Abstract
A combination of Cpl-1, a bacteriophage lytic enzyme, and penicillin, gentamicin, levofloxacin, or azithromycin was tested against Streptococcus pneumoniae strains with various susceptibilities to penicillin. Activities of Cpl-1 and gentamicin were increasingly synergistic with a decreasing penicillin MIC, while Cpl-1 and penicillin showed synergy against an extremely penicillin-resistant strain.
Streptococcus pneumoniae is a leading cause of pneumonia, otitis media, meningitis, and sepsis worldwide, and the increase in antibiotic resistance of pneumococci has limited the number of antimicrobial agents that produce reliable treatment results for these infections. Combination therapy by antimicrobials with different mechanisms of action has been used to treat infections for decades with the goal of producing a wider spectrum of action, preventing the emergence of drug-resistant subpopulations, reducing the dose of a single agent, or achieving a synergistic effect.
Lytic enzymes are produced by bacteriophages at the end of their replicative cycle and can digest the bacterial cell wall within seconds. Cpl-1 is a muramidase that specifically cleaves the glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine in the cell wall of S. pneumoniae (5). Purified recombinant Cpl-1 has shown extremely rapid killing of S. pneumoniae in vitro and can effectively eradicate these organisms from the nose or the bloodstream of mice (8, 9).
We previously reported the synergistic effect of a combination of two phage lytic enzymes with different enzymatic activities, Cpl-1 and Pal, against S. pneumoniae in vitro, and this effect has been confirmed with mouse sepsis models (6, 10). It has not been shown, however, whether lytic phage enzymes in combination with conventional antibiotics could improve their respective efficiencies against pneumococci. In this study we report the in vitro effect of the simultaneous use of Cpl-1 and penicillin, gentamicin, levofloxacin, or azithromycin against four different strains of S. pneumoniae, with MICs of penicillin indicating a range from sensitive to extremely resistant.
We produced and purified Cpl-1 from E. coli DH5α(pJML6) as described elsewhere (8, 10). Cpl-1 was lyophilized and stored at 4°C. Penicillin and gentamicin were purchased from Sigma (St. Louis, Mo.), levofloxacin was purchased from Ortho-McNeil (Raritan, N.J.), and azithromycin was purchased from Pfizer (New York, N.Y.). Stock solutions were made by resuspending the agents in sterile water.
For all the pneumococcal strains tested, we first determined MICs of each antibiotic and Cpl-1 by the macrodilution method, using 2 ml of Mueller-Hinton broth (BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.) supplemented with 5% lysed horse blood (Cleveland Scientific, Inc., Bath, Ohio). The method is described elsewhere in detail (1). The bacterial strains tested and the respective drug MICs are reported in Table 1.
TABLE 1.
MICs of drugs for pneumococcal strains
Antibiotic and Cpl-1 interactions were assessed by the checkerboard method with 96-well plates, in which Cpl-1 and the corresponding other drug were diluted twofold horizontally and vertically, with a final volume of 50 μl and an inoculum of 3 × 105 to 5 × 105 CFU of S. pneumoniae per well (3). The plates were incubated at 37°C without CO2 for 18 h and examined for growth with a reflective viewer. Each test was performed at three separate times. The results of the checkerboard testing were interpreted by the pattern they showed on an isobologram: the fractional inhibitory concentrations (FICs) of Cpl-1 and penicillin or gentamicin in each well along the inhibitory line were plotted in an x/y plot. The FIC index (FICI) was calculated as the lowest effective drug combination in a well along the 45° line with equal FICs (e.g., 1/1, 0.5/0.5, or 0.25/0.25), averaged over all repeated experiments. Synergy was defined as an FICI of ≤0.5 (3).
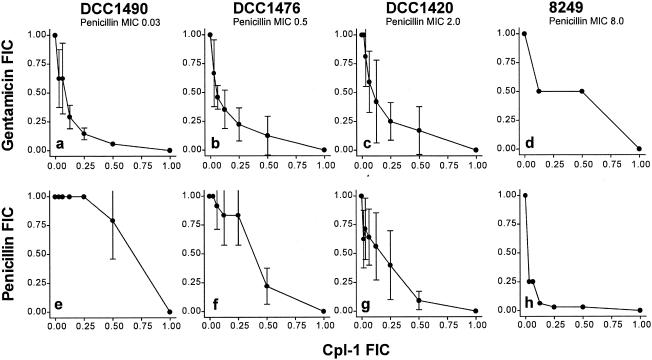
We initially tested the combination of Cpl-1 and penicillin or gentamicin against the three strains for which MICs of penicillin were between 0.03 and 2 μg/ml (12). As shown in Fig. 1a to c, the isobologram of Cpl-1 and gentamicin describes a synergistic curve for the susceptible strain DCC1490, but with increasing penicillin resistance we observed a trend away from an ideal synergistic curve. FICIs are shown in Table 2, and only with strain DCC1490 did results reach the definition of synergy. Surprisingly, with the combination of penicillin and Cpl-1 the checkerboard results showed the opposite (Fig. 1e to g). While none of the three strains revealed synergy, there appeared to be a clear shift toward an ideal synergistic curve as resistance to penicillin increased.
FIG. 1.
Synergy testing, using the checkerboard method. a-d, four increasingly penicillin-resistant strains were tested with combinations of Cpl-1 and gentamicin. Strain DCC1490 was killed synergistically (a); strains DCC1476 and DCC1420 were not quite killed synergistically (b-c) and 8249 not at all (d). e-h, the same strains were tested with combinations of Cpl-1 and penicillin. Only strain 8249 (h) was killed synergistically. The amount of the antimicrobial agents is expressed as an FIC on both scales. Error bars show standard deviations.
TABLE 2.
FIC indices for combination treatments with Cpl-1a
| Strain name | FICI for Cpl-1 and:
|
|||
|---|---|---|---|---|
| Gentamicin | Penicillin | Levofloxacin | Azithromycin | |
| DCC1490 | 0.50 | 1.67 | 1.00 | 1.00 |
| DCC1476 | 0.59 | 0.87 | 1.00 | 1.00 |
| DCC1420 | 0.61 | 0.78 | 1.00 | 1.00 |
| 8249 | 1.00 | 0.25 | 1.00 | 1.00 |
Synergistic combinations are in bold.
To confirm this observed trend, we applied the same testing method to a fourth strain, 8249 from South Africa, for which the penicillin MIC is known to be extremely high, 6 μg/ml (8 μg/ml with our testing method) (7). Here, in continuation of the trend, Cpl-1 showed synergistic killing with penicillin but only an indifferent effect with gentamicin (Fig. 1d and h, respectively; see Table 2 for FICIs).
To verify these findings, we performed time-kill assays by inoculating 4 × 106 CFU of S. pneumoniae in 2 ml of Mueller-Hinton broth supplemented with 5% lysed horse blood, with each antibiotic and enzyme alone or in combination at 0.25 MIC. Tubes were incubated at 37°C without CO2 for 19 h. Samples (10 μl) were removed after 4, 8, and 19 h, serially diluted, and plated on Columbia agar with 5% sheep blood for titer determination (detection limit, 104 CFU/ml). Experiments with penicillin were stopped at 8 h because of autolysis. The interaction was considered to be synergistic when the combination of two agents demonstrated a ≥100-fold-lower final titer compared to the most efficient single agent and compared to the inoculum titer. Each assay was performed at least twice, and results are reported as means and standard deviations.
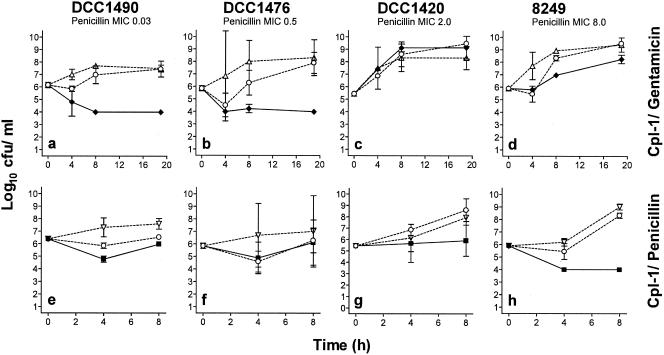
Similar to the checkerboard results, time-kill assays with Cpl-1 and gentamicin showed synergistic activity against the more susceptible strains, DCC1490 and DCC1476 (Fig. 2a and b), with a more than 1,000-fold-lower final titer in combination than with the most active agent alone, as well as a decrease of ≥100-fold compared to the initial titer. This combination did not prove to be synergistic against the more penicillin-resistant strains, DCC1420 and 8249 (Fig. 2c to d).
FIG. 2.
Synergy testing using the time-kill assay. a-d, four increasingly penicillin-resistant strains are treated with 0.25 MIC of Cpl-1 (open circles), 0.25 MIC of gentamicin (open triangles), or a combination of both at 0.25 MIC of each (filled diamonds) for 20 h. The penicillin-susceptible strain DCC1490 (a) and the intermediate resistant strain DCC1476 (b) show synergistic killing. e-h, the same four strains are treated with 0.25 MIC of Cpl-1 (open circles), 0.25 MIC of penicillin (open inverted triangles), or a combination of both at 0.25 MIC of each (filled squares) for 8 h. The extremely penicillin-resistant strain 8249 (h) shows synergistic killing; the resistant DCC1420 (g) is not quite synergistic.
Whereas Cpl-1 and penicillin revealed no synergy in the penicillin-susceptible and intermediate strains (Fig. 2 e-f), we could show a more than 100-fold lower final titer in the highly resistant strain, although the titer did not decrease compared to the inoculum (Fig. 2 g). The final titer of the extremely resistant strain was 10,000-fold lower than with the most active single agent and 100-fold lower than the inoculum and the reaction was considered synergistic (Fig. 2 h).
We also performed synergy assays with the checkerboard method for the combination of Cpl-1 and levofloxacin or azithromycin. MICs are reported in Table 1 and FICIs in Table 2. Because neither combination was synergistic or antagonistic, time-kill assays were not carried out.
Purified recombinant bacteriophage lytic enzymes are novel and original agents that are highly effective, both in vitro and in animal models, against gram-positive bacteria (9, 11, 13). Potential synergistic interaction between phage enzymes and conventional antibiotics may have important clinical implications, particularly in situations where effective drug concentrations are unable to be achieved without concomitant toxicity, e.g., in highly antibiotic-resistant bacteria. Also, while Cpl-1 is awaiting clinical trials, its short half-life has already been determined in animal models (9), and combination treatments could be a way to alleviate this limitation.
In this study we were able to show that Cpl-1 acts synergistically with gentamicin against the strain for which the penicillin MICs are lowest, while the combination of Cpl-1 and penicillin shows a synergistic effect against the strain for which the penicillin MIC is highest. Moreover, when the 4 strains were considered in sequence as shown in Fig. 1 or Table 2, synergy (in a larger sense) of Cpl-1 and gentamicin disappeared with increasing penicillin MICs and synergy of Cpl-1 and penicillin emerged. Neither combination was found to be antagonistic against any of the four strains tested.
Cell wall-active antibiotics such as penicillin and vancomycin have been shown to act synergistically with gentamicin, a positively charged antibiotic, by improving gentamicin's penetration into the cell (2). On the other hand it is also possible that gentamicin improves the penetration of the cell wall active antibiotics toward their targets. In the case of Cpl-1, a similar mechanism is likely to be responsible for the observed synergy with gentamicin in penicillin susceptible strains. However, with the increasing changes in the bacterial cell wall that have been described in highly penicillin-resistant strains (4), this may no longer be possible.
Previously we established that the combination of two bacteriophage lytic enzymes with different cleavage specificities have a synergistic effect and is highly lethal against S. pneumoniae (10). We presumed that the increased access of these enzymes to their respective cleavage sites or the enhanced destructive effect of a two-dimensional digestion in the three-dimensional peptidoglycan was responsible for the observed synergy. A synergistic effect of the combination of Cpl-1 and penicillin could therefore be expected on similar grounds, but surprisingly this only became apparent with increasing penicillin-resistance, the exact reason for which is not entirely clear. Our observed synergistic effect of Cpl-1 and antibiotics was found to be antibiotic-specific, since we did not find any effect when levofloxacin or azithromycin were used with Cpl-1. We hesitate to speculate why this is so until we more fully understand the observed synergy with certain antibiotics.
Despite the fact that in vitro synergy results do not always translate in vivo, the particular susceptibility of penicillin-resistant pneumococci to the combination treatment may have an important clinical impact by improving therapy of invasive pneumococcal infections.
REFERENCES
- 1.Amsterdam, D. 1991. Susceptibility testing of antimicrobials in liquid media, p. 53-105. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 2.Cottagnoud, P., M. Cottagnoud, and M. G. Tauber. 2003. Vancomycin acts synergistically with gentamicin against penicillin-resistant pneumococci by increasing the intracellular penetration of gentamicin. Antimicrob. Agents Chemother. 47:144-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliopoulos, G., and R. Moellering. 1991. Antimicrobial combinations, p. 432-492. InV. Lorain (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 4.Filipe, S. R., E. Severina, and A. Tomasz. 2001. The role of murMN operon in penicillin resistance and antibiotic tolerance of Streptococcus pneumoniae. Microb. Drug Resist. 7:303-316. [DOI] [PubMed] [Google Scholar]
- 5.Garcia, P., A. C. Martin, and R. Lopez. 1997. Bacteriophages of Streptococcus pneumoniae: a molecular approach. Microb. Drug Resist. 3:165-176. [DOI] [PubMed] [Google Scholar]
- 6.Jado, I., et al. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52:967-973. [DOI] [PubMed] [Google Scholar]
- 7.Liu, H. H., and A. Tomasz. 1985. Penicillin tolerance in multiply drug-resistant natural isolates of Streptococcus pneumoniae. J. Infect. Dis. 152:365-372. [DOI] [PubMed] [Google Scholar]
- 8.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 9.Loeffler, J. M., S. Djurkovic, and V. A. Fischetti. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeffler, J. M., and V. A. Fischetti. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sa-Leao, R., et al. 2000. Carriage of internationally spread clones of Streptococcus pneumoniae with unusual drug resistance patterns in children attending day care centers in Lisbon, Portugal. J. Infect. Dis. 182:1153-1160. [DOI] [PubMed] [Google Scholar]
- 13.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]