Abstract
Melioidosis is a life-threatening bacterial infection caused by Burkholderia pseudomallei. Some antibiotics used to treat melioidosis can induce filamentation in B. pseudomallei. Despite studies on the mechanism of virulence of the bacteria, the properties of B. pseudomallei filaments and their impact on virulence have not been investigated before. To understand the characteristics of antibiotic-induced filaments, we performed in vitro assays to compare several aspects of virulence between normal, nonfilamentous and filamentous B. pseudomallei. Normal, nonfilamentous B. pseudomallei could cause the lysis of monocytic cells, while filaments induced by sublethal concentrations of ceftazidime, ofloxacin, or trimethoprim show decreased lysis of monocytic cells, especially after prolonged antibiotic exposure. The motility of the filamentous bacteria was reduced compared to that of nonfilamentous bacteria. However, the filamentation was reversible when the antibiotics were removed, and the revertant bacteria recovered their motility and ability to lyse monocytic cells. Meanwhile, antibiotic resistance developed in revertant bacteria exposed to ceftazidime at the MIC. Our study highlights the danger of letting antibiotic concentration drop to the MIC or sub-MICs during antibiotic treatment of melioidosis. This could potentially give rise to a temporary reduction of bacterial virulence, only to result in bacteria that are equally virulent but more resistant to antibiotics, should the antibiotics be reduced or removed.
Melioidosis is a potentially life-threatening disease in humans and animals. The disease is endemic in Southeast Asia, Northern Australia, and many other tropical countries (18). Its etiological agent is Burkholderia pseudomallei, a gram-negative saprophytic bacterium found in the soil and water of tropical zones where it is endemic. Infection can occur by ingestion; inhalation of infectious particles; or contact of wounds or damaged skin with contaminated soil, water, or other poorly studied routes (10, 19). Melioidosis is difficult to treat due to its diverse clinical presentations, frequent association with underlying diseases, and recurrent nature. Despite intensive investigation in the fields of epidemiology, diagnosis, and therapeutics, in recent years new cases have continuously been reported both within and outside the zones of endemicity, with mortality rates remaining high (18).
Present antibiotic therapy regimens for the treatment of melioidosis typically involve the β-lactam ceftazidime in various combinations with chloramphenicol, co-trimoxazole, doxycycline, and amoxicillin-potassium clavulanate (31). Some antibiotics on this list have been known to induce morphological changes in the bacteria, such as filamentation. Among these are the β-lactam antibiotics (8, 24, 29), fluoroquinolones (14), and antibiotics that inhibit the synthesis of thymidine (1). The ability of certain β-lactam antibiotics to induce filamentation in gram-negative bacteria has been attributed to the binding of antibiotics to penicillin-binding protein 3 (PBP 3) (34), which is required for the synthesis of septa during cell division. The exact mechanism of fluoroquinolone-induced filamentation is unknown.
In the course of our study of B. pseudomallei, we found extensive formation of bacterial filaments in vitro with antibiotics used clinically. Filamentation occurred mainly at sub-MICs. At these concentrations, bacteria were not rapidly killed but remained as viable filaments. With intermittent antibiotic administration for the treatment of melioidosis, antibiotic concentrations in the body fluctuate on the basis of the dosing schedule and can drop to subinhibitory concentrations between doses. This is particularly true in cases of melioidosis involving tissue, because antibiotic concentrations in tissue are frequently lower than those in blood. In the in vitro study described here, we investigated the possibility that induction of bacterial filamentation due to the use of clinically relevant antibiotics modifies bacterial virulence, which would have important implications for the pathogenesis and treatment of the disease.
MATERIALS AND METHODS
Bacterial isolate.
B. pseudomallei isolate KHW was originally isolated from a Singapore Armed Forces national serviceman who died from melioidosis (22).
Cell line and growth condition.
The human monocytic cell line THP-1 (ATCC TIB-202) was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml in a 5% CO2 incubator at 37°C.
Antibiotics.
Aqueous stock solutions of active antibiotics were prepared from powders of ceftazidime (Cheil Jedang, Seoul, Korea), chloramphenicol (Sigma, St. Louis, Mo.), doxycycline (Sigma), gentamicin (Sigma), ofloxacin (Sigma), sulfamethoxazole (Sigma), and trimethoprim (Sigma), according to the instructions presented elsewhere (25). Trimethoprim-sulfamethoxazole (co-trimoxazole) was made by mixing trimethoprim and sulfamethoxazole at a ratio of 1:5 or a ratio of 2:1 (wt/wt). Aliquots of these antibiotics were stored at −80°C before use.
Antibiotic susceptibility testing.
In vitro susceptibility testing was performed by a standard broth microdilution method (25). Briefly, microtiter plates containing twofold serial dilutions of antibiotics in cation-adjusted Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) were inoculated with the appropriate dilution of a log-phase culture. The MICs of antibiotics for the bacterial filaments were tested by inducing log-phase bacteria to form filaments in 0.5 μg of ceftazidime per ml, 4 μg of ofloxacin per ml, or 8 μg of trimethoprim per ml for 6 or 16 h and directly reexposing them to various concentrations of these antibiotics. Viable counts were determined immediately after inoculation to verify that the actual inoculum size was between 105 and 106 CFU/ml. The plates were incubated at 35°C for 24 h. The MIC was defined as the lowest concentration of antibiotic that completely inhibited visible growth for 24 h. The minimal bactericidal concentration (MBC) was defined as the lowest concentration of antibiotic that achieved 99.9% killing by plating the cultures on Trypticase soy agar (Becton Dickinson) plates after appropriate dilution at the end of 24 h.
Filamentation.
A log-phase B. pseudomallei culture of approximately 5 × 105 CFU/ml was exposed to various concentrations (see Tables 2 and 3 and the legends to Fig. 1 to 4) of ceftazidime, chloramphenicol, doxycycline, gentamicin, ofloxacin, sulfamethoxazole, trimethoprim, or co-trimoxazole at 35°C. Filaments in the bacterial cultures were observed under a light microscope at 6 and 16 h and by phase-contrast microscopy and transmission electron microscopy (TEM) at 16 h.
TABLE 2.
Predominant morphology of B. pseudomallei after 6 h antibiotic exposure
| Antibiotic | Morphology at different MICsa
|
||||||
|---|---|---|---|---|---|---|---|
| 1/16 | 1/8 | 1/4 | 1/2 | 1 | 2 | ≥4 | |
| Ceftazidime | N | N | F | F | F | F | F |
| Chloramphenicol | N | N | N | N | N | N | N |
| Doxycycline | N | N | N | N | N | N | N |
| Gentamicin | N | N | N | N | N | N | N |
| Ofloxacin | N | F | F | F | F | N | N |
| Sulfamethoxazole | N | N | N | N | N | N | N |
| Trimethoprim | F | F | F | F | F | N | N |
| Co-trimoxazole (1:5)b | N | N | N | F | F | F | N |
| Co-trimoxazole (2:1)b | F | F | F | F | N | N | N |
The values in the subheads indicate the fractions or multiples of MIC of each antibiotic, with a value of 1 being the MIC of the antibiotic tested. N, nonfilamentous form; F, filamentous form.
Ratio of trimethoprim to sulfamethoxazole (wt/wt).
TABLE 3.
MICs of antibiotics for revertants of ceftazidime-induced B. pseudomallei filaments
| Antibiotic | MIC (μg/ml) for revertants previously exposed to the following concn of ceftazidimea:
|
MIC (μg/ml) for untreated control | |
|---|---|---|---|
| 1 μg/ml | 0.5 μg/ml | ||
| Ceftazidime | 4 | 1 | 1 |
| Chloramphenicol | 8 | 8 | 8 |
| Doxycycline | 1 | 1 | 1 |
| Gentamicin | 250 | 125 | 125 |
| Ofloxacin | 16 | 8 | 8 |
| Sulfamethoxazole | 250 | 250 | 250 |
| Trimethoprim | 64 | 64 | 64 |
| Co-trimoxazole (1:5)b | 64 | 64 | 64 |
| Co-trimoxazole (2:1)b | 32 | 32 | 32 |
Data in boldface indicate an increase in the MIC of the antibiotic for the revertants compared to that for the untreated, nonfilamentous control bacteria (data are presented in the last column) in the same experiment.
Ratio of trimethoprim to sulfamethoxazole (wt/wt).
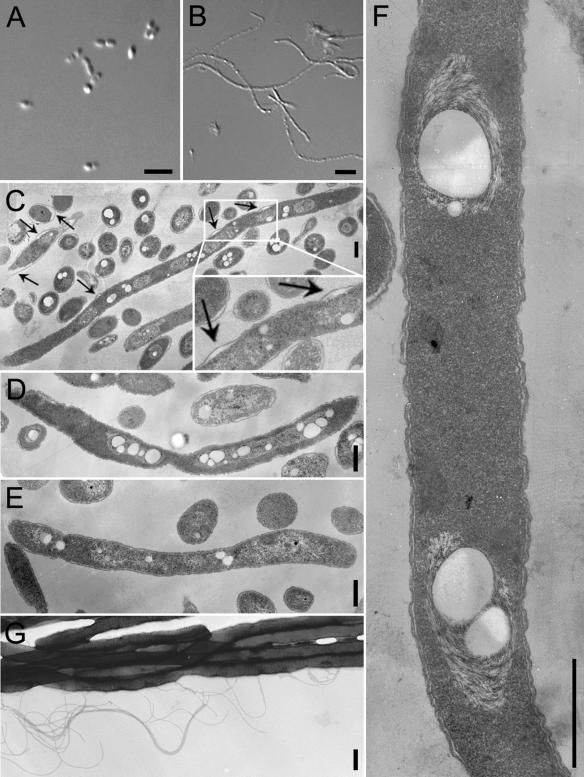
FIG. 1.
Phase-contrast and TEM of antibiotic-induced B. pseudomallei filaments. (A and B) Phase-contrast microscopy pictures of B. pseudomallei not treated with antibiotics (A) or treated with 0.5 μg ceftazidime per ml (B) for 16 h; (C to E) TEM pictures of thin sections of B. pseudomallei filaments induced by 0.5 μg of ceftazidime per ml (C), 4 μg of ofloxacin per ml (D), or 8 μg of trimethoprim per ml (E) for 16 h; (F) a higher-magnification picture of filaments induced by 0.5 μg of ceftazidime per ml; note the absence of any visible septa in the filament; (G) TEM picture of filaments induced by 0.5 μg of ceftazidime per ml without sectioning, which shows the presence of flagella. The arrows in panel C indicate points of periplasmic space enlargement. Bars in panels A and B, 5 μm; bars in panels C to G, 500 nm.
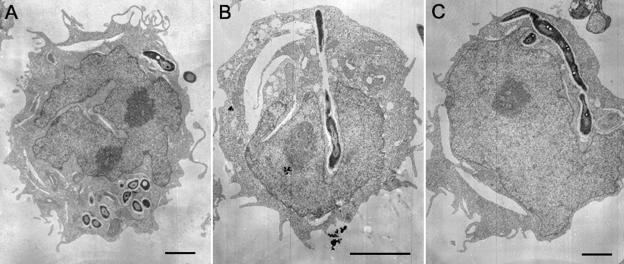
FIG. 4.
THP-1 cells infected with B. pseudomallei. THP-1 cells were infected with B. pseudomallei that were either not exposed to antibiotics (A) or exposed to 0.5 μg of ceftazidime per ml for 6 h (B and C). The cells were processed for TEM at 6 h after infection. Bars, 2 μm.
(i) Phase-contrast microscopy.
Clean coverslips were coated with 100 μg of ml poly-l-lysine solution per ml at room temperature overnight and were washed twice with saline. A total of 50 μl of each of the culture suspensions was added onto the coverslips, and the coverslips were incubated for 2 h. The bacterial suspensions were drained, and the coverslips were washed twice with saline. The coverslips were fixed sequentially with 3.7% paraformaldehyde at 4°C for 2 h and with 100% methanol at −20°C for 30 min. They were washed twice with saline, mounted onto glass slides, and observed with a DMLB microscope (Leica Microsystems, Wetzlar, Germany). The images were created with Leica Qfluoro (version 1.2.0) software.
(ii) TEM.
Bacterial filaments in the culture suspensions were pelleted at 3,000 × g for 5 min and washed once with saline. The samples were fixed in 100 mM cacodylate buffer with 2% paraformaldehyde and 4% glutaraldehyde for 3 h and were then fixed in 100 mM sodium cacodylate buffer with 2% osmium tetroxide at 4°C for 1 h. The samples were then dehydrated through a series of 30, 50, 70, 90, and 100% ethanol and finally in propylene oxide prior to infiltration with Spurr resin (32). The samples were embedded in 100% Spurr resin and polymerized at 65°C overnight. Ultrathin sections were cut on a Reichert-Jung ultramicrotome (Leica Microsystems) and stained with uranyl acetate and lead nitrate. Sections were examined with a JEM-1010 transmission electron microscope (JEOL, Peabody, Mass.) at 100 kV. Images were saved and processed with Photoshop software (version 7.0; Adobe Systems, San Jose, Calif.).
PAE determination.
A log-phase B. pseudomallei inoculum of approximately 7 × 105 CFU/ml was exposed to ceftazidime at 0.5, 1, and 2 μg/ml in Mueller-Hinton broth at 35°C for 24 h. A control culture with no drugs was included in parallel. Drug activity was removed by washing the bacterial cultures three times with saline (which gave a >104-fold reduction in the drug concentrations). The cultures were then appropriately diluted to start regrowth at 35°C. The starting concentration was between 7 × 105 and 1.5 × 106 CFU/ml. A growth curve was constructed by doing viability counts at the time of drug removal and at 2-h intervals thereafter. Aliquots were removed from all cultures, serially diluted in saline, and plated on Trypticase soy agar plates. After incubation for 24 to 36 h, the numbers of CFU were counted. The postantibiotic effect (PAE) was calculated as described previously (9).
Filament reversion.
A log-phase B. pseudomallei culture was induced to form filaments with 0.5 μg of ceftazidime per ml, 4 μg of ofloxacin per ml, or 8 μg of trimethoprim per ml for 6 or 16 h. The filaments were washed three times with saline. The cultures were then appropriately diluted to start regrowth in antibiotic-free broth at a starting concentration between 7 × 105 and 1.5 × 106 CFU/ml. At 10 h after antibiotic removal, cultures of the revertants were used for antibiotic susceptibility testing, motility assay, and infection of THP-1 cells.
Motility assay.
A log-phase B. pseudomallei culture was exposed to 0.5 μg of ceftazidime per ml, 4 μg of ofloxacin per ml, or 8 μg of trimethoprim per ml for 6 or 16 h. A control culture not treated with antibiotics was included. Bacterial motility was assayed as described previously (6). After 6 or 16 h of exposure, the antibiotics were removed by washing and the filaments were allowed to revert. At 10 h after antibiotic removal, the motilities of the filament revertants were assayed in the same way (6).
Infection of THP-1 cells.
Four hours before infection, THP-1 cells were washed three times with infection medium (RPMI 1640 medium with 2% fetal bovine serum and no antibiotics) and seeded in 48-well plates at 2 × 105 cells in 0.5 ml of infection medium per well. Log-phase B. pseudomallei cultures were exposed to 0.5 μg of ceftazidime per ml, 4 μg of ofloxacin per ml, or 8 μg of trimethoprim per ml for 6 or 16 h to induce filament formation. At the end of the 6 or 16 h of exposure or 10 h after antibiotic removal, the bacteria were transferred from the broth to the infection medium. The bacterial suspensions were adjusted to appropriate concentrations and serially diluted twofold. A total of 100 μl of each bacterial dilution was added to infect the cells in each well in duplicate. For infection by bacterial filaments, ceftazidime, ofloxacin, and trimethoprim were added to final concentrations of 0.5, 4, and 8 μg/ml, respectively, to keep the bacteria in their filamentous form. Infection by filament revertants was performed in the same way, but with no antibiotics added to the infection cultures. The viable counts of the bacteria were determined immediately to determine the multiplicities of infection (MOIs). One hour after addition of the bacteria, tetracycline was added to 40 μg/ml to suppress the growth of the bacteria in the medium. Supernatants were collected at 8 h after infection. The lactate dehydrogenase (LDH) level in the supernatant was assayed with a cytotoxicity detection kit (Roche Applied Science, Penzburg, Germany), according to the instructions of the manufacturer. The tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) levels in the supernatant were measured by enzyme-linked immunosorbent assay (Bender Medsystems, Vienna, Austria).
TEM of infected THP-1 cells.
THP-1 cells (5 × 105) were seeded in each well and infected at a range of MOIs, as described above. Six hours after infection, the cells were pelleted at 200 × g for 3 min, washed once with saline, fixed, and prepared for TEM. A parallel experiment performed to measure the amount of LDH released helped us to identify wells with the highest relative amount of LDH release at 8 h. Only corresponding wells containing cells infected with the MOI that gave the largest amount of LDH release were chosen for TEM.
RESULTS
MICs and MBCs of antibiotics.
Antibiotics belonging to several distinct categories were chosen for susceptibility testing (Table 1). The relatively high MICs of the two co-trimoxazole combinations indicate that the B. pseudomallei isolate is resistant to co-trimoxazole. It has susceptibilities to ceftazidime, chloramphenicol, and doxycycline similar to those of other B. pseudomallei strains (36).
TABLE 1.
MICs and MBCs of various antibiotics for B. pseudomallei
| Antibiotic | Category | MIC (μg/ml) | MBC (μg/ml) |
|---|---|---|---|
| Ceftazidime | Cephalosporin | 1 | 2 |
| Chloramphenicol | Protein synthesis inhibitor | 8 | >125 |
| Doxycycline | Tetracycline | 0.5 | 16 |
| Gentamicin | Aminoglycoside | 125 | 250 |
| Ofloxacin | Fluoroquinolone | 8 | 32 |
| Sulfamethoxazole | Sulfonamide | 250 | >16,000 |
| Trimethoprim | Folate synthesis inhibitor | 64 | 500 |
| Co-trimoxazole (1:5)a | 64 | 1,000 | |
| Co-trimoxazole (2:1)a | 32 | 64 |
Ratio of trimethoprim to sulfamethoxazole (wt/wt).
Antibiotic-induced filamentation.
Among the antibiotics tested, ceftazidime, ofloxacin, and trimethoprim induced filamentation (Table 2). Filamentation induced by ceftazidime occurred at 1/4 the MIC and at up to 16 times the MIC (higher concentrations were not tested) and was most extensive at 1/2 the MIC (0.5 μg/ml) (Fig. 1B). Ofloxacin- and trimethoprim-induced filamentation occurred mainly at the MICs and sub-MICs and was most extensive at one-half the MIC of ofloxacin (4 μg/ml) and one-eighth the MIC of trimethoprim (8 μg/ml).
On the basis of phase-contrast microscopy and TEM, short filaments were usually from 7 to 10 μm in length and long filaments could be up to 20 to 30 μm in length. Ceftazidime-induced filaments were generally longer than ofloxacin- or trimethoprim-induced ones at any time point.
Representative pictures of the ultrastructures of thin sections of filaments (Fig. 1C to F) revealed the absence of any visible septa or constrictions in all three types of filaments (i.e., those induced by the three antibiotics). The filaments induced by ofloxacin had a rugged outer surface (data not shown) and greatly expanded DNA regions. Interestingly, some ceftazidime-induced filaments seemed to have enlarged periplasmic spaces at certain points (Fig. 1C).
Motility.
We assayed the motilities of filaments induced by 6 or 16 h of antibiotic exposure and their revertants 10 h after antibiotic removal. After 6 h of antibiotic exposure, the filamentous form was almost as motile as the untreated nonfilamentous control. However, the motility of the filamentous form markedly decreased after 16 h of antibiotic exposure (Fig. 2). The loss of motility appeared to be transient and was restored by antibiotic removal.
FIG. 2.
Motility of B. pseudomallei filaments on semisolid agar. Filaments were induced with 0.5 μg of ceftazidime per ml, 4 μg of ofloxacin per ml, or 8 μg of trimethoprim per ml for either 6 or 16 h. At the end of the 6 or 16 h, the antibiotics were removed and the filaments were allowed to revert in antibiotic-free broth for 10 h. Controls not treated with any antibiotics were tested in parallel and were processed in the same way as the treated bacteria. Bars, 1 cm.
Cytotoxicity.
We have observed that untreated B. pseudomallei is able to lyse phagocytic cells (unpublished data). Thus, we investigated the effect of filamentation on the ability to induce monocytic cell cytotoxicity in vitro. Our result shows that normal bacteria caused a dose-dependent lysis of THP-1 cells (Fig. 3A). Filaments induced by 6 h of exposure to subinhibitory concentrations of ceftazidime or ofloxacin are as capable as the normal bacteria of causing THP-1 cell lysis. Sixteen hours of exposure to ceftazidime or ofloxacin significantly decreased the cytotoxicities of the filaments to THP-1 cells. Filaments induced by trimethoprim had decreased cytotoxicities at both time points. Antibiotics alone did not induce cytotoxicity in THP-1 cells at either of the two time points (data not shown). The decrease in lysis consistently observed for both normal bacteria and the filaments when MOIs were increased beyond optimal values is likely due to bacterial aggregation at high concentrations.
FIG. 3.
Lysis and cytokine secretion of THP-1 cells caused by B. pseudomallei. THP-1 cells were infected either with B. pseudomallei filaments induced by 6 or 16 h of exposure to 0.5 μg of ceftazidime per ml (•), 4 μg of ofloxacin per ml (▵), or 8 μg of trimethoprim per ml (⋄) or with their respective revertants across a range of MOIs. Bacteria not exposed to antibiotics were included as controls (▪). Cell lysis after 8 h infection (A) was expressed as the amount of LDH released in the supernatants normalized to that caused by 1% Triton X-100. TNF-α (B) and IL-1β (C) levels in the supernatants were measured and are expressed as picograms per 106 THP-1 cells. The experiments were performed in duplicate. Horizontal axes are log MOIs.
Cytokine production.
Following entry into phagocytes, pathogens can either induce or inhibit the release of proinflammatory cytokines (26). We therefore studied the ability of filamentous B. pseudomallei to induce TNF-α and IL-1β release from THP-1 cells in comparison with the induction ability of the nonfilamentous form (Fig. 3B and C). The TNF-α level correlated well with the cell lysis induced by filaments exposed to antibiotics for 6 h. Filaments induced by 16 h of antibiotic exposure could effectively induce TNF-α release, although they were less effective in causing cell lysis. The filamentous and nonfilamentous forms of B. pseudomallei were equally capable of inducing IL-1β release. The abilities of the revertant and normal bacteria to induce TNF-α and IL-1β release were very similar. The presence of antibiotics in the infection medium did not affect cytokine production (data not shown).
Intracellular B. pseudomallei filaments.
Many have reported on the altered adherence and phagocytosis of antibiotic-induced filaments in other bacteria (3, 4, 27). Decreased phagocytosis of these filaments was likely due to mechanical constraints. However, the shorter filaments induced by 6 h of antibiotic exposure still caused significant cell lysis. We examined if these bacterial filaments could enter THP-1 cells and remain as filaments inside the cells. TEM of infected THP-1 cells showed that the cells can internalize the filaments (Fig. 4). The filaments remained in the vacuoles like sessile bacteria.
Filament reversion.
Because of the recurrent nature of melioidosis, we examined if B. pseudomallei filaments revert to normal forms once the antibiotic pressure is removed. We washed the bacteria after 6, 16, or 24 h of exposure to subinhibitory levels of ceftazidime, ofloxacin, or trimethoprim and allowed the cultures to regrow in antibiotic-free broth. Gradual shortening of the filaments and the breaking off of individual cells from the ends were observed by light microscopy. Within 10 h, the majority of the bacteria in the cultures were nonfilamentous. The revertants were just as motile (Fig. 2) and equally capable of causing lysis (Fig. 3A) and inducing proinflammatory cytokine secretion (Fig. 3B and C) from THP-1 cells as normal bacteria.
Antibiotic resistance.
If filaments induced by 6 or 16 h of exposure to sub-MICs of ceftazidime (0.5 μg/ml) and trimethoprim (8 μg/ml) were directly reexposed to antibiotics, they were not more resistant to these antibiotics, but the MICs for those induced by a sub-MIC of ofloxacin (4 μg/ml) increased twofold (data not shown). However, if ceftazidime-induced filaments were exposed to antibiotics after they reverted to the normal forms, some resistance and cross-resistance developed (Table 3). The ceftazidime MIC for revertants of filaments induced by 1 μg of ceftazidime per ml consistently increased fourfold and the ofloxacin and gentamicin MICs consistently increased twofold in three independent experiments. We detected no PAE on B. pseudomallei after 24 h of exposure to 1 or 0.5 μg of ceftazidime per ml, similar to previous studies with several other B. pseudomallei strains (36). Although determination of the PAEs of antibiotics that induced filamentation neglected other biochemical and physiological impacts caused by antibiotic exposure (23, 30), we were concerned only about whether the increased antibiotic resistance was due to growth delay and not to the PAE per se. Thus, the increased level of resistance was not due to growth delay after the initial exposure.
Häuβbler et al. (16) observed the presence of resistant small-colony variants (SCVs) after repeated exposure to the MICs of antibiotics in vitro. We found few SCVs after a single 6-, 16-, or 24-h exposure to sub-MICs of ceftazidime (0.5 μg/ml), ofloxacin (4 μg/ml), or trimethoprim (8 μg/ml) and very low frequencies (<5%) of SCVs after a single 24-h exposure to the MIC of ceftazidime (1 μg/ml). However, if the culture exposed to an antibiotic for 24 h was allowed to revert completely and be reexposed to ceftazidime, many SCVs were isolated after plating.
DISCUSSION
It is now clear that sublethal concentrations of various antibiotics not only leave the bacteria unkilled but also cause many morphological and physiological changes in the bacteria. In the context of bacterial pathogenesis and the host-pathogen interaction, many studies (3, 4, 15, 37) have found that various virulence mechanisms of bacterial pathogens are affected. In this study, we investigated the effects of inhibitory and subinhibitory concentrations of some clinically relevant antibiotics on B. pseudomallei, which produced a common morphological alteration, filamentation. As current antibiotic treatments for melioidosis often involve the discontinuous administration of antibiotics to the patients, the concentrations of antibiotics can drop to near or below the MIC, at which residual bacteria are not eradicated but have modified structural and functional characteristics. It is thus possible for filamentation to frequently occur in vivo during antibiotic treatment for melioidosis, and the bacterial filaments induced during treatment could potentially influence the disease outcome due to altered virulence. To understand the characteristics of these bacterial filaments, we used in vitro assays to approximate bacterial behavior and the interaction of the bacteria with host cells in vivo.
The filamentation induced by ceftazidime was affected by several parameters. The filaments were generally longer if they were exposed to ceftazidime for a longer period. Interestingly, some filaments induced by ceftazidime had enlarged periplasmic spaces at certain points. Such enlargements were not seen in ofloxacin- or trimethoprim-induced filaments or untreated bacteria. Because increased levels of endotoxin production have been observed to be induced by both B. pseudomallei (31) and many other filamentous gram-negative bacteria (5, 6, 11, 17) during antibiotic treatment for sepsis caused by gram-negative bacteria and because most of these antibiotics affect the bacterial cell wall through PBP 3, we think that the enlargement of the periplasmic space observed in ceftazidime-induced filaments could be structural features resulting from increased levels of endotoxin production.
Ofloxacin and trimethoprim induced filamentation mainly at MICs and sub-MICs. Ofloxacin binds primarily to DNA gyrase in gram-negative bacteria to form gyrase-DNA-ofloxacin complexes, so that the DNA cleaved by gyrase cannot be religated. A bacteriostatic effect can be caused by inhibition of DNA and RNA synthesis due to complex formation, which may eventually interfere with the expression of genes involved in septum formation. The bactericidal effect could be due to the release of double-stranded DNA segments (12). Trimethoprim-induced filamentation occurs in a strain-specific manner and has been attributed to an SOS response that leads to the synthesis of an inhibitor of septum formation (1). However, although the filamentation induced by all these different antibiotics resulted from inhibited septum formation, the different modes of action and different filamentation-inducing kinetics of the antibiotics could result in filaments with qualitatively different virulence characteristics.
We demonstrated in this paper that B. pseudomallei isolate KHW can cause the lysis of the monocytic cell line THP-1. Phagocytes are the first line of defense in the fight against invading pathogens, in which the phagocytes actively engulf and kill the pathogens intracellularly. They also have a potent ability to recruit and activate the appropriate types of immune cells. The ability to kill macrophages has been shown to be an essential virulence mechanism of Salmonella enterica serovar Typhimurium (21), Shigella flexneri (38), and Yersinia enterocolitica (28). B. pseudomallei is an intracellular pathogen and has been found to be able to resist intracellular killing by phagocytes (13, 35). Many studies have demonstrated that subinhibitory concentrations of antibiotics increase the susceptibilities of bacteria to phagocytosis or killing by phagocytes (3, 4, 27, 30). Not only is B. pseudomallei resistant to killing by monocytes, but it also kills monocytes. All filaments except trimethoprim-induced filaments induced after 6 h of exposure to antibiotics could still kill monocytes. From our TEM pictures, it was evident that filaments could still be internalized into the cells. At 16 h after exposure to antibiotics at sub-MICs, the length of the filaments may have impeded their internalization into cells, which correlates with decreased killing. Ceftazidime-induced filaments were longer than ofloxacin- and trimethoprim-induced filaments and, thus, caused less killing. This is in agreement with our unpublished data that lysis of cells is dependent on internalization of the bacteria. It is likely that trimethoprim-induced filaments are qualitatively different from the rest of the filaments and that their inability to exert lysis at 6 h is due to perturbations in aspects of metabolism which interfere with cell lysis. Thus, we have demonstrated an example of altered virulence induced by bacterial filamentation which is due not simply to the physical constraints imposed by the filament length but to altered biochemistry.
Our results show that high levels of TNF-α were released during THP-1 cell death. The level of TNF-α has been shown to be strongly predictive of a lethal outcome in patients with severe melioidosis (33). Thus, it is possible that death due to severe melioidosis is preceded by massive phagocytic cell death and the release of large amounts of TNF-α, which impairs the development of a protective immune response and causes septic shock. Lipopolysaccharide has been shown to be the major mediator of inflammation in the supernatants of antibiotic-treated bacteria (20). However, it is unclear what the in vivo significance is concerning the increased levels of release of endotoxin due to antibiotic-induced filamentation. A study examining the treatment of melioidosis with ceftazidime, which causes higher levels of release of endotoxin compared to that caused by imipenem, which causes lower levels of release, did not show any correlation between endotoxin release and disease prognosis (31). Yet, our finding that at 16 h filaments could induce an increase in TNF-α and IL-1β production independent of cell lysis and internalization suggests that filaments could potentially contribute to increased levels of inflammation in patients.
Another aspect of virulence that we examined is bacterial motility. We have previously shown that the flagellum is an important virulence factor in bacterial pathogenesis in mice (7). The decreased motility observed with filaments is not due to suppressed flagellum synthesis, as flagella could be observed on filaments (Fig. 1G), but is more likely due to suppression of the global energy metabolism that drives the movement of flagella. Decreased motility would likely result in decreased virulence of the bacteria by limiting their spread.
An important aspect of altered bacterial properties after antibiotic treatment is the development of antibiotic resistance. We found that filaments are generally not more resistant during reexposure to higher concentrations of antibiotics, except for a twofold increase in resistance to ofloxacin by ofloxacin-induced filaments. However, revertants of bacteria exposed to ceftazidime at its MIC developed resistance to ceftazidime as well as cross-resistance to gentamicin and ofloxacin. We detected the presence of SCVs only after exposing the revertant bacteria to ceftazidime at its MIC. These results are similar to the findings of Häuβbler et al. (16), who found that higher concentrations of antibiotics and more passages favored the isolation of SCVs. Thus, the presence of SCVs seems to contribute to the increase in MICs.
Angus et al. (2) previously performed pharmacokinetic-pharmacodynamic evaluations and found that a continuous infusion of ceftazidime was superior to the conventional 8-h intermittent dosing for septicemic melioidosis due to significant dose reductions and cost savings. Furthermore, they found that with the 8-h intermittent dosing, plasma ceftazidime concentrations could fall below the target concentration for patients with normal renal function and clearance. Although our study was conducted in a static environment and it is not known under what conditions in the dynamic in vivo environment filaments would arise, it does suggest that a continuous infusion regimen could reduce the possibility of filament formation. The formation of filaments which could revert is problematic not only because of the development of antibiotic resistance but also because it could potentially lead to a temporary reduction in virulence and disease severity, as shown by the decreases in motility and monocyte killing, only to recur with a vengeance upon removal of the antibiotic selection pressure. Therefore, one should exercise caution with the premature discontinuation of antibiotic administration or a reduction in the dosage upon observation of an improvement in a patient's disease condition.
Acknowledgments
This work was funded by the Academic Research Fund R-183-000-080-112 from the National University of Singapore.
We thank Soh Chan Lim and Yu Seng Er for technical assistance and the EM unit in Temasek Life Sciences Laboratory for excellent technical support.
REFERENCES
- 1.Ahmad, S. I., S. H. Kirk, and A. Eisenstark. 1998. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu. Rev. Microbiol. 52:591-625. [DOI] [PubMed] [Google Scholar]
- 2.Angus, B. J., M. D. Smith, Y. Suputtamongkol, H. Mattie, A. L. Walsh, V. Wuthiekanun, W. Chaowagul, and N. J. White. 2000. Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis. Br. J. Clin. Pharmacol. 50:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga, P. C., M. T. Sala, and M. Dal Sasso. 1999. Pharmacodynamic effects of subinhibitory concentrations of rufloxacin on bacterial virulence factors. Antimicrob. Agents Chemother. 43:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braga, P. C., M. D. Sasso, and M. T. Sala. 2000. Sub-MIC concentrations of cefodizime interfere with various factors affecting bacterial virulence. J. Antimicrob. Chemother. 45:15-25. [DOI] [PubMed] [Google Scholar]
- 5.Bucklin, S. E., Y. Fujihara, M. C. Leeson, and D. C. Morrison. 1994. Differential antibiotic-induced release of endotoxin from gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 13:S43-S51. [DOI] [PubMed] [Google Scholar]
- 6.Bucklin, S. E., and D. C. Morrison. 1995. Differences in therapeutic efficacy among cell wall-active antibiotics in a mouse model of gram-negative sepsis. J. Infect. Dis. 172:1519-1527. [DOI] [PubMed] [Google Scholar]
- 7.Chua, K. L., Y. Y. Chan, and Y. H. Gan. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71:1622-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comber, K. R., R. J. Boon, and R. Sutherland. 1977. Comparative effects of amoxycillin and ampicillin on the morphology of Escherichia coli in vivo and correlation with activity. Antimicrob. Agents Chemother. 12:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, W. A., and S. Gudmundsson. 1991. The postantibiotic effect, p. 403-431. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. The Wilkins & Wilkins Co., Baltimore, Md.
- 10.Dance, D. A. B. 2002. Melioidosis. Curr. Opin. Infect. Dis. 15:127-132. [DOI] [PubMed] [Google Scholar]
- 11.Dofferhoff, A. S., J. H. Nijland, H. G. de Vries-Hospers, P. O. Mulder, J. Weits, and V. J. Bom. 1991. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in-vitro and in-vivo study. Scand J. Infect. Dis. 23:745-754. [DOI] [PubMed] [Google Scholar]
- 12.Drlica, K., and C. Hooper. 2003. Mechanisms of quinolone action, p. 19-39. In D. C. Hooper and E. Rubinstein (ed.), Quinolone antimicrobial agents, 3rd ed. American Society for Microbiology, Washington, D.C.
- 13.Egan, A. M., and D. L. Gordon. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott, T. S., A. Shelton, and D. Greenwood. 1987. The response of Escherichia coli to ciprofloxacin and norfloxacin. J. Med. Microbiol. 23:83-88. [DOI] [PubMed] [Google Scholar]
- 15.Gemmell, C. G. 1991. Antibiotics and expression of microbial virulence factors: implications for host defense. J. Chemother. 3:105-111. [PubMed] [Google Scholar]
- 16.Häuβbler, S., M. Rohde, and I. Steinmetz. 1999. Highly resistant Burkholderia pseudomallei small colony variants isolated in vitro and in experimental melioidosis. Med. Microbiol. Immunol. 188:91-97. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, J. J., and H. Kropp. 1992. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 165:1033-1041. [DOI] [PubMed] [Google Scholar]
- 18.Leelarasamee, A. 2004. Recent development in melioidosis. Curr. Opin. Infect. Dis. 17:131-136. [DOI] [PubMed] [Google Scholar]
- 19.Leelarasamee, A., and S. Bovornkitti. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413-425. [DOI] [PubMed] [Google Scholar]
- 20.Leeson, M. C., Y. Fujihara, and D. C. Morrison. 1994. Evidence for lipopolysaccharide as the predominant proinflammatory mediator in supernatants of antibiotic-treated bacteria. Infect. Immun. 62:4975-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindgren, S. W., I. Stojiljkovic, and F. Heffron. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, B., G. C. Koo, E. H. Yap, K. L. Chua, and Y. H. Gan. 2002. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect. Immun. 70:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majcherczyk, P. A. 1996. The issue of the true postantibiotic effect. J. Antimicrob. Chemother. 37:188-189. [DOI] [PubMed] [Google Scholar]
- 24.Nakao, M., T. Nishi, and K. Tsuchiya. 1981. In vitro and in vivo morphological response of Klebsiella pneumoniae to cefotiam and cefazolin. Antimicrob. Agents Chemother. 19:901-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 6th ed., NCCLS document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell. Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 27.Ramadan, M. A., A. F. Tawfik, A. M. Shibl, and C. G. Gemmell. 1995. Post-antibiotic effect of azithromycin and erythromycin on streptococcal susceptibility to phagocytosis. J. Med. Microbiol. 42:362-366. [DOI] [PubMed] [Google Scholar]
- 28.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan, D. M., and D. Monsey. 1981. Bacterial filamentation and in vivo efficacy: a comparison of several cephalosporins. J. Antimicrob. Chemother. 7:57-63. [DOI] [PubMed] [Google Scholar]
- 30.Shibl, A. M., J.-C. Pechère, and M. A. Ramadan. 1995. Postantibiotic effect and host-bacteria interactions. J. Antimicrob. Chemother. 36:885-887. [DOI] [PubMed] [Google Scholar]
- 31.Simpson, A. J. H., S. M. Opal, B. J. Angus, J. M. Prins, J. E. Palardy, N. A. Parejo, W. Chaowagul, and N. J. White. 2000. Differential antibiotic-induced endotoxin release in severe melioidosis. J. Infect. Dis. 181:1014-1019. [DOI] [PubMed] [Google Scholar]
- 32.Spurr, A. R. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-34. [DOI] [PubMed] [Google Scholar]
- 33.Suptuuamongkol, Y., D. Kwiatkowski, D. A. B. Dance, W. Chaowagul, and N. J. White. 1992. Tumor necrosis factor in septicemic melioidosis. J. Infect. Dis. 165:561-564. [DOI] [PubMed] [Google Scholar]
- 34.Tomasz, A. 1979. From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev. Infect. Dis. 1:434-467. [DOI] [PubMed] [Google Scholar]
- 35.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, A. L., M. D. Smith, V. Wuthiekanun, and N. J. White. 1995. Postantibiotic effects and Burkholderia (Pseudomonas) pseudomallei: evaluation of current treatment. Antimicrob. Agents Chemother. 39:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wozniak, D. J., and R. Keyser. 2004. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 125:62S-69S. [DOI] [PubMed] [Google Scholar]
- 38.Zychlinsky, A., M. C. Prevos, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]