Abstract
An experimental rat pneumonia model using two amoxicillin-susceptible (MICs, ≤0.015 and 2 μg/ml) and two non-amoxicillin-susceptible (MIC, 4 μg/ml) Streptococcus pneumoniae strains was developed for testing the efficacy of amoxicillin administered to simulate human serum kinetics after treatment with amoxicillin-clavulanate (2,000 and 125 mg, respectively, twice a day, for 2.5 days). The end points for efficacy were reductions in bacterial loads in the lungs and reductions in levels of pulmonary damage. For the amoxicillin-susceptible strains (serotypes 23F and 14), a decrease greater than 4.5 log10 CFU/pair of lungs was obtained, and the time for which the serum antibiotic concentration (SAC) was higher than the MIC (TSAC>MIC) was greater than 60% of the dosing interval. For non-amoxicillin-susceptible strains, the decrease in bacterial load was 1.34 to 1.75 log10 CFU/pair of lungs, with a TSAC>MIC of 46.7% of the dosing interval. An in vitro study showed that serotype 9V non-amoxicillin-susceptible strains behaved as tolerant-like to concentrations similar to those in the in vivo model. The high and maintained SACs (TSAC>MIC, >46% for all strains) significantly diminished lung injury (affected area of the lung and lung weight), compared to that in controls, by all strains, regardless of the MIC, bactericidal behavior in in vitro killing curves, or the serotype of the infecting strain. These results show the importance of host therapeutic end points in the evaluation of antibiotic efficacy. The antibiotic was more efficacious, for one nonsusceptible strain tested, when the treatment was started early (1 h postinoculation [p.i.]) than when treatment was delayed (24 h p.i.).
Acute pneumonia is the most frequent cause of death from infectious diseases in developed countries (16). Streptococcus pneumoniae is the leading cause of community-acquired pneumonia (2). Despite the availability of potent antimicrobial agents, pneumococcal pneumonia has a very high mortality, reaching 38% in some areas (3, 19). In addition, the appearance of pneumococcal strains with decreased susceptibility to β-lactams and other antibiotics has caused concern because in some areas as many as 35% of pneumococcal pneumonias are caused by non-penicillin-susceptible organisms (3). In spite of this, β-lactams are widely and successfully used for respiratory tract infections caused by S. pneumoniae strains, even non-penicillin-susceptible strains. However, there has been little experience with the treatment of pneumococcal pneumonia caused by strains for which amoxicillin MICs are higher than 2 μg/ml, because the probability of pathogens with such a reduced susceptibility profile is too low (11) and this warrants the use of animal models for testing efficacy. To cover pneumococcal strains for which amoxicillin MICs are 4 μg/ml for approximately 50% of the dosing interval with a twice-a-day (b.i.d.) regimen, a new sustained-release amoxicillin-clavulanate formulation has been developed (12). This antibiotic treatment has shown clinical efficacy in community-acquired pneumonia (8, 11, 22), but there was a small number of patients infected with pneumococci for which amoxicillin MICs were high.
The aim of this study was to compare the efficacies of amoxicillin in an experimental model of pneumonias caused by four pneumococcal strains with different amoxicillin susceptibilities. The antibiotic was administered so as to attain an amoxicillin pharmacokinetic profile similar to that obtained in humans after administration of the new sustained-release amoxicillin-clavulanic acid formulation of 2,000 and 125 mg, respectively, b.i.d. A secondary aim was to evaluate the effect of the time from inoculation to treatment initiation on antibiotic efficacy.
MATERIALS AND METHODS
Bacteria.
Four strains of S. pneumoniae with different in vitro susceptibilities to amoxicillin were selected based on their similar virulence in experimental animals. Strain AR30118 (serotype 23F) was fully susceptible (MIC, ≤0.015 μg/ml); the MIC for strain FJD70 (serotype 14) was at the susceptibility breakpoint (2 μg/ml); and two strains (strains AR06016 and AR09164, both belonging to serotype 9V) exhibited no susceptibility to amoxicillin (MIC, 4 μg/ml).
Antibiotic.
Amoxicillin trihydrate of known potency (SmithKline Beecham Pharmaceuticals, Worthing, England) was used in the in vitro studies. For in vivo (therapeutic) use, amoxicillin in commercial vials (Clamoxyl; SmithKline Beecham S.A., Madrid, Spain) was reconstituted in sterile solutions in 0.85% sodium chloride to the desired concentrations.
In vitro studies.
MICs of amoxicillin were determined by microdilution as previously described (17). Modal values from three separate determinations were considered. Conventional time-kill experiments were performed with the three strains that exhibited lower susceptibility to amoxicillin, at drug concentrations equivalent to 2 or 4 times the MIC, or without antibiotic, in shaking tubes containing 10 ml of Mueller-Hinton broth (Becton Dickinson and Co., Cockeysville, Md.) by following previously described methods (6). All time-kill curves were determined in duplicate.
Animals.
Specific-pathogen-free female Sprague-Dawley rats weighing approximately 300 g were purchased from the Centre d'Élevage R. Janvier (Le Genest, St.-Isle, France). Animals were individually housed in cages with free access to food and water.
The study was performed in accordance with prevailing regulations regarding the care and use of laboratory animals in the European Community and was approved by our ethical committee for animal experimentation.
Experimental pneumonia.
For each experiment, a stock inoculum was thawed, spread onto four agar plates containing 5% defibrinated sheep blood (BioMerieux, Marcy-l'Étoile, France), and incubated overnight at 37°C. The growth from all plates was suspended in 4 ml of Todd-Hewitt broth (Oxoid Ltd., Basinsgtoke, Hampshire, England) to reach approximately 2 × 109 CFU/ml. Immediately prior to infection, the suspension was diluted 1:10 in molten nutrient agar (Becton Dickinson and Co.) maintained at 40°C to give a final bacterial concentration of approximately 2 × 108 CFU/ml.
The day before bacterial inoculation, the animals were anesthetized with 95 mg of ketamine (Ketolar; Parke Davis, Barcelona, Spain)/kg of body weight and 10 mg of xylazine (Rompun; Bayer, Barcelona, Spain)/kg, and the jugular vein was cannulated for antibiotic administration as previously described (26).
To proceed with bacterial inoculation, the animals were again anesthetized and then infected by intratracheal instillation (23) of 100 μl, in cooled molten agar, containing the organism (approximately 2 × 107 CFU). Animals were maintained with an enriched O2 atmosphere for 1 min to prevent early mortality.
Amoxicillin administration.
The antibiotic was administered by continuous intravenous infusion adjusted to simulate the concentrations of amoxicillin achieved in the plasma of adults after b.i.d. administration of the enhanced formulation of 2,000 and 125 mg, respectively, of amoxicillin-clavulanate (Augmentin SR). The pharmacokinetic software was programmed to administer the antibiotic at the calculated rates in 12-h cycles over 2.5 days. Antimicrobial treatment was initiated 8 h postinoculation (p.i.) in experiments carried out with the four strains, and animals were sacrificed 72 h p.i. (four groups). Additionally, a study on the effect of the time from inoculation to treatment initiation was performed with one of the non-amoxicillin-susceptible strains used (AR09164), by dividing infected animals into two groups initiating antibiotic treatment at 1 and 24 h p.i., respectively, and sacrificing animals at 65 and 88 h p.i., respectively. Thus, in total, there were six experimental groups. Untreated control animals receiving 0.85% sodium chloride at a rate similar to that of antibiotic treatment were included in all experiments. Twelve to 20 rats were included in each experimental group.
Evaluation of therapy.
Four hours after the cessation of infusion (i.e., 65, 72, and 88 h after bacterial challenge for antibiotic treatment starting 1, 8, and 24 h p.i, respectively), the animals were killed with a 0.5-ml intraperitoneal injection of sodium pentobarbital (Euta-Lender; Normon Laboratories, Madrid, Spain) and weighed. Later, both lungs were removed aseptically, weighed, and processed as previously described (23). An overall blinded macroscopic examination of lungs to evaluate the extent (expressed as a percentage) of the affected area and histopathological studies were performed. The number of viable S. pneumoniae organisms present was evaluated as previously described (23). The lower limit of detection was 25 (1.4 log10) CFU/pair of lungs.
Antibiotic simulation profile.
Calculation of intravenous drug administration rates in order to simulate in rats the amoxicillin concentrations obtained in humans after oral administration of the amoxicillin-clavulanate (2,000- and 125-mg) sustained-release formulation was performed by using target concentrations obtained from a previous publication (12). The total area under the concentration-time curve from 0 to 12 h (AUC0-12) and partial areas at 17 different time intervals were calculated. These concentrations and the total AUC were considered the target concentrations for simulation (AUCtarget). The data on the pharmacokinetics of amoxicillin in rats that were used to determine dosing parameters were obtained from concentrations measured in uninfected animals after intravenous administration of a bolus dose of 44.5 mg/kg (the test dose). The total dose to be administered was calculated as (AUCtarget × test dose)/AUCtest, where AUCtest is the AUC resulting from the test dose. Taking into account all the previous data (human target concentrations and the amoxicillin elimination rate constant obtained in rats), the fraction of bioavailable drug, or “cumulated (absorbed) fraction at each interval,” was calculated by the Wagner-Nelson method (24). The cumulated fraction (absorbed) equals the cumulated dose administered at each time interval used, as the administration was performed with an intravenous catheter. Assuming, as previously calculated, that the total cumulated dose required was 56.13 mg/kg, calculation of the dose at each interval was straightforward. Concentrations found after this test was applied showed a departure from target concentrations in the first 2 h, and therefore a new schedule was calculated, adjusting new doses proportionally to changes desired in partial AUCs. The final total dose used was 374.60 mg/kg administered in 12-h cycles over 2.5 days (74.92 mg per kg and cycle).
Pharmacokinetic and pharmacodynamic analyses.
Animals were prepared as described under “Experimental pneumonia” above. The carotid artery and jugular vein were cannulated for blood sampling and antibiotic administration, respectively, as previously described (26). Flow rates were controlled with infusion pumps (PHD 2000; Harvard Instruments, Edenbridge, United Kingdom) linked serially to a microcomputer. The system was programmed to administer the calculated rates in a 12-h cycle. Blood samples were taken from the carotid artery cannulae of nine animals at eight intervals (30, 60, 90, 210, 300, 420, 600, and 720 min) during antimicrobial infusion for determination of serum antibiotic concentrations (SACs). The concentration of amoxicillin was determined by a bioassay according to previously described techniques (13) using Micrococcus luteus ATCC 9341 as the indicator organism. The antimicrobial concentrations in the samples were derived from a standard solution prepared in pooled rat serum. The within-day and between-day coefficients of variation for amoxicillin were 2.9 to 7.9% and 4.0 to 6.2%, respectively. The lower limit of detection was 0.06 μg/ml.
The antibiotic concentration-time curve was analyzed by a noncompartmental approach using the Win-Nonlin program (Pharsight, Mountain View, Calif.). Times for which the SAC exceeded the MIC (TSAC>MIC) were calculated graphically from the semilogarithmic representation of the concentration-time curve and the regression line representing the apparent elimination rate constant.
Data analysis.
Mean values and standard deviations (SD) for loss of total body weight, affected area, relative weight of lungs, and colony counts in untreated and treated groups by strain and time p.i. of treatment initiation were calculated. Reductions in colony counts per pair of lungs versus infecting inocula were evaluated by using log10 CFU per pair of lungs. Mean reductions, SD, and standard errors were afterwards calculated for treated and untreated groups by strain and time after the start of antibiotic administration. Statistical comparison of the affected area and colony counts were done by the Student t test. Relative lung weights and loss of body weight in treated animals versus controls were compared by the Kruskal-Wallis test.
RESULTS
The following serum amoxicillin concentrations (mean ± SD, in micrograms per milliliter) were achieved at the specified time period: 5.1 ± 0.9 (30 min), 13.5 ± 1.8 (60 min), 15.1 ± 3.7 (90 min), 11.0 ± 1.7 (210 min), 7.0 ± 1.7 (300 min), 2.6 ± 1.1 (420 min), 0.8 ± 0.2 (600 min), and 0.4 ± 0.1 (720 min). Experimental SACs achieved from 30 min to 12 h are very close to previously defined target concentrations.
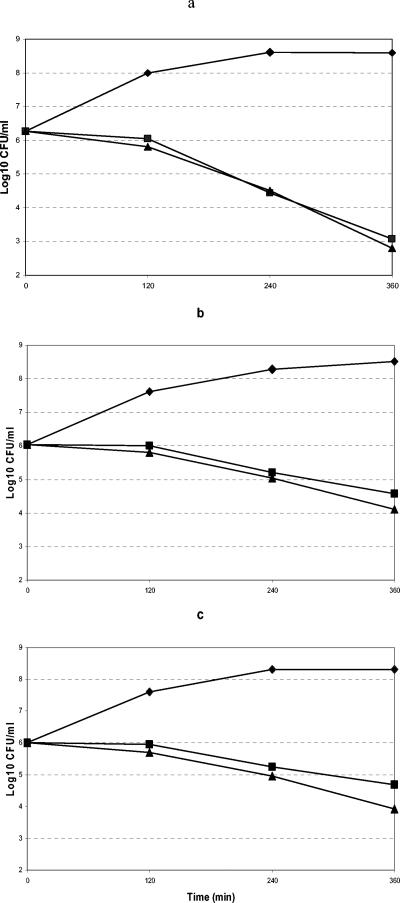
Figure 1 shows the bactericidal activity of amoxicillin against S. pneumoniae strains for which MICs are ≥2 μg/ml that had been exposed for as long as 6 h to amoxicillin concentrations equivalent to 2 or 4 times the MIC. After a 6-h incubation, the decreases in bacterial loads with concentrations 2 and 4 times the MIC were 3.19 and 3.47 log10 CFU for strain FJD70, 1.47 and 1.7 log10 CFU for strain AR06016, and 1.33 and 2.09 log10 CFU for strain AR09164, respectively.
FIG. 1.
Bactericidal activity of amoxicillin against S. pneumoniae strains FJD70 (a), AR06016 (b), and AR09164 (c) at concentrations equivalent to twice (▪) and four times (▴) the MIC compared with bacterial loads in controls (♦).
Following infection with any strain, untreated rats developed bilateral pneumococcal pneumonia. Animals were hypoactive and tachypneic and presented a moderate weight loss over the time the experiment lasted. The mortality rate in untreated animals was 6.8% (3 out of 44 animals). Lungs of increased size and weight, with areas of red and gray congestion affecting between 40 and 50% of the pair of lungs, were observed. Lung histopathology of macroscopic affected areas at necropsy showed intense infiltration by polymorphonuclear leukocytes, macrophages, and erythrocytes within the alveoli and bronchiolar lumen; the alveolar spaces showed fibrinous exudates. The bacterial loads in these animals at the end of the experiment remained at concentrations similar to those inoculated (differences, from +0.22 to −1.02 log10 CFU/pair of lungs). No differences in bacterial clearance, lung injury, or loss of total body weight were found between control animals infected with different strains.
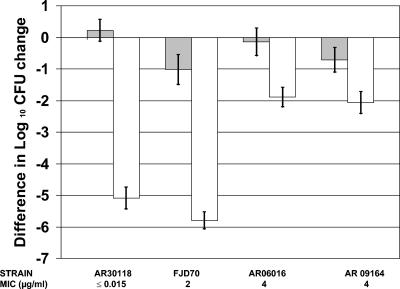
No mortality was observed among the 49 amoxicillin-treated animals. Figure 2 shows reductions in bacterial loads (log10 CFU) in lungs at 72 h p.i. for treated (beginning at 8 h p.i.) and untreated animals inoculated with the four pneumococcal strains. Differences of more than 4.5 log10 CFU in bacterial loads in the lungs were observed between control and treated animals for strains AR30118 (amoxicillin MIC, ≤0.015 μg/ml) and FJD70 (amoxicillin MIC, 2 μg/ml), while the difference was less than 1.8 log10 CFU for strains AR06016 and AR09164 (amoxicillin MIC for both strains, 4 μg/ml). Nevertheless, the difference between control and treated animals was statistically significant for all strains (P < 0.001).
FIG. 2.
Difference in log10 CFU change in lung tissue at 72 h p.i. between rats treated with amoxicillin starting at 8 h p.i. (white bars) and untreated rats (gray bars). P < 0.001 for untreated versus treated animals in each group.
Table 1 summarizes the changes in lungs and body weight in untreated and treated animals inoculated with the four pneumococcal strains. The extent of lung damage was significantly lower in treated animals than in untreated controls for all four pneumococcal strains (P < 0.05). The relative weight of lungs was lower in treated than untreated animals (P < 0.05). The loss of body weight was greater in untreated than treated animals, although the difference was significant (P < 0.05) only for strains AR30118 and AR09164. TSAC>MIC was 100, 61.6, and 46.7% of the dosing interval for strains for which MICs were ≤0.015, 2, and 4 μg/ml, respectively.
TABLE 1.
Pulmonary lesions and loss of body weight in rats with pneumonia caused by four S. pneumoniae strains and treated with simulated human treatment-like slow-release amoxicillin therapy
| Parametera | Pneumococcal strain (amoxicillin MIC, μg/ml) [no. of animals in untreated/treated group]
|
|||
|---|---|---|---|---|
| AR30118 (≤0.015) [6/8] | FJD70 (2) [6/6] | AR06016 (4) [10/10] | AR09164 (4) [9/11] | |
| Extent of lung damage (%) | ||||
| Untreated | 49.17 ± 27.18b | 42.92 ± 22.58b | 49.37 ± 20.93b | 38.89 ± 12.80b |
| Treated | 10.47 ± 5.82 | 18.33 ± 6.74 | 21.00 ± 8.70 | 15.11 ± 14.16 |
| Relative lung weight (% of body weight) | ||||
| Untreated | 2.02 ± 0.97b | 1.51 ± 0.65b | 1.55 ± 0.73b | 1.45 ± 0.40b |
| Treated | 0.85 ± 0.10 | 0.77 ± 0.18 | 1.00 ± 0.30 | 0.92 ± 0.25 |
| Loss of body weight (%) | ||||
| Untreated | 13.90 ± 4.92b | 8.29 ± 4.04 | 10.59 ± 6.18 | 13.10 ± 4.25b |
| Treated | 4.87 ± 2.62 | 5.10 ± 2.56 | 8.25 ± 4.78 | 7.34 ± 6.00 |
Values are means ± SD.
Values for untreated versus treated groups are significantly different (P < 0.05).
Table 2 summarizes the differences in colony counts, lung injury, and body weight in animals inoculated with strain AR09164 (MIC, 4 μg/ml) and either left untreated or treated beginning at different times p.i. When untreated animals were sacrificed at 88 h p.i., lower numbers of organisms were recovered than were obtained from untreated animals killed 65 h p.i. The extent of lung damage and loss of total body weight were also more favorable when animals were sacrificed at 88 h p.i., suggesting a spontaneous improvement in relation to time. The antibiotic diminished the number of organisms in lungs compared to those in untreated controls regardless of the starting time for treatment (P < 0.02). Antibiotic treatment also improved the other parameters evaluated compared with those for untreated controls, although statistical differences (P < 0.05) were found in those parameters representing lung injury only when treatment was initiated 1 h after bacterial challenge, not when treatment was initiated 24 h after inoculation. No bactericidal effect (≥3 log10 reduction in CFU) against this strain was observed when amoxicillin was administered at either 1, 8, or 24 h p.i. (Table 2; Fig. 2), times when microorganisms probably encountered different growth and environmental conditions.
TABLE 2.
Reductions in bacterial load, pulmonary lesions, and loss of body weight in rats with pneumonia caused by one S. pneumoniae strain (AR09164) in relation to the time elapsed between challenge and initiation of amoxicillin treatment
| Parametera | Time elapsed between bacterial challenge and treatment (time from challenge to necropsy) [no. of animals in untreated/treated group]
|
|
|---|---|---|
| 1 h (65 h) [6/6] | 24 h (88 h) [7/8] | |
| Difference in log10 CFU/pair of lungs compared to initial inoculum | ||
| Untreated | −0.48 ± 1.03b | −1.11 ± 0.90b |
| Treated | −2.27 ± 1.09 | −2.43 ± 1.00 |
| Extent of lung damage (%) | ||
| Untreated | 53.96 ± 13.24c | 29.29 ± 6.92 |
| Treated | 21.25 ± 9.97 | 21.70 ± 9.97 |
| Relative lung weight (% of body weight) | ||
| Untreated | 1.63 ± 0.45c | 1.01 ± 0.26 |
| Treated | 0.97 ± 0.36 | 0.87 ± 0.15 |
| Loss of body weight (%) | ||
| Untreated | 11.80 ± 3.99 | 9.78 ± 3.84 |
| Treated | 7.54 ± 4.02 | 7.33 ± 4.10 |
Values are means ± SD.
Values for untreated and treated groups are significantly different (P < 0.02).
Values for untreated and treated groups are significantly different (P < 0.05).
DISCUSSION
Two of the pneumococcal strains used in this study are representative of MIC50s (MICs at which 50% of isolates are inhibited) and MIC90s (≤0.015 and 2 μg/ml, respectively) obtained in many Western countries (21, 25). The other two strains represent less than 10% of the clinical isolates of pneumococci but are a real challenge for new antibiotics or formulations. All strains belong to serotypes commonly involved in pneumonia (7). Amoxicillin at concentrations 2 and 4 times the MIC was bactericidal (≥3 log10 reduction in CFU) after a 6-h incubation against the strain for which the amoxicillin MIC was 2 μg/ml as determined by the killing curve studies. However, for both serotype 9V strains (MIC, 4 μg/ml), the antibiotic, under the same experimental conditions (2 and 4 times the MIC), was not bactericidal after a 6-h incubation (decrease in CFU, ≤ 2.1 log10 units), although the inoculum size was significantly reduced, by approximately 99.0%. A typical penicillin-tolerant culture of S. pneumoniae has been described as one that is not lysed or is only partially lysed by penicillin at 10 times the MIC after 4 h (18). In this study, time-kill curves were performed using concentrations equivalent to 2 and 4 times the MIC in order to assess in vitro activity under the conditions used in the in vivo model, where amoxicillin concentrations were not higher than 4 times the MIC, for strains for which the MIC was 4 μg/ml. Under these conditions, the nonsusceptible strains showed a tolerant-like profile, since cultures were only partially lysed after 6 h. Penicillin tolerance has been described previously as a common finding among penicillin-resistant pneumococci (14).
Two parameters are classically used for efficacy evaluation of antibiotics in animal models: bacterial counts in tissues and the animal survival rate (10). While the latter is commonly used to show differences in antibiotic behavior (9), the reduction in bacterial counts seems a natural way of comparing in vivo with in vitro effects that may not be related to the animal survival rate (9). In any case, antibiotics are designed to decrease the bacterial load, and this may translate into a decrease in tissue damage, which logically is related to survival.
From the bacterial (inoculum reduction) perspective, this pneumococcal pneumonia model was reproducible, and the bacterial inoculum in the lungs was very stable. The administration of amoxicillin simulating the human pharmacokinetics of slow-release amoxicillin-clavulanic acid produced an in vivo bactericidal effect against the two amoxicillin-susceptible strains; the bacterial inoculum decreased more than 4.5 log10 CFU/pair of lungs. For strains for which the amoxicillin MIC was 4 μg/ml, no in vivo bactericidal effect (≥3 log10 or 99.9% reduction) was observed, although the reduction in the inoculum was close to 95.0%. Such an effect could be related to the tolerant-like behavior of the strains in the killing curve experiments. The in vivo significance of tolerance is not clear, although under some experimental conditions, treatment failure with amoxicillin has been reported in a mouse pneumonia model caused by amoxicillin-resistant and -tolerant strains (1, 5). It is clear that tolerant organisms are more difficult to eradicate, especially if a foreign body (as the agar is) is present in the infectious foci. Nevertheless, antibiotics with bacteriostatic effects have been demonstrated to be useful in many experimental and human infections, but their efficacy should be demonstrated by parameters other than colony counts.
From the host perspective, the antibiotic was efficacious against the four strains inoculated, as evaluated by taking into account the extent of lung damage, relative lungs weight, and loss of body weight. The microorganism and the inflammatory exudate define the consolidation and are related to an increase in lung weight. For this reason, reductions in the affected area per lung and in lungs weight can be considered therapeutic end points of treatment evaluation. The extent of lung damage caused by any of the four strains was lower in all treated animals than in untreated controls, and as a consequence, the weight of lungs did not increase, as did that of lungs from untreated animals. A significant reduction in lung damage in treated animals versus controls was found for the four strains, regardless of the reduction in the bacterial load or the susceptibility of the strain. For the four strains, the TSAC>MIC (a parameter related to therapeutic-clinical efficacy for β-lactams) (4) was higher than 46%. These results confirm that other therapeutic parameters, and not only the antibiotic effect on colony counts, should be taken into account in evaluating the in vivo efficacy of antibiotics, because as in this model, lower reductions in bacterial loads in lungs may not imply lower efficacy when other therapeutic parameters are also considered.
The results of experiments performed in order to evaluate the effect that early or delayed treatment may have on antibiotic efficacy indicate that early treatment is associated with a better outcome than delayed treatment, as observed in other experimental infection models (20) as well as in humans (15).
In summary, amoxicillin administered to immunocompetent rats with pneumonia caused by pneumococcus strains for which amoxicillin MICs range from ≤0.015 to 4 μg/ml, and achieving a TSAC>MIC higher than 46%, was able to reduce lung damage by all strains to a significant degree, although in vivo bactericidal activity (99.9% reduction in bacterial load in lungs) was achieved only against amoxicillin-susceptible strains, not against nonsusceptible strains (where a 95% reduction was obtained). Reductions of ≥95% in bacterial load produced therapeutic efficacy regardless of the MIC, bactericidal behavior in in vitro killing curves, or the serotype of the infecting strain.
Acknowledgments
We thank A. Drozdowskyj (Pivotal, Madrid, Spain) for the data analysis and A Fenoll (Instituto de Salud Carlos III, Madrid, Spain) for serotyping of organisms.
This study was supported by a grant from SmithKline Beecham (now GlaxoSmithKline). M. Gracia, C. Martínez-Marín, and L. Huelves were aided by scholarships from the Fundación Conchita Rábago, Madrid, Spain.
REFERENCES
- 1.Azoulay-Dupuis, E., P. Moine, J. P. Bedos, V. Rieux, and E. Vallee. 1996. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis, and tolerance properties of the strains. Antimicrob. Agents Chemother. 40:941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., and L. M. Mundy. 1995. Community-acquired pneumonia. N. Engl. J. Med. 333:1618-1624. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., R. F. Breiman, L. A. Mandell, T. M. File, Jr., et al. 1998. Community-acquired peneumonia in adults: guidelines for management. Clin. Infect. Dis. 26:811-838. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Darras-Joly, C., J. P. Bédos, C. Sauve, P. Moine, E. Vallée, C. Carbon, and E. Azoulay-Dupuis. 1996. Synergy between amoxicillin and gentamicin in combination against a highly penicillin-resistant and -tolerant strain of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob. Agents Chemother. 40:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Hollander, J. G., J. D. Knudsen, J. W. Mouton, K. Fuursted, N. Frimodt-Moller, H. A. Verbrugh, and F. Espersen. 1998. Comparison of pharmacodynamics of azithromycin and erythromycin in vitro and in vivo. Antimicrob. Agents Chemother. 42:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenoll, A., C. M. Bourgon, R. Muñoz, D. Vicioso, and J. Casal. 1991. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979-1989. Rev. Infect. Dis. 13:56-60. [DOI] [PubMed] [Google Scholar]
- 8.File, T. M., Jr., M. R. Jacobs, M. D. Poole, B. Wynne, et al. 2002. Outcome of treatment of respiratory tract infections due to Streptococcus pneumoniae, including drug-resistant strains, with pharmacokinetically enhanced amoxicillin/clavulanate. Int. J. Antimicrob. Agents 20:235-247. [DOI] [PubMed] [Google Scholar]
- 9.Frimodt-Moller, N. 1988. Correlation of in vitro activity and pharmacokinetic parameters with effect in vivo for antibiotics. Observations from experimental pneumococcus infection in mice. Danish Med. Bull. 35:422-437. [PubMed] [Google Scholar]
- 10.Frimodt-Moller, N. 1993. The mouse peritonitis model: present and future use. J. Antimicrob. Chemother. 31(Suppl. D):55-60. [DOI] [PubMed] [Google Scholar]
- 11.Garau, J., M. Twynholm, E. García-Mendez, B. Siquier, A. Rivero, and the 557 Clinical Study Group. 2003. Oral pharmacokinetically enhanced co-amoxiclav 2000/125 mg, twice daily, compared with co-amoxiclav 875/125 mg, three times daily, in the treatment of community-acquired pneumonia in European adults. J. Antimicrob. Chemother. 52:826-836. [DOI] [PubMed] [Google Scholar]
- 12.Kaye, C. M., A. Allen, S. Perry, M. McDonagh, M. Davy, K. Storm, N. Bird, and O. Dewit. 2001. The clinical pharmacokinetics of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate. Clin. Ther. 23:578-584. [DOI] [PubMed] [Google Scholar]
- 13.Klassen, M., and K. A. Nash. 1996. Measurement of antibiotics in human body fluids: techniques and significance, p. 230-295. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 14.Liu, H. H., and A. Tomasz. 1985. Penicillin tolerance in multiply drug-resistant natural isolates of Streptococcus pneumoniae. J. Infect. Dis. 152:365-372. [DOI] [PubMed] [Google Scholar]
- 15.Lodise, T., P. S. McKinnon, L. Swiderski, and M. J. Rybak. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36:1418-1423. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. 1992. Advanced report of final mortality statistics, vol. 42. National Center for Health Statistics, Hyattsville, Md.
- 17.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Normark, B. H., and S. Normark. 2002. Antibiotic tolerance in pneumococci. Clin. Microbiol. Infect. 8:613-622. [DOI] [PubMed] [Google Scholar]
- 19.Pallarés, R., J. Liñares, M. Vadillo, C. Cabellos, F. Manresa, P. F. Viladrich, R. Martín, and F. Gudiol. 1995. Resistance to penicillin and cephalosporin and mortality from severe penumococcal pneumonia in Barcelona, Spain. N. Engl. J. Med. 333:474-480. [DOI] [PubMed] [Google Scholar]
- 20.Parra, A., C. Ponte, C. Cenjor, C. Martínez-Marín, F. Soriano, and the Spanish Pneumococcal Infection Study Network. 2004. Effect of antibiotic treatment delay on therapeutic outcome of experimental acute otitis media caused by Streptococcus pneumoniae strains with different susceptibilities to amoxicillin. Antimicrob. Agents Chemother. 48:860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Trallero, E., C. Fernández-Mazarrasa, C. García-Rey, E. Bouza, L. Aguilar, J. García-de-Lomas, F. Baquero, and the Spanish Surveillance Group for Respiratory Pathogens. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob. Agents Chemother. 45:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petitpretz, P., C. Chidiac, F. Soriano, J. Garau, K. Stevenson, E. Ruffiac, and the 556 Clinical Study Group. 2002. The efficacy and safety of oral pharmacokinetically enhanced amoxicillin-clavulanate 2000/125 mg, twice daily, versus oral amoxicillin-clavulanate 1000/125 mg, three times daily, for the treatment of bacterial community-acquired pneumonia in adults. Int. J. Antimicrob. Agents 20:119-129. [DOI] [PubMed] [Google Scholar]
- 23.Ponte, C., A. Parra, E. Nieto, and F. Soriano. 1996. Development of experimental pneumonia by infection with penicillin-insensitive Streptococcus pneumoniae in guinea pigs and their treatment with amoxicillin, cefotaxime, and meropenem. Antimicrob. Agents Chemother. 40:2698-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowland, M., and T. Tozer. 1995. Clinical pharmacokinetics: concepts and applications, 3rd ed. Williams & Wilkins, Baltimore, Md.
- 25.Sahm, D. F., J. A. Karlowsky, L. J. Kelly, I. A. Critchley, M. E. Jones, C. Thornsberry, Y. Mauriz, and J. Kahn. 2001. Need for annual surveillance of antimicrobial resistance in Streptococcus pneumoniae in the United States: 2-year longitudinal analysis. Antimicrob. Agents Chemother. 45:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodnutt, G., and V. Berry. 1999. Efficacy of high-dose amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]