCP analog dizocilpine on sensory gating: potential relevance to clinical subtypes of schizophrenia. Biol Psychiatry. 1996;40:744–754. doi: 10.1016/0006-3223(95)00485-8. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Andersen KK, Nielsen EØ, Mathiasen L, Mirza NR. Nicotine and clozapine selectively reverse a PCP-induced deficit of PPI in BALB/cByJ but not NMRI mice: comparison with risperidone. Behav Brain Res. 2006;167:118–127. doi: 10.1016/j.bbr.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonises phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- Ballmaier M, Zoli M, Mazzoncini R, Gennarelli M, Spano F. Combined alpha 2-adrenergic/D2 dopamine receptor blockade fails to reproduce the ability of clozapine to reverse phencyclidine-induced deficits in prepulse inhibition of startle. Psychopharmacology. 2001;159:105–110. doi: 10.1007/s002130100905. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang W, Feldon J, White IM. Effects of MK801 and neuroleptics on prepulse inhibition: re-examination in two strains of rats. Pharmacol Biochem Behav. 2000;67:647–658. doi: 10.1016/s0091-3057(00)00409-3. [DOI] [PubMed] [Google Scholar]
- Bignami G. Selection for high rates and low rates of avoidance conditioning in the rat. Anim Behav. 1965;13:221–227. doi: 10.1016/0003-3472(65)90038-2. [DOI] [PubMed] [Google Scholar]
- Breier MR, Lewis B, Shoemaker J, Light G, Swerdlow NR. Sensory and sensorimotor gating-disruptive effects of apomorphine in Sprague Dawley and long Evans rats. Behav Brain Res. 2010;208:560565. doi: 10.1016/j.bbr.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeníková V, Votava M, Horáček J, Páleníček T, Dockery C. The effect of zotepine, risperidone, clozapine and olanzapine on MK-801-disrupted sensorimotor gating. Pharmacol Biochem Behav. 2005;80:591–596. doi: 10.1016/j.pbb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Cilia J, Gartlon JE, Shilliam C, Dawson LA, Moore SH, Jones DNC. Further neurochemical and behavioural investigation of Brattleboro rats as a putative model of schizophrenia. J Psychopharmacol. 2010;24:407–419. doi: 10.1177/0269881108098787. [DOI] [PubMed] [Google Scholar]
- Corda MG, Piras G, Lecca D, Fernández-Teruel A, Driscoll P, Giorgi O. The psychogenetically selected roman rat lines differ in the susceptibility to develop amphetamine sensitization. Behav Brain Res. 2005;157:147–156. doi: 10.1016/j.bbr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortexlimbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm. 2009;116:941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F, Mohammed AH, Fuxe K. Stimulation of D2 receptors in the prefrontal cortex reduces PCP-induced hyperactivity, acetylcholine release and dopamine metabolism in the nucleus accumbens. J Neural Transm. 2007;114:185–193. doi: 10.1007/s00702-006-0533-3. [DOI] [PubMed] [Google Scholar]
- Del Río C, Oliveras I, Cañete T, Blázquez G, Tobeña A, Fernández-Teruel A. Genetic rat models of schizophrenia-relevant symptoms. World J Neurosci. 2014;4(3):261–278. [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, et al. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62:2184–2191. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Bättig K. Behavioural, emotional and neurochemical profiles of rats selected for extreme differences in active, two-way avoidance performance. In: Lieblich I, editor. Genetics of the brain. Elsevier; Amsterdam: 1982. pp. 95–123. [Google Scholar]
- Driscoll P, Ferré P, Fernández-Teruel A, Tobeña A, Escorihuela RM, Levi de Stein M, et al. Effects of prenatal diazepam on two-way avoidance behavior, swimming navigation and brain levels of benzodiazepine-like molecules in male roman high- and low-avoidance rats. Psychopharmacology. 1995;122:51–57. doi: 10.1007/BF02246441. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Fernández-Teruel A, Corda MG, Giorgi O, Steimer T. Some guidelines for defining personality differences in rats. In: Yong-Kyu K, editor. Handbook of behavior genetics. Springer; New York, NY: 2009. pp. 281–300. [Google Scholar]
- Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol. 1989;161:151–157. doi: 10.1016/0014-2999(89)90837-6. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Geyer MA, Cools AR. The behavior of APO-SUS rats in animal models with construct validity for schizophrenia. J Neurosci. 1995;15:7604–7611. doi: 10.1523/JNEUROSCI.15-11-07604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorihuela RM, Tobeña A, Fernández-Teruel A. Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (roman high-avoidance) and hyperemotional (roman low-avoidance) rats. Learning & Memory. 1995;2:40–48. doi: 10.1101/lm.2.1.40. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernández-Teruel A, Gil L, Aguilar R, Tobeña A, Driscoll P. Inbred roman high- and low-avoidance rats: differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol Behav. 1999;67:19–26. doi: 10.1016/S0031-9384(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Esnal A, Sánchez-González A, Río-Álamos C, Oliveras I, Cañete T, Blázquez G, et al. Prepulse inhibition and latent inhibition deficits in roman high-avoidance vs. roman low-avoidance rats: modeling schizophrenia-related features. Physiol Behav. 2016;163:267273. doi: 10.1016/j.physbeh.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Farid M, Martinez ZA, Geyer MA, Swerdlow NR. Regulation of sensorimotor gating of the startle reflex by serotonin 2A receptors. Ontogeny and strain differences. Neuropsychopharmacology. 2000;23:623–632. doi: 10.1016/S0893-133X(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Feifel D. Individual differences in prepulse inhibition of startle as a measure of individual dopamine function. Behav Neurosci. 1999;113:1020–1029. doi: 10.1037//0735-7044.113.5.1020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Priebe K, Shilling PD. Startle and sensorimotor gating in rats lacking CCK-A receptors. Neuropsychopharmacology. 2001;24:663670. doi: 10.1016/S0893-133X(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Teruel A, Blázquez G, Perez M, Aguilar R, Cañete T, Guitart M, et al. Latent inhibition threshold in roman high-avoidance rats: a psychogenetic model of abnormalities in attentional filter? Actas Esp. Psiquiatr. 2006;34:257–263. [PubMed] [Google Scholar]
- Fijał K, Popik P, Nikiforuk A. Co-administration of 5-HT6 receptor antagonists with clozapine, risperidone, and a 5-HT2A receptor antagonist: effects on prepulse inhibition in rats. Psychopharmacology. 2014;231:269–281. doi: 10.1007/s00213-013-3234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya Y, Kagaya T, Ogura H, Nishizawa Y. Competitive NMDA receptor antagonists disrupt prepulse inhibition without reduction of startle amplitude in a dopamine receptor-independent manner in mice. Euro J Pharmacol. 1999;364:133–140. doi: 10.1016/S0014-2999(98)00839-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Castillo-Ruiz MM, Put T, Tobeña A, Fernández-Teruel A. Differential hippocampal neuron density between inbred roman high- (low anxious) and low-avoidance (high anxious) rats. Neurosci Lett. 2012;522:41–46. doi: 10.1016/j.neulet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giménez-Llort L, Cañete T, Guitart-Masip M, Fernández-Teruel A, Tobeña A. Two distinctive apomorphine-induced phenotypes in the roman high- and low-avoidance rats. Physiol Behav. 2005;86:458466. doi: 10.1016/j.physbeh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Lecca D, Piras G, Driscoll P, Corda MG. Dissociation between mesocortical dopamine release and fear-related behaviours in two psychogenetically selected lines of rats that differ in coping strategies to aversive conditions. Eur J Neurosci. 2003;17:2716–2726. doi: 10.1046/j.1460-9568.2003.02689.x. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Piras G, Corda MG. The psychogenetically selected roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci Biobehav R. 2007;31:148–163. doi: 10.1016/j.neubiorev.2006.07.008. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M, Johansson B, Cañete T, Fernández-Teruel A, Tobeña A, Terenius L, Giménez-Llort L. Regional adaptations in PSD-95, NGFI-A and secretogranin gene transcripts related to vulnerability to behavioral sensitization to amphetamine in the roman rat strains. Neuroscience. 2008a;151:195–208. doi: 10.1016/j.neuroscience.2007.09.072. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Johansson B, Fernández-Teruel A, Tobeña A, Giménez-Llort L. Divergent effect of the selective D3 receptor agonist PD-128,907 on locomotor activity in roman high- and low-avoidance rats: relationship to NGFI-A gene expression in the Calleja islands. Psychopharmacology. 2008b;196:39–49. doi: 10.1007/s00213-007-0925-6. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Harich S, Koch M, Schwabe K. Deficient prepulse inhibition induced by selective breeding of rats can be restored by the dopamine D2 antagonist haloperidol. Behav Brain Res. 2007;177:364367. doi: 10.1016/j.bbr.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Serotonergic hallucinogens as translational models relevant to schizophrenia. Int J Neuropsychoph. 2013;16:2165–2180. doi: 10.1017/S1461145713000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19:105–113. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-X. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Brit J Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AB, Ultved L, Adamsen D, Santini MA, Tobeña A, Fernandez-Teruel A, et al. 5-HT(2A) and mGlu2 receptor binding levels are related to differences in impulsive behavior in the roman low-(RLA) and high-(RHA) avoidance rat strains. Neuroscience. 2014;263:36–45. doi: 10.1016/j.neuroscience.2013.12.063. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkötter J, Kuhn J. Prepulse inhibition in psychiatric disorders-apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Lannes B, Micheletti G, Warter JM, Kempf E, Di Scala G. Behavioural, pharmacological and biochemical effects of acute and chronic administration of ketamine in the rat. Neurosci Lett. 1991;128:177–181. doi: 10.1016/0304-3940(91)90255-r. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Caldwell DP. Nicotine and clozapine actions on pre-pulse inhibition deficits caused by N-methyl-D-aspartate (NMDA) glutamatergic receptor blockade. Prog Neuro-Psychoph. 2005;29:581–586. doi: 10.1016/j.pnpbp.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Levin ED, Caldwell DP, Perraut C. Clozapine treatment reverses dizocilpine-induced deficits of pre-pulse inhibition of tactile startle response. Pharmacol Biochem Behav. 2007;86:597–605. doi: 10.1016/j.pbb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn GS, Negi SS, Gerum SV, Javitt DC. Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology. 2003;169:234–239. doi: 10.1007/s00213-003-1533-8. [DOI] [PubMed] [Google Scholar]
- Lipina T, Labrie V, Weiner I, Roder J. Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology. 2005;179:54–67. doi: 10.1007/s00213-005-2210-x. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/S0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology. 2011;213:289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- Meyza KZ, Boguszewski PM, Nikolaev E, Zagrodzka J. Diverse sensitivity of RHA/Verh and RLA/Verh rats to emotional and spatial aspects of a novel environment as a result of a distinct pattern of neuronal activation in the fear/anxiety circuit. Behav Genet. 2009;39:4861. doi: 10.1007/s10519-008-9234-z. [DOI] [PubMed] [Google Scholar]
- Moreno M, Cardona D, Gómez MJ, Sánchez-Santed F, Tobeña A, Fernández-Teruel A, et al. Impulsivity characterization in the roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology. 2010;35:1198–1208. doi: 10.1038/npp.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nil R, Bättig K. Spontaneous maze ambulation and Hebb-Williams learning in roman high-avoidance and roman low-avoidance rats. Behav Neural Biol. 1981;33:465–475. doi: 10.1016/S0163-1047(81)91833-1. [DOI] [PubMed] [Google Scholar]
- Oliveras I, Río-Alamos C, Cañete T, Blázquez G, Martínez-Membrives E, Giorgi O, et al. Prepulse inhibition predicts spatial working memory performance in the inbred roman high- and low-avoidance rats and in genetically heterogeneous NIH-HS rats: relevance for studying pre-attentive and cognitive anomalies in schizophrenia. Front Behav Neurosci. 2015;9:213. doi: 10.3389/fnbeh.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiat. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon GC. Differential effects of apomorphine on prepulse inhibition of acoustic startle reflex in two rat strains. Psychopharmacology. 1990;102:419–421. doi: 10.1007/BF02244115. [DOI] [PubMed] [Google Scholar]
- Rio-Alamos C, Oliveras I, Cañete T, Blázquez G, Martínez-Membrives E, Tobeña A, Fernández-Teruel A. Neonatal handling decreases unconditioned anxiety, conditioned fear, and improves two-way avoidance acquisition: a study with the inbred roman high (RHA-I)- and low-avoidance (RLA-I) rats of both sexes. Front Behav Neurosci. 2015;9:174. doi: 10.3389/fnbeh.2015.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallés J, López de Jeúus M, Goñi O, Fernández-Teruel A, Driscoll P, Tobeña A, Escorihuela RM. Transmembrane signaling through phospholipase C in cortical and hippocampal membranes of psychogenetically selected rat lines. Psychopharmacology. 2001;154:115–125. doi: 10.1007/s002130000621. [DOI] [PubMed] [Google Scholar]
- Sánchez-González A, Esnal A, Río-Álamos C, Oliveras I, Cañete T, Blázquez G, et al. Association between prepulse inhibition of the startle response and latent inhibition of two-way avoidance acquisition: a study with heterogeneous NIH-HS rats. Physiol Behav. 2016;155:195–201. doi: 10.1016/j.physbeh.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Sanna F, Corda MG, Melis MR, Piludu MA, Löber S, Hübner H, et al. Dopamine agonist-induced penile erection and yawning: a comparative study in outbred roman high- and low-avoidance rats. Pharmacol Biochem Behav. 2013;109:59–66. doi: 10.1016/j.pbb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Sanna F, Corda MG, Melis MR, Piludu MA, Giorgi O, Argiolas A. Male roman high and low avoidance rats show different patterns of copulatory behaviour: comparison with Sprague Dawley rats. Physiol Behav. 2014a;127:27–36. doi: 10.1016/j.physbeh.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Sanna F, Piludu MA, Corda MG, Argiolas A, Giorgi O, Melis MR. Dopamine is involved in the different patterns of copulatory behaviour of roman high and low avoidance rats: studies with apomorphine and haloperidol. Pharmacol Biochem Behav. 2014b;124:211219. doi: 10.1016/j.pbb.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT(2A) and not by 5-HT(2C) receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/S0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Steimer T, Driscoll P. Divergent stress responses and coping styles in psychogenetically selected roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress. 2003;6:87–100. doi: 10.1080/1025389031000111320. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA, Koob GF. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biol Psychiatry. 1986;21:23–33. doi: 10.1016/0006-3223(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology. 1990;100:413–416. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff D, Taiid N, Geyer MA. Assessing the validity of an animal model of sensorimotor gating deficits in schizophrenia. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Bakshi V, Geyer M. Seroquel restores sensorimotor gating in phencyclidine-treated rats. J Pharmacol Exp Ther. 1996;279:1290–1299. [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibanez V, Ginovart N. Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol. 2013;16:1819–1834. doi: 10.1017/S1461145713000205. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Aho HEA, Koch M. DOI-induced deficits in prepulse inhibition in Wistar rats are reversed by mGlu2/3 receptor stimulation. Pharmacol Biochem Behav. 2012;102:6–12. doi: 10.1016/j.pbb.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Wood CM, Nicolas CS, Choi SL, Roman E, Nylander I, Fernandez-Teruel A, et al. Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.03.020. (In press) [DOI] [PubMed] [Google Scholar]
- Zangrando J, Carvalheira R, Labbate G, Medeiros P, Longo BM, Melo-Thomas L, Silva RCB. Atypical antipsychotic olanzapine reversed deficit on prepulse inhibition of the acoustic startle reflex produced by microinjection of dizocilpine (MK-801) into the inferior colliculus in rats. Behav Brain Res. 2013;257:77–82. doi: 10.1016/j.bbr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Zeier H, Baettig K, Driscoll P. Acquisition of DRL-20 behavior in male and female, roman high- and low-avoidance rats. Physiol Behav. 1978;20:791–793. doi: 10.1016/0031-9384(78)90307-4. [DOI] [PubMed] [Google Scholar]


