Abstract
Staphylococcus aureus is an important pathogen of humans and animals, and antibiotic resistance is a public health concern. Biofilm formation is essential in virulence and pathogenesis, and the ability to resist antibiotic treatment results in difficult-to-treat and persistent infections. As such, novel antimicrobial approaches are of great interest to the scientific, medical, and agriculture communities. We recently proposed that modulating levels of the cyclic dinucleotide signaling molecule, c-di-GMP (cyclic diguanylate [3′,5′-cyclic diguanylic acid], cGpGp), has utility in regulating phenotypes of prokaryotes. We report that extracellular c-di-GMP shows activity against human clinical and bovine intramammary mastitis isolates of S. aureus, including methicillin-resistant S. aureus (MRSA) isolates. We show that chemically synthesized c-di-GMP is soluble and stable in water and physiological saline and stable following boiling and exposure to acid and alkali. Treatment of S. aureus with extracellular c-di-GMP inhibited cell-to-cell (intercellular) adhesive interactions in liquid medium and reduced (>50%) biofilm formation in human and bovine isolates compared to untreated controls. c-di-GMP inhibited the adherence of S. aureus to human epithelial HeLa cells. The cyclic nucleotide analogs cyclic GMP and cyclic AMP had a lesser inhibitory effect on biofilms, while 5′-GMP had no major effect. We propose that cyclic dinucleotides such as c-di-GMP, used either alone or in combination with other antimicrobial agents, represent a novel and attractive approach in the development of intervention strategies for the prevention of biofilms and the control and treatment of infection.
Staphylococcus aureus is an important human and animal pathogen (2, 21, 27, 57). S. aureus is found on the skin and mucosal surfaces of humans, particularly in the anterior nares. Approximately 20% of the human population are persistent carriers; 60% are intermittent carriers, while 20% of the population will never be colonized (45). S. aureus is a common cause of both community-acquired and hospital-acquired nosocomial infections. Patients with indwelling medical devices, patients on hemodialysis, patients who use intravenous drugs, and patients with dermatologic disease and diabetes mellitus have higher rates of colonization than the general population (26, 59, 60), and S. aureus isolates causing infection are often endogenous in origin (16, 28, 39, 62, 64). According to the National Nosocomial Infection Surveillance System of the Center for Disease Control and Prevention, S. aureus is the most common cause of surgical site infection and the second most common cause of nosocomial bacteremia (38). Infections caused by multiple-antibiotic-resistant S. aureus strains (e.g., methicillin-resistant S. aureus [MRSA]) are particularly difficult to treat, and MRSA infections are often associated with higher mortality and increased healthcare costs compared to methicillin-sensitive strain infections (10). S. aureus is also a common cause of intramammary infections (IMI) in lactating females; such infections often result in chronic mastitis, with annual losses associated with subclinical mastitis in dairy cows across the United States being estimated at approximately $1 billion (43).
Biofilm formation is a key factor in the establishment and persistence of staphylococcal infections in humans and animals, and biofilm formation on tissues or on medical devices is an important first step in the pathogenesis of S. aureus infection in humans (8, 9, 12-14, 19, 22, 27, 33, 36, 45, 46, 49). It is thought that upwards of 60% of all nosocomial infections involve biofilms; these biofilm-based infections can increase hospital stays by up to 2 to 3 days and can result in upwards of $1 billion per year in added costs. Another compelling reason to prevent colonization and biofilm formation is to prevent the transmission of S. aureus to others (37). Therefore, novel intervention strategies that prevent biofilm formation are needed.
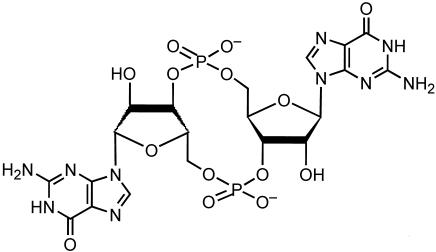
Cyclic nucleotides, such as cyclic AMP (cAMP) and cyclic GMP (cGMP), are well recognized as important low-molecular-weight signaling molecules in eukaryotes. In bacteria, cAMP has a role in alleviating glucose catabolite repression (23, 40), and cGMP has been shown to act as a signaling molecule in Synechocystis cyanobacteria (41, 42). Another novel guanosine nucleotide, the cyclic dinucleotide GMP (c-di-GMP; cyclic diguanylate [3′,5′-cyclic diguanylic acid], cGpGp) (Fig. 1), has been reported to be an intracellular bacterial signaling molecule whose structure is known and consists of two cGMP molecules joined by a 3′,5′-phosphodiester bond (24, 53). c-di-GMP was first identified in Acetobacter xylinum (renamed Gluconacetobacter xylinus) and was shown to regulate cellulose production in this species (1, 31, 53, 54) in which c-di-GMP binds to and activates the cellulose synthase BcsB. Cellulose production is modulated by the opposing effects of two proteins with GGDEF domains, diguanylate cyclase (Dgc) and c-di-GMP phosphodiesterase (PdeA), each controlling the level of c-di-GMP in the cell. Thus, c-di-GMP is thought to be a signaling molecule.
FIG. 1.
Structure of the cyclic dinucleotide, c-di-GMP.
Judging on the basis of studies by us and others, it is now increasingly reported that biofilm formation by many pathogens, including Vibrio cholerae, Yersinia pestis, Salmonella enteritidis serovar Typhimurium, and Pseudomonas aeruginosa, is associated with GGDEF proteins and, therefore, might be regulated by c-di-GMP (7, 15, 25, 51). On the basis of the results of our studies in V. cholerae, we recently proposed that modulation of c-di-GMP levels might have applications in regulating various phenotypes in bacteria such as biofilm formation and virulence (47).
The increasing emergence of antimicrobial resistance in bacterial pathogens and the importance of biofilms in the infection process requires that alternate antimicrobial strategies be developed. We explored a novel approach in which c-di-GMP treatment can be used as a novel intervention strategy to control S. aureus biofilm formation and virulence. We present evidence that c-di-GMP is a stable molecule and that extracellular treatment inhibits biofilm formation in both human and bovine S. aureus clinical isolates, including MRSA strains.
MATERIALS AND METHODS
c-di-GMP, cGMP, and 5′-GMP nucleotides used.
The c-di-GMP used in these studies was chemically synthesized in pure form by the use of a recently described novel synthesis method that produces a pure and high-yield preparation of c-di-GMP diammonium salt (20). Before use, the purity and stability of lyophilized c-di-GMP were determined by first resuspending the molecule in 0.9% NaCl, creating a 2 mM solution; the results were then confirmed by high-performance liquid chromatography (HPLC) analysis and electrospray ionization (ESI)-time of flight (mass spectrometry). The cGMP (guanosine 3′,5′-cyclic monophosphate; Sigma catalog no. G7504), cAMP (adenosine 3′,5′-cyclic monophosphate; Sigma catalog no. A-9501), and 5′-GMP (GMP; TCI, Tokyo Kasei Kogyo Co.) nucleotides were also used. Unless otherwise stated, a 4 mM stock solution of each nucleotide in 0.9% NaCl was prepared and stored at 4°C until needed.
Bacterial strains and growth media used.
Clinical isolates of S. aureus used in this study were stored at −70°C in 50% glycerol. S. aureus strain DK825 was isolated in 2003 from the blood culture of a patient at the Veterans Affairs Medical Center (approximately 200 acute care beds) in Baltimore, Md. Strain DK825 was isolated by the diagnostic microbiology laboratory at the Veterans Affairs Medical Center and was confirmed as methicillin-resistant S. aureus (MRSA) by the use of standard plating techniques and latex agglutination using a BactiStaph Latex 150 test kit (Remel) for the detection of coagulase and by antibiotic susceptibility tests (Sensititre plates; Microbiology Systems). S. aureus strain 15981 is a highly adherent hyperbiofilm human clinical strain that was isolated in 1999 from an otitis patient by the clinical laboratory at the Universitaria de Navarra, Pamplona, Spain (Iñigo Lasa, personal communication) (61). S. aureus 15981 is a natural agr mutant and is susceptible to methicillin, amoxicillin, clindamycin, erythromycin, doxycycline, fosfomycin, vancomycin, and ciprofloxacin and is resistant to gentamicin. The wild-type bovine mastitis strains used in this study were V329 (hyperbiofilm strain) (12), V299 (bap negative and icaADBC positive) (14), and V315 (bap negative and icaADBC negative) (14). S. aureus strain M556 (an isogenic transposon insertional bap mutant of V329) was also used (12), Unless otherwise noted, S. aureus strains were grown at 37°C on sheep blood agar plates or in tryptic soy broth (TSB; Difco) supplemented with 0.25% glucose.
c-di-GMP stability tests.
Although the term c-di-GMP is used here, c-di-GMP diammonium salt (and not the free diphosphoric acid) was used for these stability tests. (i) A 2 mM solution of c-di-GMP was prepared in boiling water by dissolving 2.42 mg (3.3 μmol) of c-di-GMP in 1.65 ml of ion-exchanged, ion-free water (prepared by passing distilled water through ion-exchange resins). This solution was heated at 100°C for 10 min and then concentrated under reduced pressure. The resulting residual was subjected to the HPLC analysis under the conditions described below. (ii) A total of 500 μl of the 2 mM c-di-GMP aqueous solution described above (containing 1 μM of c-di-GMP) was dissolved in 20 ml of 1 mM HCl to produce a pH 3 solution. The resulting solution was stirred at room temperature for 1 h and then neutralized by the addition of 200 ml of 0.1 mM NaOH. Water was evaporated under reduced pressure, and the resulting residue was subjected to HPLC analysis. (iii) A total of 500 μl of the 2 mM c-di-GMP aqueous solution described above (containing 1 μM of c-di-GMP) was dissolved in 200 ml of a 0.1 mM NaOH aqueous solution to produce a pH 10 solution. The resulting solution was stirred at room temperature for 1 h. The reaction was quenched by addition of 20 ml of a 1 mM HCl. Concentration of the resulting neutral solution under reduced pressure gave a residual material that was subjected to the HPLC analysis. The HPLC analysis was performed on a Waters 2695 separation module with a Waters 2996 photodiode array detector under the following conditions: column, Nacalai Tesque COSMOSIL 5C18-AR-II column (4.6 mm in diameter by 250 mm in length); detection, 254-nm UV light; temperature, 40°C; eluent A, 0.9% NaCl; eluent B, a 20:80 mixture of water:acetonitrile; flow rate, 1 ml/min; during min 0 to 10, the eluent A linear gradient value was 100%; during min 10 to 60, linear gradient values for eluent A and eluent B changed from 100 and 0% to 40 and 60%, respectively.
Effect on growth rate of S. aureus.
S. aureus DK825 was subcultured from glycerol stocks onto a blood agar plate and incubated at 37°C for 18 h. A single colony was then inoculated into 5 ml of TSB (supplemented with 0.25% glucose) and incubated at 37°C for 24 h with shaking at 250 rpm. From a 10−3 dilution of the overnight culture a 100-μl aliquot was inoculated into tubes containing 5 ml of TSB, resulting in an initial cell count of 105 CFU/ml (confirmed by plating). For “treated” samples, an appropriate aliquot of c-di-GMP was added to give 200 μM (final concentration) c-di-GMP. As a negative (untreated) control, tubes containing TSB and a similar volume of 0.9% NaCl were included in the studies. At time 0, 50 μl from each sample diluted appropriately was plated on blood agar plates to determine the initial number of CFU/ml. The tubes were then incubated at 37°C for 8 h under shaking conditions, with aliquots being plated every 30 min from all tubes following appropriate dilution.
Tube agglutination assay and light microscopy.
Colonies from an 18-h blood agar plate were inoculated into 1 ml of phosphate-buffered saline (PBS) to achieve a 0.5 McFarland standard containing ∼5 × 108 CFU/ml. A 5-μl aliquot from the 0.5 McFarland standard was then inoculated into 5-ml polystyrene tubes containing 1 ml of TSB representing ∼105 CFU/ml. These tubes containing 1 ml of TSB were inoculated with 50 μl of c-di-GMP to give a 200 μM c-di-GMP final concentration representing a treated sample or with 50 μl of 0.9% NaCl as an untreated control. Cultures were incubated at 37°C for 24 h statically. Following incubation, these cultures were examined macroscopically and microscopically (Zeiss Axioskop) for the presence or absence of visible cell-to-cell clumping.
Effect of c-di-GMP on S. aureus biofilm formation.
S. aureus strains were subcultured from glycerol stocks onto blood agar plates and incubated at 37°C for 18 h. A single colony was inoculated into 5 ml of TSB with a sterilized loop and incubated at 37°C for 18 h with shaking at 250 rpm and until the optical density at 660 nm (OD660) reached 3.0, as measured spectrophotometrically using a spectrophotometer (SpectraMAX 250; Molecular Devices). Following incubation, the culture was diluted 1:250 with fresh TSB and a 200 μl of the diluted culture was transferred into wells of a flat-bottom polystyrene microtiter plate (Evergreen Scientific). To test the effect of c-di-GMP treatment on biofilm formation, a series of treated samples containing 10-fold dilutions of c-di-GMP were set up in TSB which contained the following final concentrations of c-di-GMP: 0, 2, 20, and 200 μM. In these biofilm experiments, similar volumes of 0.9% NaCl were added to a set of different wells representing the untreated control samples. The microtiter plates were then incubated statically at 37°C for 24 or 48 h. Following incubation, the supernatant was carefully discarded and the wells were washed twice with 260 μl of PBS. The plate was then kept for drying on a paper towel for 30 min, after which 260 μl of 0.1% crystal violet was added to each well and the plates were incubated at room temperature for 30 min. The crystal violet was discarded, and the plate was washed gently with water, the wells were allowed to dry for 30 min, and then 260 μl of dimethyl sulfoxide was added to each well and gently agitated for 1 h and the OD570 was measured by using a spectrophotometer (SpectraMAX 250; Molecular Devices). The results of these biofilm assays were based on data obtained from at least three independent colonies tested in duplicate.
Effect of c-di-GMP on preformed S. aureus biofilms.
S. aureus DK825 was subcultured from glycerol stocks onto blood agar plate and incubated at 37°C statically. A single colony was inoculated into 5 ml of TSB (containing 0.25% glucose) and incubated at 37°C overnight with shaking at 250 rpm until the culture reached an OD660 of ∼3.0. Following incubation, the culture was diluted to 1:250 with fresh TSB, 200 μl of the diluted culture was transferred into each well of microtiter plate, and the plate containing wells with S. aureus cultures was incubated statically at 37°C for 24 h. After 24 h, an appropriate volume of c-di-GMP was added to give a final concentration of 200 μM; this represented the treated sample. As an untreated control, an identical volume of 0.9% NaCl was added to independent wells. The plates were then incubated statically at 37°C for an additional 24 h. Following incubation, the culture was discarded and the microtiter plate was washed twice with 1× PBS. An equal volume (260 μl) of 1× PBS was added to each well for washing. The plates were then kept for drying on paper towel for ∼30 min. A total of 260 μl of 0.1% crystal violet was added to each well to stain the cells in the biofilm, and the plates were incubated at room temperature for 30 min. The crystal violet was discarded, and the plate was gently washed with tap water and kept on a paper towel to dry for 30 min. A total of 260 μl of dimethyl sulfoxide was added to each well and gently rocked for 1 h. To quantitatively assay the amount of biofilm, the OD570 was measured with a spectrophotometer (SpectraMAX 250; Molecular Devices). The results were based on experiments with at least three independent colonies tested in duplicate.
Epithelial cell assay.
HeLa cells (ATCC CCL2) were grown to confluence in complete medium (10% FBS [Sigma], Dulbecco's modified Eagle's medium [DMEM]-F12 with glutamine [Invitrogen], 50 μg of gentamicin/ml) and were trypsinized with 0.1% trypsin-EDTA. Approximately 105 HeLa cells were seeded in each well of a chamber slide, and then the HeLa cells were incubated at 37°C in 5% CO2 for at least 18 h until they were 85% confluent. Prior to infection, the HeLa cells were washed twice with warm PBS and then 500 μl of warm FMEM-F12 was added to each well. For the bacterial adherence assay, a fresh colony of S. aureus strain DK825 was grown overnight in 5 ml of Luria-Bertani broth at 37°C with shaking at 250 rpm. Following incubation, 1 ml of the overnight culture was pelleted, washed twice with PBS, and resuspended in 1 ml of PBS. The HeLa cells in the wells were washed twice with 500 μl of HBSS, and aliquots of DMEM with the desired final concentration of c-di-GMP (0, 2, 20, or 200 μM) were prepared. DMEM (500 μl) containing the respective concentrations of c-di-GMP was inoculated into the wells containing HeLa cells followed by the addition of 10 μl (∼107 cells) of S. aureus (multiplicity of infection: HeLa:bacteria ∼ 1:100). The epithelial cell assay mixture was incubated in CO2 for 45 min. Following incubation, the cells were washed twice with 500 μl of PBS and fixed with 2% formalin for 20 min. The cells were again washed twice with PBS and stained with Giemsa stain for 10 s and washed three times with PBS, and then the cells covered with 40 μl of 90% glycerol in water and a coverslip was placed. The slides were observed under a light microscope (Zeiss Axioskop) at ×630 magnification. The level of bacterial adherence to 100 individual HeLa cells was calculated for each duplicate treatment, and the average was determined.
RESULTS AND DISCUSSION
Identification of GGDEF domains in S. aureus suggests a link to c-di-GMP.
c-di-GMP is associated with proteins containing GGDEF protein domains (3, 24, 44, 50, 53, 55). The GGDEF domains are ∼180-amino-acid protein fragments that have an adenylate cyclase-like fold and work as a cyclic diguanylate synthetase. These domains have a conserved GG(D/E)EF motif but also many other conserved residues (17, 18). GGDEF proteins are increasingly being found to be important in the regulation of bacterial exopolysaccharide and biofilm formation (7, 15, 25, 51). The presence of GGDEF proteins is widespread in bacteria (17, 18), suggesting that a broad range of species and phenotypes might be potentially targeted and modulated by c-di-GMP. Interestingly, a search of the COG database shows that S. aureus has only one protein (SA0701 [COG2199]) with a C-terminal GGDEF domain and another protein (SA0013 [COG3887]) with a modified GGDEF domain (58). According to a Pfam analysis (http://pfam.wustl.edu), the N-terminal fragment of SA0701 is predicted to be an integral membrane sensor domain of the 5TM-5TMR_LYT type (five predicted transmembrane segments; Pfam entry PF07694) and therefore is predicted to be a membrane receptor with a diguanylate cyclase output domain. Unfortunately, the role of these putative signal transduction proteins in S. aureus and whether they are potentially linked to c-di-GMP, whether c-di-GMP is made by S. aureus, and whether the regulatory effects of c-di-GMP are similar in all species are not yet known but should be studied.
c-di-GMP treatment prevents S. aureus cell-to-cell interactions.
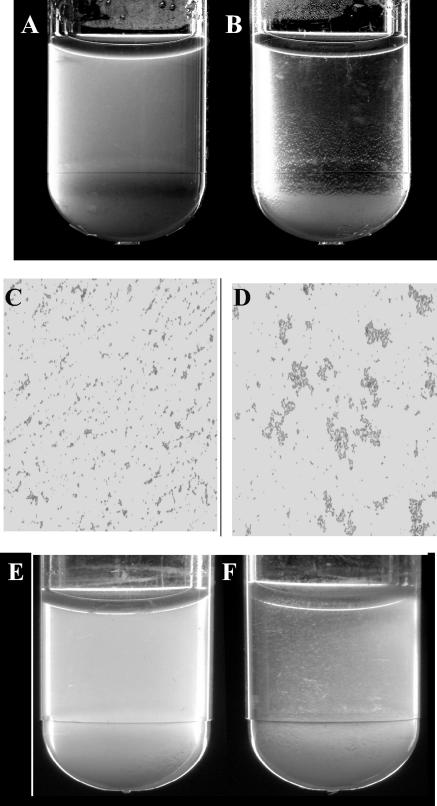
Our initial experiments on the effect of c-di-GMP on S. aureus examined whether c-di-GMP had any effect on the growth rate of S. aureus. Hourly examination of the growth rate for up to 8 h showed that 200 μM c-di-GMP had no obvious effect on growth rate. We then tested whether c-di-GMP treatment affected the macroscopic growth and appearance of S. aureus cells after 24 h of static incubation in liquid culture. Following incubation, the treated and untreated cultures were visually examined for visible cell-cell clumping and aggregation. The results seen with S. aureus DK825 showed that the 200 μM c-di-GMP-treated culture exhibited no obvious visible cell aggregation or pellet at the bottom of the tube whereas the untreated culture showed obvious cell aggregation and a pellet (Fig. 2). Plating of c-di-GMP-treated and untreated cultures showed no difference in final cell counts (6 × 108 CFU/ml) between the cultures, further suggesting that the inhibition of cell-to-cell interactions is not due to major differences in growth rate or final cell numbers. Similar effects on cell aggregation at the bottom of the tube in response to c-di-GMP were observed with several independent wild-type S. aureus bovine mastitis strains (V329, V299, and V315) (data not shown).
FIG. 2.
Effect of c-di-GMP on S. aureus cell-to-cell aggregation. (A and B) Results for a 24-h culture of strain DK825 treated with 200 μM c-di-GMP (A) and for an untreated control (B) are shown. (C and D) The results of Gram staining of c-di-GMP treated cells (C) and untreated cells (D) are shown. (E and F) The results obtained with a c-di-GMP-treated culture of bap mutant M556 (E) and an untreated control (F) are shown. Magnification in panels C and D is ×630.
The regulatory mechanisms involved in S. aureus biofilm formation are not fully understood. However, S. aureus biofilm formation is known to be mediated through the production of the extracellular polysaccharide intercellular adhesin (PIA/PNAG/PSA) that is synthesized by the icaADBC genes and also has a role in cell aggregation (11, 29, 32). The SarA regulator has been shown to be important for biofilm formation (5, 6, 61), as has the Bap protein (12, 13). A recent study by Cucarella et al. (12) resulted in the report that accumulation of cell aggregates at the bottom of the tube was macroscopically only observed for wild-type V329 and not with the isogenic bap mutant M556. While we observed much less visible clumping with strain M556, our results clearly suggested that c-di-GMP treatment inhibits cell aggregation of M556 cell at the bottom of the tube compared to untreated culture results (Fig. 2). It is also important to note that strain M556, while being a bap mutant, is ica positive (12). Two possibilities that might explain the findings in these two studies are that the results of Cucarella et al. (12) were obtained on the basis of shaking cultures and that TSB obtained from different sources might influence cell growth and cell interactions. Inhibition of cell aggregation was consistent and was observed following similar c-di-GMP treatment in which the c-di-GMP used was independently synthesized. The results from this macroscopic analysis indicate that S. aureus cells respond to extracellular c-di-GMP and that c-di-GMP treatment inhibits S. aureus cell aggregation in human and animal isolates.
To further study the basis underlying the macroscopic difference observed between treated and untreated cultures described above, the cells in these cultures were vortexed, Gram stained, and visualized by light microscopy. The name “Staphylococcus” is derived from Greek and means “bunch of grapes.” However, examination of the strain DK825 c-di-GMP-treated cultures after Gram staining revealed dramatically fewer cell-to-cell interactions and clumping in liquid medium compared to untreated cell results, which showed typical grape-like clusters (Fig. 2). Consistent with these findings, fewer intercellular interactions and less clumping was observed by Gram staining and light microscopy for the wild-type bovine mastitis strains (V329, V299, and V315) (data not shown). Note that wild-type strain V329 is bap positive and icaADBC positive, while wild-type strain V299 is bap negative and icaADBC positive and wild-type strain V315 is bap negative and icaADBC negative (14). While most bovine mastitis isolates appear to be bap negative, as the bap gene seems to be only present in a small percentage of bovine mastitis isolates, bap appears to be absent from human isolates (12). In our studies, although cell aggregation in the isogenic bap mutant strain M556 was much less than that seen with its parent V329 strain, the data suggest that c-di-GMP treatment inhibits cell clumping in mutant strain M556 (Fig. 2). These data from analyses of wild-type and mutant strains seem to imply that the inhibition of cell interactions is independent of the presence of bap and the icaADBC gene clusters. Importantly, the results of the microscopic analysis correlated with the macroscopic observations and further indicate that c-di-GMP treatment can inhibit S. aureus cell-to-cell (intercellular) adhesive interactions in human and bovine isolates.
c-di-GMP inhibits biofilm formation in human and bovine S. aureus.
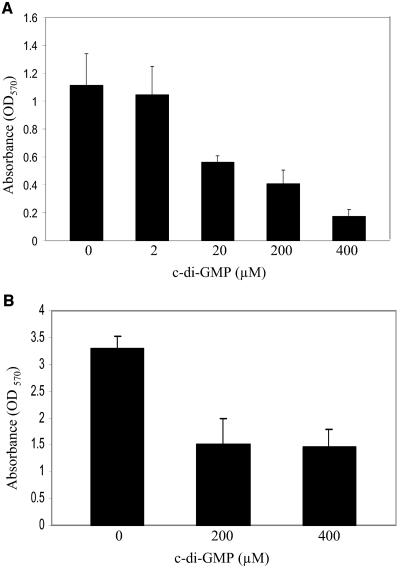
Given that c-di-GMP treatment inhibits S. aureus cell-to-cell interactions, we speculated that c-di-GMP could inhibit biofilm formation. The biofilm results showed that c-di-GMP treatment inhibits S. aureus DK825 biofilm formation on abiotic polystyrene surfaces at 24 h in a dose-dependent manner (Fig. 3). The inhibitory effect of extracellular c-di-GMP was seen at 20 μM (∼50% reduction), 200 μM (∼65% reduction), and 400 μM (∼85% reduction). A similar difference in biofilm formation results between treated and untreated cultures was observed after measurement at 48 h, suggesting that selection for resistance to treatment did not occur (data not shown). Our results also showed similar results for c-di-GMP inhibition of biofilm formation at 24 h in the highly adherent hyperbiofilm S. aureus strain 15981 at the concentrations tested (Fig. 3). Although coagulase has been shown to be important for S. aureus colonization of host tissues, we did not find any difference in coagulase production (bound or free) for strain DK825 between treated and untreated cells (data not shown). The biofilm results correlate with the macroscopic and microscopic cell-to-cell aggregation data, suggesting that extracellular c-di-GMP inhibits cell interactions and biofilm formation in human isolates.
FIG. 3.
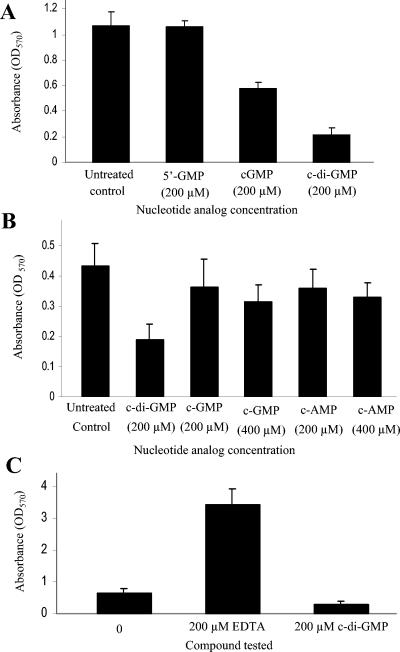
Effect of c-di-GMP on the ability of S. aureus human clinical isolates to form biofilms on a polystyrene surface. The results were obtained using microtiter plates. (A) Inhibition of biofilm formation in S. aureus strain DK825 in TSB-0.25% glucose treated with various concentrations of c-di-GMP for 24 h and stained with crystal violet. (B) Quantitative analysis of the inhibition of biofilm formation in hyperbiofilm S. aureus strain 15981 treated with c-di-GMP. Error bars represent ± standard errors.
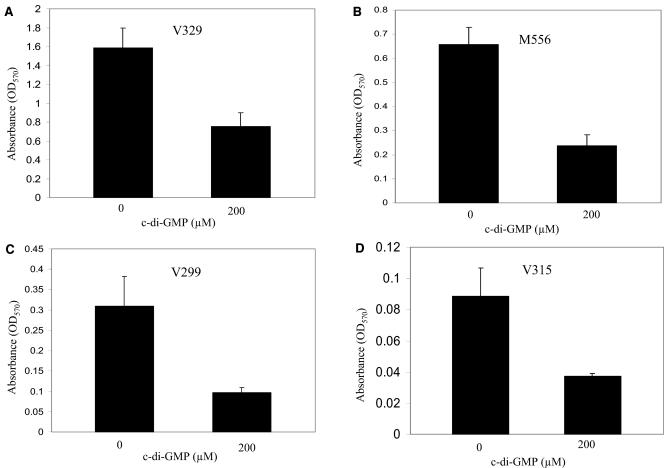
Our quantitative biofilm analysis also demonstrated that c-di-GMP inhibits biofilm formation of bovine mastitis strains (Fig. 4). The wild-type bovine strain V329 was previously shown to be a strong biofilm producer on polystyrene surfaces, whereas the isogenic bap mutant M556 was attenuated in this biofilm ability (12). Our analysis supports this finding but also importantly shows that in similarity to human isolate results, c-di-GMP dramatically inhibits biofilm formation (∼50 to 70% reduction) in these wild-type and mutant bovine strains as well as in the wild-type bap-negative strain V299 and the wild-type bap-negative icaADBC-negative strain V315, which formed very low levels of biofilm (Fig. 4). Together, these results provide further compelling evidence to indicate that c-di-GMP treatment inhibits S. aureus cell-to-cell interactions and cell-to-surface interactions involved in biofilm formation. While the exact mechanism of action remains to be determined, our results for strain 15981 suggest the mechanism might be independent of the presence of agr and our earlier studies with the bovine isolates suggested that the mechanism might be independent of the presence of bap and icaADBC. These findings also suggest that cyclic dinucleotides such as c-di-GMP might be useful in preventing biofilms on clinically relevant surfaces such as medical devices and, potentially, in the control of human and animal infection.
FIG. 4.
Quantitative analysis on the effect of c-di-GMP on the ability of S. aureus bovine mastitis isolates to form biofilms on a polystyrene surface.
Effect of nucleotide analogs, cations, and EDTA on biofilm formation.
Proceeding on the basis of the observed effects for strain DK825 with the cyclic dinucleotide c-di-GMP, we then tested whether treating cultures with extracellular cyclic nucleotide analogs such as cGMP (guanosine 3′,5′-cyclic monophosphate), cAMP (adenosine 3′,5′-cyclic monophosphate), and 5′-GMP at 200 or 400 μM could inhibit S. aureus biofilm formation. These experiments were performed to test the specificity of c-di-GMP as a biofilm blocker and to rule out the possibility that the effects on biofilms we observed were merely due to the presence of extracellular nucleotides in general or to that of cyclic mononucleotide (guanosine or adenosine) analogs in particular. The two nucleotides 5′-GMP and cGMP were also chosen, as the structure of c-di-GMP is somewhat similar to those of the two cGMP molecules, being linked by a 3′-5′ phosphodiester bond, and as 5′-GMP is a known breakdown product of c-di-GMP (53).
As expected, while addition of 200 μM c-di-GMP significantly blocked biofilm formation in strain DK825, the addition of 200 μM cGMP and cAMP to the growth medium inhibited biofilm formation, albeit to a much lesser extent (Fig. 5A and B). Similar results were observed with the other strains tested, strain 15981 (human) and strain V329 (bovine) (data not shown). The data showed that the presence of 200 μM 5′-GMP had no major effect on biofilm formation (Fig. 5A). In addition, we compared a dose response to the presence of 200 μM c-di-GMP to that of 400 μM cGMP and 400 μM cAMP. We did this primarily because 200 μM c-di-GMP has twice as much of a “cGMP module” as cGMP, and we wanted to know whether doubling the concentration of these cyclic nucleotide analogs would result in a biofilm blocking activity similar to that of c-di-GMP. The results shown in Fig. 5B, which are based on two independent experiments performed in triplicate, clearly demonstrate that doubling the concentration of the cGMP and cAMP cyclic nucleotides to 400 μM does not greatly affect their ability to inhibit biofilm formation in DK825 compared to 200 μM c-di-GMP. Similar results were also observed with strains 15981 and V329 (data not shown). These findings show that in contrast to the results seen with cGMP, cAMP, and 5′-GMP, the significant inhibitory effect observed with c-di-GMP is not due to the molecule merely having a guanosine base or merely being cyclic in nature but is somehow unique and specific to its cyclic dinucleotide structure.
FIG. 5.
Quantitative biofilm analysis of the effect of guanosine nucleotide analogs and of general EDTA chelating activity on S. aureus biofilm formation on polystyrene surfaces. (A) Effect of 200 μM 5′-GMP, cGMP, and c-di-GMP treatment on biofilm formation. (B) Effect of 400 μM cGMP and cAMP treatment compared with that of 200 μM c-di-GMP treatment on biofilm formation. (C) Effect of 200 μM EDTA on biofilm formation. Error bars represent ± standard errors.
Given its unusual structure, it is possible that the hydroxyl-phosphodiester ring of c-di-GMP might form physical complexes with some extracellular material, such as metal cations (e.g., MgCl2 and CaCl2), that is needed or promotes biofilm formation by S. aureus. However, repeated experiments showed that addition of 200 and 800 μM MgCl2 or CaCl2 in combination with c-di-GMP did not prevent the inhibition of biofilm formation by c-di-GMP. Furthermore, the addition of 200 μM EDTA alone to TSB caused a dramatic (fourfold) increase in biofilm formation (Fig. 5C). Under these conditions and with the concentrations tested, these results indicate that the mechanism of action of c-di-GMP with respect to inhibition of S. aureus biofilm formation does not appear to be due to a general sequestering ability or to involve a general chelating effect. These results highlight the importance, novelty, and perhaps specificity and affinity of c-di-GMP in its mechanism of action and effect on the cell.
Effect of c-di-GMP treatment on S. aureus preformed biofilms.
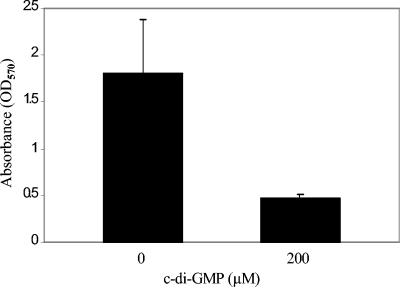
Since our data showed that c-di-GMP treatment inhibited biofilm formation in S. aureus strains DK825 and 15981, we tested the hypothesis that extracellular c-di-GMP has an effect on preformed established biofilms. Our results showed that c-di-GMP treatment (200 μM) of a 24-h preformed biofilm blocks further biofilm development (∼75% reduction) compared to the untreated control results (Fig. 6). Judging on the basis of these data, it appears that c-di-GMP inhibits both the initial formation of biofilms and further development of preformed biofilms.
FIG. 6.
Quantitative biofilm analysis of the effect of c-di-GMP on S. aureus 24-h preformed biofilms.
c-di-GMP treatment inhibits S. aureus adherence to human epithelial cells.
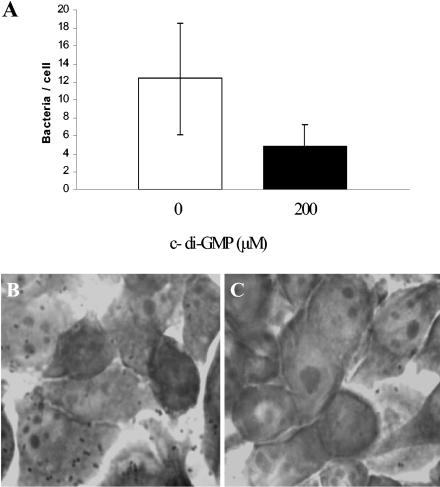
Studies examining the adherence of S. aureus to epithelial cell monolayers and the effect of potential therapeutic agents to inhibit adherence have been performed previously (4, 13, 30, 34, 35, 48, 63). The data from our studies showed that treatment with 2 and 20 μM c-di-GMP did not show any obvious effect on adherence. However, compared to untreated control results, treatment with 200 μM c-di-GMP reduced the numbers of S. aureus cells adhering to HeLa cells (Fig. 7). The data indicated that c-di-GMP treatment results in an average reduction in adherence from 12 bacteria/cell to 4 bacteria/cell (∼66% reduction). Experiments examining the effects of various concentrations (0, 25, 50, 100, 200, and 400 μM) of c-di-GMP on HeLa cells prepared in complete medium (but in the absence of bacteria) as described above showed no obvious visible effect on HeLa cell morphology examined microscopically at 12, 24, and 48 h of incubation. While the molecular basis for c-di-GMP inhibition of S. aureus epithelial cell adherence is not yet understood, these in vitro data are clearly consistent with our previous biofilm results obtained using polystyrene (abiotic) surfaces and suggest that c-di-GMP can also be used to inhibit biofilm formation of epithelial cell (biotic) surfaces.
FIG. 7.
Effect of c-di-GMP treatment on S. aureus DK825 adherence to HeLa epithelial cells. (A) Graph showing level of epithelial cell adherence. (A and B) Photographs of Giemsa-stained untreated (B) and treated (C) S. aureus cells adhering to HeLa cells.
Stability of c-di-GMP.
The stability of c-di-GMP under various physical conditions and treatments is not well understood and so if c-di-GMP is to be used as part of an antimicrobial strategy or therapeutic agent, its stability needs to be better studied. We determined the stability of c-di-GMP under several storage and various exposure conditions, including heat, acid (pH 3), and alkali (pH 10) treatment.
Initially, we determined the stability of chemically synthesized undiluted (powdered form) c-di-GMP following several days of storage at −78°C. Immediately prior to analysis, the dry compound was resuspended in ion-free distilled water (water prepared by passing distilled water through an ion-exchange resin column) to produce a 2 mM stock. HPLC analysis of the 2 mM stock c-di-GMP indicated that storage of the undiluted form of c-di-GMP for several days at −78°C resulted in the formation of aggregate molecules whose structure is unknown at present but is being determined (data not shown). Furthermore, storage of a 2 mM stock solution in water at ambient temperature (10 to 20°C) for several days resulted in the formation of an aggregated product, interestingly, however, adjustment of the solution to a 0.9% concentration of NaCl was found to cause the aggregated molecules to revert to the monomeric form, as determined by HPLC and ESI-time of flight (mass spectrometry) (data not shown). c-di-GMP was stable in a 100 mM phosphate buffer for at least 1 month at −78, 4, and 25°C and did not undergo any structural changes (data not shown). HPLC analysis showed that c-di-GMP was very stable in a 0.9% NaCl solution with respect to the monomeric structure following storage at −78, 4, or 25°C for at least 3 months. We found that c-di-GMP was also stable in a 100 mM ammonium acetate buffer for at least 1 month at −78, 4, and 25°C and did not undergo any structural changes. These results suggest that stock solutions of c-di-GMP should be prepared in 0.9% NaCl such that c-di-GMP will be stable and remain in monomeric form for at least several months.
Consistent with a previous study by Ross et al. (52), our HPLC analysis demonstrated that chemically synthesized c-di-GMP is stable following 10 min of exposure at 100°C. That study found that (as measured by cellulose-activating activity) c-di-GMP is labile after treatment in relatively strong alkali (0.2 N NaOH [∼pH 13.5] at 37°C for 24 h), and our HPLC analysis suggests that c-di-GMP is stable following treatment in mild alkali (0.0001 N NaOH [pH 10] at 20 to 25°C for 1 h). Consistent with the findings from the previous study by Ross et al. (52), our data showed that chemically synthesized c-di-GMP is stable following acid treatment (0.001 N HCl [pH 3] at 20 to 25°C for 1 h). Judging on the basis of the results of these studies and the physicochemical properties of the molecule, c-di-GMP is a stable and soluble low-molecular-weight molecule that possesses antimicrobial activity against S. aureus.
Possible mechanism of action of c-di-GMP on S. aureus.
Studies of the gram-negative bacterial species G. xylinus showed that c-di-GMP is an intracellular signaling molecule. Judging on the basis of our findings obtained with S. aureus, a gram-positive species, we speculate that S. aureus can respond to the presence of extracellular c-di-GMP. It is still possible that c-di-GMP might form complexes with or sequester certain extracellular molecules that are important for S. aureus biofilm formation. Another possibility, however, is that c-di-GMP does not enter cells and that extracellular c-di-GMP treatment and the inhibition of cell-to-cell interactions and biofilm formation could involve c-di-GMP binding to a surface receptor which then might trigger signaling events modulating gene and protein expression. c-di-GMP has been reported to be able to enter eukaryotic cells (56), and no published reports have described the ability or inability of c-di-GMP to act extracellularly on bacteria or bind to or enter cells. Therefore, another possibility is that c-di-GMP might be able to enter S. aureus and trigger changes in protein expression. Regardless of the molecular mechanism involved, which is presently being investigated by us, our findings clearly indicate that c-di-GMP treatment inhibits biofilm formation in S. aureus. This ability would be a valuable auxiliary property for present antimicrobial treatments, as it might potentially increase the availability of the bacterial target site to the antibiotic.
Conclusion.
A recent review by Jenal states that “we are only beginning to understand the role of c-di-GMP signaling in bacteria; indeed, several important issues remain to be addressed” (24). That review also raises the following question: “Does c-di-GMP act exclusively intracellularly or could the compound, similar to cAMP, also be used for extracellular signaling?” The evidence presented in this report strongly suggests that extracellular c-di-GMP inhibits S. aureus cell-to-cell (intercellular) adhesive interactions and biofilm formation. The ability of c-di-GMP to inhibit cell-to-cell interactions and potentially act as a “biofilm blocker” was observed for a variety of human and animal pathogenic strains, suggesting that the mechanism of action might be independent of S. aureus host specificity. The mechanism of action is still not yet understood but is being studied by us; these studies include the identification of genes and proteins that could be differentially expressed by extracellular c-di-GMP as well as potential surface receptors that might potentially lead to future antimicrobial drug targets.
Since the structure of c-di-GMP is known and so analogs can be synthesized and since the molecule shows several ideal drug-like properties, cyclic dinucleotides potentially represent a drug platform for use against a variety of diseases. Judging on the basis of our in vitro results presented here, further studies with c-di-GMP and testing in animal models seem warranted. We propose that treatment with cyclic dinucleotides, such as c-di-GMP, used either alone or in combination with other antimicrobial agents, represents an innovative approach that might be developed as a novel antimicrobial strategy to combat biofilm formation and infection of certain bacterial pathogens.
Acknowledgments
We are very grateful to Iñigo Lasa for supplying the human clinical strain 15981 and to José Penadés for supplying the wild-type and mutant bovine mastitis strains used in this study. We also thank Michael Galperin for helpful discussions and Jan Powell, Mary-Claire Roghmann, and Glenn Morris for critically reviewing the manuscript and providing useful suggestions. We also thank the two anonymous reviewers for providing very useful comments.
REFERENCES
- 1.Amikam, D., and M. Benziman. 1989. Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J. Bacteriol. 171:6649-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer, G. L. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179-1181. [DOI] [PubMed] [Google Scholar]
- 3.Ausmees, N., R. Mayer, H. Weinhouse, G. Volman, D. Amikam, M. Benziman, and M. Lindberg. 2001. Genetic data indicate that proteins containing the GGDEF domain possess diguanylate cyclase activity. FEMS Microbiol. Lett. 204:163-167. [DOI] [PubMed] [Google Scholar]
- 4.Balaban, N., Y. Gov, A. Bitler, and J. R. Boelaert. 2003. Prevention of Staphylococcus aureus biofilm on dialysis catheters and adherence to human cells. Kidney Int. 63:340-345. [DOI] [PubMed] [Google Scholar]
- 5.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomchil, N., P. Watnick, and R. Kolter. 2003. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley, S. F., M. S. Terpenning, M. A. Ramsey, L. T. Zarins, K. A. Jorgensen, W. S. Sottile, D. R. Schaberg, and C. A. Kauffman. 1991. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann. Intern. Med. 115:417-422. [DOI] [PubMed] [Google Scholar]
- 9.Cole, A. M., S. Tahk, A. Oren, D. Yoshioka, Y. H. Kim, A. Park, and T. Ganz. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 11.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cucarella, C., M. A. Tormo, E. Knecht, B. Amorena, I. Lasa, T. J. Foster, and J. R. Penades. 2002. Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process. Infect. Immun. 70:3180-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cucarella, C., M. A. Tormo, C. Ubeda, M. P. Trotonda, M. Monzon, C. Peris, B. Amorena, I. Lasa, and J. R. Penades. 2004. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 72:2177-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ena, J., J. R. Boelaert, L. D. Boyken, H. W. Van Landuyt, C. A. Godard, and L. A. Herwaldt. 1994. Epidemiology of Staphylococcus aureus infections in patients on hemodialysis. Infect. Control Hosp. Epidemiol. 15:78-81. [DOI] [PubMed] [Google Scholar]
- 17.Galperin, M. Y. 2004. Bacterial signal transduction network in a genomic perspective. Environ. Microbiol. 6:552-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galperin, M. Y., A. N. Nikolskaya, and E. V. Koonin. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 203:11-21. [DOI] [PubMed] [Google Scholar]
- 19.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa, Y., R. Nagata, A. Hirata, M. Hyodo, and R. Kawai. 2003. A facile synthesis of cyclic bis(3′-5′)diguanylic acid. Tetrahedron 59:6465-6471. [Google Scholar]
- 21.Hermans, K., L. A. Devriese, and F. Haesebrouck. 2003. Rabbit staphylococcosis: difficult solutions for serious problems. Vet. Microbiol. 91:57-64. [DOI] [PubMed] [Google Scholar]
- 22.Huang, S. S., and R. Platt. 2003. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin. Infect. Dis. 36:281-285. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, D. W., J. W. Simecka, and T. Romeo. 2002. Catabolite repression of Escherichia coli biofilm formation. J. Bacteriol. 184:3406-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenal, U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185-191. [DOI] [PubMed] [Google Scholar]
- 25.Jones, H. A., J. W. Lillard, Jr., and R. D. Perry. 1999. HmsT, a protein essential for expression of the haemin storage (Hms+) phenotype of Yersinia pestis. Microbiology 145(Pt. 8):2117-2128. [DOI] [PubMed] [Google Scholar]
- 26.Kirmani, N., C. U. Tuazon, H. W. Murray, A. E. Parrish, and J. N. Sheagren. 1978. Staphylococcus aureus carriage rate of patients receiving long-term hemodialysis. Arch. Intern. Med. 138:1657-1659. [PubMed] [Google Scholar]
- 27.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luzar, M. A., G. A. Coles, B. Faller, A. Slingeneyer, G. D. Dah, C. Briat, C. Wone, Y. Knefati, M. Kessler, and F. Peluso. 1990. Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N. Engl. J. Med. 322:505-509. [DOI] [PubMed] [Google Scholar]
- 29.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuura, T., Y. Miyake, S. Nakashima, H. Komatsuzawa, Y. Akagawa, and H. Suginaka. 1996. Isolation and characterization of teichoic acid-lake substance as an adhesin of Staphylococcus aureus to HeLa cells. Microbiol. Immunol. 40:247-254. [DOI] [PubMed] [Google Scholar]
- 31.Mayer, R., P. Ross, H. Weinhouse, D. Amikam, G. Volman, P. Ohana, R. D. Calhoon, H. C. Wong, A. W. Emerick, and M. Benziman. 1991. Polypeptide composition of bacterial cyclic diguanylic acid-dependent cellulose synthase and the occurrence of immunologically crossreacting proteins in higher plants. Proc. Natl. Acad. Sci. USA 88:5472-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 33.Mest, D. R., D. H. Wong, K. J. Shimoda, M. E. Mulligan, and S. E. Wilson. 1994. Nasal colonization with methicillin-resistant Staphylococcus aureus on admission to the surgical intensive care unit increases the risk of infection. Anesth. Analg. 78:644-650. [DOI] [PubMed] [Google Scholar]
- 34.Miyake, Y., A. Kohada, I. Fujii, M. Sugai, and H. Suginaka. 1989. Aminoglycosides enhance the adherence of Staphylococcus aureus to HeLa cells. J. Antimicrob. Chemother. 23:79-86. [DOI] [PubMed] [Google Scholar]
- 35.Miyake, Y., A. Kohada, M. Sugai, and H. Suginaka. 1991. Mechanism of aminoglycoside enhancement of Staphylococcus aureus adherence to HeLa cells. J. Antimicrob. Chemother. 28:811-817. [DOI] [PubMed] [Google Scholar]
- 36.Muder, R. R., C. Brennen, M. M. Wagener, R. M. Vickers, J. D. Rihs, G. A. Hancock, Y. C. Yee, J. M. Miller, and V. L. Yu. 1991. Methicillin-resistant staphylococcal colonization and infection in a long-term care facility. Ann. Intern. Med. 114:107-112. [DOI] [PubMed] [Google Scholar]
- 37.Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, H. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect. Control Hosp. Epidemiol. 24:362-386. [DOI] [PubMed] [Google Scholar]
- 38.National Nosocomial Infections Surveillance System. 1998. National Nosocomial Infections Surveillance (NNIS) System report, data summary from October 1986-April 1998, issued June 1998. Am. J. Infect. Control 26:522-533. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen, M. H., C. A. Kauffman, R. P. Goodman, C. Squier, R. D. Arbeit, N. Singh, M. M. Wagener, and V. L. Yu. 1999. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann. Intern. Med. 130:221-225. [DOI] [PubMed] [Google Scholar]
- 40.Notley-McRobb, L., A. Death, and T. Ferenci. 1997. The relationship between external glucose concentration and cAMP levels inside Escherichia coli: implications for models of phosphotransferase-mediated regulation of adenylate cyclase. Microbiology. 143(Pt. 6):1909-1918. [DOI] [PubMed] [Google Scholar]
- 41.Ochoa De Alda, J. A., G. Ajlani, and J. Houmard. 2000. Synechocystis strain PCC 6803 cya2, a prokaryotic gene that encodes a guanylyl cyclase. J. Bacteriol. 182:3839-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochoa de Alda, J. A., and J. Houmard. 2000. Genomic survey of cAMP and cGMP signalling components in the cyanobacterium Synechocystis PCC 6803. Microbiology 146(Pt. 12):3183-3194. [DOI] [PubMed] [Google Scholar]
- 43.Ott, S. L. 1999. Costs of herd-level production losses associated with subclinical mastitis in U.S. dairy cows. Natl. Mastitis Council 38th Annu. Meet., p. 152.
- 44.Paul, R., S. Weiser, N. C. Amiot, C. Chan, T. Schirmer, B. Giese, and U. Jenal. 2004. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605-610. [DOI] [PubMed] [Google Scholar]
- 46.Pujol, M., C. Pena, R. Pallares, J. Ariza, J. Ayats, M. A. Dominguez, and F. Gudiol. 1996. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am. J. Med. 100:509-516. [DOI] [PubMed] [Google Scholar]
- 47.Rashid, M. H., C. Rajanna, A. Ali, and D. K. R. Karaolis. 2003. Identification of genes involved in the switch between the smooth and rugose phenotypes of Vibrio cholerae. FEMS Microbiol. Lett. 227:113-119. [DOI] [PubMed] [Google Scholar]
- 48.Roche, F. M., M. Meehan, and T. J. Foster. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759-2767. [DOI] [PubMed] [Google Scholar]
- 49.Roghmann, M. C., A. Siddiqui, K. Plaisance, and H. Standiford. 2001. MRSA colonization and the risk of MRSA bacteraemia in hospitalized patients with chronic ulcers. J. Hosp. Infect. 47:98-103. [DOI] [PubMed] [Google Scholar]
- 50.Römling, U. 2002. Molecular biology of cellulose production in bacteria. Res. Microbiol. 153:205-212. [DOI] [PubMed] [Google Scholar]
- 51.Römling, U., M. Rohde, A. Olsen, S. Normark, and J. Reinkoster. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-23. [DOI] [PubMed] [Google Scholar]
- 52.Ross, P., Y. Aloni, C. Weinhouse, D. Michaeli, P. Weinberger-Ohana, R. Meyer, and M. Benziman. 1991. An unusual guanyl oligonucleotide regulates cellulose synthesis in Acetobacter xylinum. FEBS Lett. 186:191-196. [DOI] [PubMed] [Google Scholar]
- 53.Ross, P., R. Mayer, and M. Benziman. 1991. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 55:35-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross, P., R. Mayer, H. Weinhouse, D. Amikam, Y. Huggirat, M. Benziman, E. de Vroom, A. Fidder, P. de Paus, L. A. Sliedregt, et al. 1990. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J. Biol. Chem. 265:18933-18943. [PubMed] [Google Scholar]
- 55.Simm, R., M. Morr, A. Kader, M. Nimtz, and U. Romling. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123-1134. [DOI] [PubMed] [Google Scholar]
- 56.Steinberger, O., Z. Lapidot, Z. Ben-Ishai, and D. Amikam. 1999. Elevated expression of the CD4 receptor and cell cycle arrest are induced in Jurkat cells by treatment with the novel cyclic dinucleotide 3′,5′-cyclic diguanylic acid. FEBS Lett. 444:125-129. [DOI] [PubMed] [Google Scholar]
- 57.Sutra, L., and B. Poutrel. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:79-89. [DOI] [PubMed] [Google Scholar]
- 58.Tatusov, R. L., D. A. Natale, I. V. Garkavtsev, T. A. Tatusova, U. T. Shankavaram, B. S. Rao, B. Kiryutin, M. Y. Galperin, N. D. Fedorova, and E. V. Koonin. 2001. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 29:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuazon, C. U., A. Perez, T. Kishaba, and J. N. Sheagren. 1975. Staphylococcus aureus among insulin-injecting diabetic patients. An increased carrier rate. JAMA 231:1272. [PubMed] [Google Scholar]
- 60.Tuazon, C. U., and J. N. Sheagren. 1974. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. J. Infect. Dis. 129:725-727. [DOI] [PubMed] [Google Scholar]
- 61.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 62.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters.2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 63.Wyatt, J. E., S. M. Poston, and W. C. Noble. 1990. Adherence of Staphylococcus aureus to cell monolayers. J. Appl. Bacteriol. 69:834-844. [DOI] [PubMed] [Google Scholar]
- 64.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N. Engl. J. Med. 315:91-96. [DOI] [PubMed] [Google Scholar]