Abstract
The protease inhibitor (PI) indinavir may be used in the management of human immunodeficiency virus (HIV) infection during pregnancy. Poor maternal-to-fetal transfer of indinavir has been reported previously, but the mechanisms of transfer remain unknown. The bidirectional transfer of indinavir was assessed in dually perfused, isolated human placentae. Term placentae (n = 5) were obtained from non-HIV-infected pregnant women. To investigate transport mechanisms, the steady-state transfer of indinavir was compared to those of antipyrine (a marker of passive diffusion) and [3H]vinblastine (a marker of P-glycoprotein [P-gp] transport) in the maternal-to-fetal and fetal-to-maternal directions in each placenta. Indinavir and antipyrine perfusate concentrations were determined by using reverse-phase, high-performance liquid chromatography; [3H]vinblastine concentrations were measured by liquid scintillation. The antipyrine transfer clearance in each direction did not differ (P = 0.76), a finding consistent with passive diffusion. However, the maternal-to-fetal transfer clearance of vinblastine, normalized to that of antipyrine (clearance index) (0.31 ± 0.05), was significantly lower than the fetal-to-maternal clearance index of vinblastine (0.67 ± 0.17; P = 0.017), suggesting the involvement of placental P-gp. Similarly, the maternal-to-fetal clearance index of indinavir (0.39 ± 0.09) was significantly lower than its fetal-to-maternal clearance index (0.97 ± 0.12; P < 0.001). These results represent the first evidence for differential transfer of a xenobiotic in the intact human placenta. The use of transport modulators to increase the maternal-to-fetal transfer of PIs as a possible strategy to reduce mother-to-child transmission of HIV warrants investigation.
Mother-to-child transmission (MTCT) is the principal cause of human immunodeficiency virus (HIV) infections in infants (17). Transmission occurs primarily intrapartum, and maternal viral load is a strong risk factor for transmission (29). However, cases of transmission have been reported for women with undetectable viral loads at delivery (16). In addition, zidovudine monotherapy reduces MTCT independently of reducing maternal viral load (12). It is believed that the efficacy of zidovudine is a result, at least in part, of placental transfer providing preexposure and postexposure prophylaxis to the infant (9). Abbreviated zidovudine regimens establish the importance of antepartum dosing (18), further suggesting that antiretroviral prophylaxis in the fetus is important for decreasing transmission. Consequently, a novel prophylactic strategy has been proposed in which HIV type 1 (HIV-1) protease inhibitors (PIs), as part of an antiretroviral regimen, are administered intrapartum to “preload” the fetus via placental transfer (15). The aim of this approach is to achieve therapeutic concentrations in the fetus with minimum toxicity.
PIs within highly active antiretroviral therapy are used increasingly in pregnancy due to their potency (21, 24, 26); however, there are limited data on placental transfer of these drugs. Previous studies with isolated perfused human placentae demonstrated low maternal-to-fetal transfer of amprenavir, ritonavir, and saquinavir (4, 6, 11). It is not clear whether this low placental transfer was the result of poor diffusional permeability or of some other mechanism. Because these perfusions were performed in the maternal-to-fetal direction only, comparison with fetal-to-maternal transfer was not possible. However, the low maternal-to-fetal transfer observed in the perfused human placenta models are consistent with the results of studies using matched maternal and umbilical cord blood samples collected at the time of delivery from women who had been medicated previously, which revealed the PI concentration in the fetal circulation to be very low (21, 23).
Huisman et al. (15) have implicated placental P-glycoprotein (P-gp) in limiting maternal-to-fetal transfer of PIs. P-gp is a membrane transporter that facilitates active efflux of a wide range of xenobiotics, including PIs (19), from certain cells. It is expressed in several epithelial barriers, including the trophoblast cells of the placenta (20), where it is present in the maternal facing cell membrane. Placental P-gp may extrude xenobiotics from trophoblast cells back into the maternal circulation and thus limit fetal exposure (37). Inhibition of placental P-gp in mice increases the maternal-to-fetal transfer of saquinavir and fetal exposure to this PI (34). It is postulated that P-gp may also influence the transfer of PIs across the placenta in humans, but this has not yet been investigated. An improved understanding of the mechanism of placental transfer of PIs, including the potential role of placental membrane transporters, is potentially important for the development of strategies involving preloading of the fetus with PIs to reduce MTCT of HIV. Therefore, the purpose of this study was to determine the placental transfer of the PI indinavir, vinblastine (a P-gp substrate) (36), and antipyrine (a marker of passive diffusion) (8, 33). We have used the dually perfused isolated human cotyledon, a technically demanding but powerful model that allows determination of transfer of these compounds in both the maternal-to-fetal and fetal-to-maternal directions within the same placenta.
MATERIALS AND METHODS
Chemicals.
Indinavir sulfate was donated by Merck Research Laboratories (Rahway, N.J.). Analytical-grade antipyrine, verapamil hydrochloride, tetramethylammonium perchlorate, and trifluoroacetic acid were obtained from Sigma-Aldrich (Castle Hill, Australia). [3H]vinblastine (specific activity, 1.85 GBq/mmol) and dextran (average molecular weight, 70,000; range, 60,000 to 90,000) were purchased from Amersham Biosciences (Castle Hill, Australia). Other chemicals used were d-glucose from Ajax Chemicals (Melbourne, Australia) and methanol from Biolab (Melbourne, Australia).
Placentae.
The study protocol was approved by the Ethics Committee of the Royal Women's Hospital and Monash University (Melbourne, Australia). Written, informed consent was obtained from participants prior to delivery. Women were excluded if they had HIV/AIDS or any other chronic diseases or were treated with indinavir or other chronic drug therapy. Placentae were obtained from women undergoing elective caesarean sections from uncomplicated, term pregnancies. Prior to perfusion, placentae were carefully inspected and those with visible tears were discarded.
Perfusion technique.
The dual in vitro perfusion of the isolated human placenta was based on the method of Penfold et al. (30) and subsequently modified by our group (7, 8, 13). Briefly, placentae were obtained within 5 min of delivery, and independent fetal and maternal circulations were established in a single, nontraumatized peripheral cotyledon within 20 min. A fetal chorionic artery and vein pair were cannulated by using polyvinyl chloride tubing (inside diameter, 1.0 mm; outside diameter, 1.5 mm; Dural Plastics and Engineering, Sydney, Australia) to establish the fetal circulation. The cotyledon was cleared of blood by flushing with heparinized (10 IU/ml) compound sodium lactate injection (at 37°C) at 3 ml/min for 5 min. The whole placenta was mounted, fetal side uppermost, onto a Perspex platform (containing a cut-out circle, 10 cm in diameter). The perfused cotyledon was centered over the opening and supported by plastic mesh (3-mm grid). The maternal circulation was established by insertion of two blunt-ended cannulas (made from 19-gauge needles) approximately 0.5 cm through the maternal decidual basal plate into the intervillous space. The maternal and fetal perfusate flow rates were increased slowly to approximately 14 and 7 ml/min, respectively.
Both the maternal and the fetal perfusate were composed of Krebs-Ringer (Henseleit) phosphate-bicarbonate electrolyte solution (pH 7.4), with the addition of 3% dextran and 0.1% glucose, as described by Penfold et al. (30). The placenta, together with the maternal and fetal perfusate reservoirs, was housed in a thermostatically controlled cabinet at 37°C. The maternal and fetal circuits were pumped separately by a peristaltic pump (Minipals 2; Gilson Medical Electronics, Villiers le Bel, France). Perfusate passed from the reservoirs, through Millipore filters (pore size, 1 μm), silastic membrane oxygenators (receiving 95% O2-5% CO2), and bubble traps (to which separate maternal and fetal manometers were attached), and finally to the inflow cannulae.
Each perfusion was commenced by using drug-free perfusate for 20 min to stabilize the placenta and determine viability. The maternal circuit, containing 10 IU of heparin in 350 ml of perfusate, was in an open, single-pass mode, while the fetal circuit was in a closed, recycling mode. The viability of the placenta was assessed by monitoring the pressure in the fetal circuit and the loss of perfusate from the fetal reservoir. The placental preparation was discarded if there was a change in pressure (>10 mm Hg) or a loss of perfusate (>3 ml/h). Temperature and pressure were monitored continuously for the entire perfusion.
Study design.
Following the stabilization period, both maternal and fetal circuits were operated in an open, single-pass mode for the remainder of the perfusion (90 min). In each placenta, the maternal-to-fetal and fetal-to-maternal transfer across the placenta of indinavir, [3H]vinblastine (a P-gp substrate) (36) in tracer quantities, and antipyrine (a marker for passive diffusion) (8) was examined.
For determining maternal-to-fetal transfer, the three analytes were added to the maternal reservoir at the following concentrations: indinavir, 7.6 mg/liter; [3H]vinblastine, 0.03 mg/liter (5 μCi/liter); antipyrine, 20 mg/liter. The fetal inflow perfusate was drug free. The perfusate indinavir concentration (7.6 mg/liter) is similar to the maximum unbound concentration in plasma encountered in a population pharmacokinetic study (8.2 mg/liter) (10) but somewhat higher than the mean maximum unbound concentration in plasma derived from the product information (22) by using a protein binding value of 54% (1) (3.6 mg/liter). Samples (3 ml) were collected from the maternal inflow reservoir and the fetal outflow perfusate at time zero and every 5 min for 40 min in order to determine the maternal-to-fetal transfer clearance at steady state. Fetal-to-maternal transfer was assessed after addition of the three analytes to the fetal reservoir at the concentrations described above; while the maternal inflow perfusate was drug free. Fetal inflow from the reservoir and the maternal outflow perfusate were sampled as before. A washout period (10 min) separated the two phases. Five out of 19 placentae were successfully perfused. The order of transport studies (maternal donor first [n = 3] or fetal donor first [n = 2]) was randomly assigned for each perfusion. Maternal and fetal perfusate samples were stored at −20°C until the time of analysis.
Analytical methods. (i) Indinavir.
Perfusate indinavir concentrations were determined by using high-performance liquid chromatography (HPLC). Briefly, indinavir and the internal standard, verapamil, were extracted from the perfusate by using solid-phase extraction (Strata X; Phenomenex, Torrance, Calif.). The cartridges were first conditioned with 2 ml of methanol followed by 2 ml of water. Perfusate samples (1 ml) were added to the cartridges and washed with 3 ml of 10% methanol in water. Indinavir and verapamil were eluted from cartridges by using 2 ml of methanol. The eluate was evaporated to dryness by using a centrifuge evaporator. The residue was reconstituted in 100 μl of methanol-water (55:45), and 30 μl was injected onto a Waters (Milford, Conn.) Symmetry C8 analytical HPLC column (3.9 by 150 mm). The mobile phase contained 25 mmol of tetramethylammonium perchlorate/liter, 0.2% trifluoroacetic acid, and methanol (50:50), at a flow rate of 0.8 ml/min. Indinavir and verapamil were detected at 205 nm by using a UV detector. Calibration standards (0.2 to 10.0 mg/liter) and quality control (QC) samples (0.25, 1.0, and 9.0 mg/liter) were used in each analytical run. The measured concentrations of the QC samples were within 15% of the nominal concentrations, and reproducibility was within 15% as assessed by coefficients of variation at 1.0 and 9.0 mg/liter. For the low QC, the corresponding values were within 20%.
(ii) Vinblastine.
Concentrations of [3H]vinblastine in perfusate were analyzed by using liquid scintillation counting (Packard Tricarb 1900CA scintillation counter) against an external quench curve. With this system, 1 ml of placental perfusate was mixed with 10 ml of Ultima Gold liquid scintillation cocktail and disintegrations per minute were counted for 15 min.
(iii) Antipyrine.
Perfusate antipyrine concentrations were determined by using the HPLC assay of Ghabrial et al. (13). The measured concentrations of the QC samples (0.5, 5.0, and 20.0 mg/liter) were within 8% of the nominal concentrations, and reproducibility was also within 8% for all QCs.
Data analysis.
When the maternal perfusate was spiked with indinavir, vinblastine, and antipyrine, samples were collected to determine the transplacental maternal-to-fetal clearance at steady state (CLM→F) as described previously (7). CLM→F was calculated as (Fout · Qf)/Min, where Qf is the fetal perfusate flow rate, Fout is the fetal outflow concentration, and Min is the maternal inflow concentration of the respective analyte.
When the fetal perfusate was the donor circuit for all analytes, samples were collected and the transplacental fetal-to-maternal clearance at steady state (CLF→M) was calculated as (Mout · Qm)/Fin, where Qm is the maternal perfusate flow rate, Mout is the maternal outflow concentration, and Fin is the fetal inflow concentration of the respective analyte.
The transmembrane clearances of indinavir and vinblastine in the maternal-to-fetal direction and vice versa were normalized to the respective transmembrane clearances of antipyrine, the marker of passive diffusion, which was the same in both directions (see below). The ratio of the clearance of indinavir or vinblastine to that of antipyrine was termed a clearance index. Clearance indexM→F was calculated as (CLM→F of X)/(CLM→F of antipyrine), and clearance indexF→M was calculated as (CLF→M of X)/(CLF→M of antipyrine), where X is indinavir or vinblastine.
Statistical analysis.
Group data are expressed as means ± standard deviations. Differences between the CLM→F and CLF→M for each analyte were analyzed by using the paired Student t test. The CLM→F/CLF→M ratio for antipyrine was compared to unity by using the one-sample t test. Student t tests (accepting a P value of <0.05 as statistically significant) and determination of 95% confidence intervals (95% CIs) were performed by using SPSS for Windows, version 11.5 (SPSS Inc., Chicago, Ill.).
RESULTS
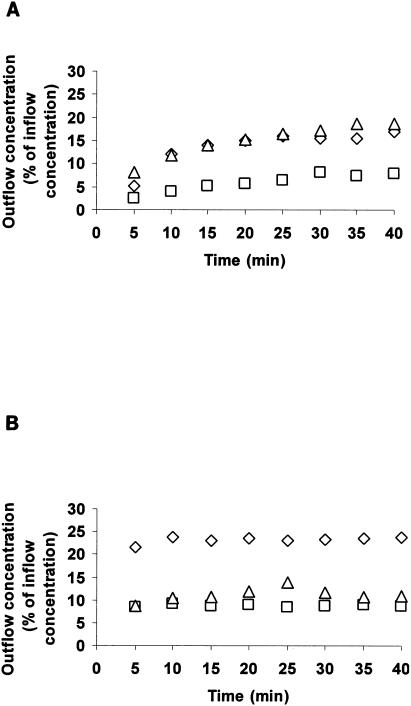
The time taken for the outflow concentration of each analyte to reach steady state (25 min) was determined visually by using concentration-versus-time plots for individual placentae (Fig. 1). The CLM→F and CLF→M for each analyte were determined over the period from 25 to 40 min in the respective perfusion phases.
FIG. 1.
Typical profile of (A) maternal and (B) fetal outflow concentration as a function of time for indinavir (▵), vinblastine (□), and antipyrine (⋄). Plot of outflow concentration in the receiver circuit as a percentage of inflow concentration in the donor reservoir versus time for a representative placenta.
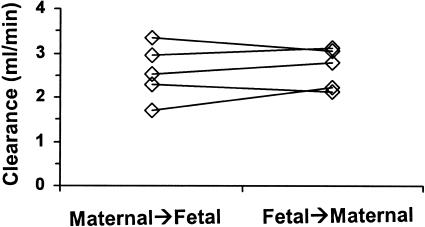
For antipyrine, maternal-to-fetal clearance (2.57 ± 0.63 ml/min) was not significantly different (P = 0.76) from fetal-to-maternal clearance (2.62 ± 0.47 ml/min) (Fig. 2). The ratio of the clearances (CLM→F:CLF→M) for antipyrine (0.98 ± 0.15; 95% CI, 0.77 to 1.15) was not significantly different (P = 0.76) from unity.
FIG. 2.
Transplacental clearance of antipyrine determined in the maternal-to-fetal and fetal-to-maternal directions for each placenta. Lines join data from the same placenta.
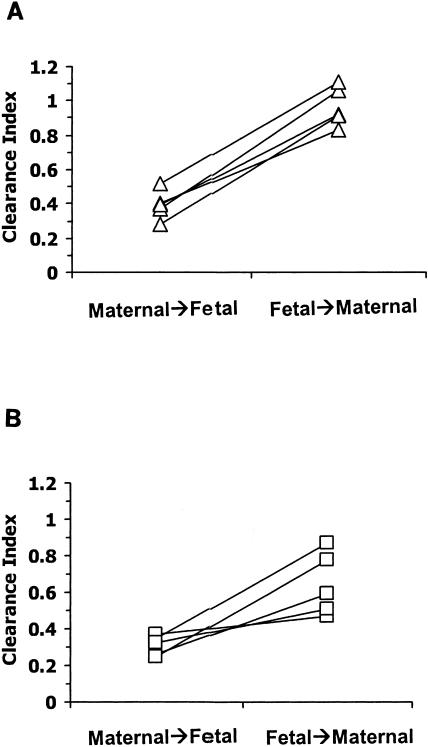
For indinavir, the mean CLM→F (± standard deviation) was 0.97 ± 0.09 ml/min while the corresponding CLF→M was 2.53 ± 0.51 ml/min (P = 0.001). The mean maternal-to-fetal clearance index for indinavir (0.39 ± 0.09) was different (P < 0.001) from the fetal-to-maternal clearance index (0.97 ± 0.12) (95% CI of the difference, −0.70 to −0.45) (Fig. 3A).
FIG. 3.
Transplacental clearance index of (A) indinavir and (B) vinblastine determined in the maternal-to-fetal and fetal-to-maternal directions for each placenta. Lines join data from the same placenta.
The mean vinblastine CLM→F was 0.79 ± 0.20 ml/min, and the mean vinblastine CLF→M was 1.75 ± 0.54 ml/min (P = 0.01). The mean vinblastine clearance index in the maternal-to-fetal direction (0.31 ± 0.05) was significantly different (P = 0.017) from the vinblastine clearance index in the fetal-to-maternal direction (0.67 ± 0.17) (95% CI of the difference, −0.61 to −0.11) (Fig. 3B).
DISCUSSION
To the best of our knowledge, this is the first study to demonstrate differential bidirectional transport of a xenobiotic in the intact human placenta. In all five placentae, the maternal-to-fetal transfer clearances of indinavir and vinblastine were approximately 40% of the corresponding fetal-to-maternal clearances, and this was also the case after normalizing to the respective antipyrine clearance. While physicochemical properties of drugs (e.g., low lipophilicity), can slow the rate of diffusion (27), this would be evident in both directions across the placenta. Clearly this was not the case for indinavir and vinblastine, suggesting that efflux transporters such as P-gp may be involved in perturbing placental transfer (37). Thus, although it has been suggested that passive diffusion is the only mechanism of placental drug transfer (5), and this appears to be the case for antipyrine in this study, it is evident that this is not the case for indinavir and vinblastine.
In the present study, the maternal-to-fetal clearance index of indinavir was significantly less than the fetal-to-maternal clearance index. This is not a result of differential distribution in the placenta, since indinavir transfer clearance was calculated at steady state, after distribution was complete. Differential protein binding in maternal and fetal perfusate cannot explain the differential bidirectional clearance, since the perfusate did not contain human serum albumin as an oncotic agent and perfusions were performed in an open, single-pass mode. It is also not likely to be due to indinavir metabolism, since the term placenta has a very limited metabolic capacity (28), and there is no reason to expect that any influence of metabolism would be dependent on the direction of placental transfer.
The differential transfer of indinavir across the placenta is consistent with a transport system(s) retarding diffusion from the mother to the fetus. PIs are substrates for the efflux transporters P-gp and multidrug resistance-associated protein (MRP1) (35), which are expressed in placental trophoblast cells (2, 37). P-gp is present on the apical membrane domain (maternal), while MRP1 is located predominantly on the fetal facing basal membrane (2). The difference in localization suggests that P-gp may be more important than MRP1 in protecting the fetus from maternal xenobiotics (2). The results of the present study provide circumstantial evidence that the placental transfer of indinavir is modulated by P-gp, since the fetal-to-maternal transfer clearance of indinavir exceeded the maternal-to-fetal transfer clearance. Extended exposure to PIs has been shown to induce expression of P-gp, with indinavir being the weakest inducer (31). Such induction is not likely in the present study, since the mothers were not treated with PIs prior to delivery and the placentae were exposed to indinavir for only 90 min during the perfusion.
Vinblastine was selected as the reference P-gp substrate (36) in this study. As with indinavir, a significant difference in the bidirectional placental transfer clearance of vinblastine was observed: the maternal-to-fetal clearance index of vinblastine was appreciably less than the fetal-to-maternal clearance index. These findings for the intact placenta are in agreement with those of studies with the BeWo cell line (human placental choriocarcinoma epithelial cells), where vinblastine transport in the apical-to-basolateral direction was significantly less than that in the opposite direction due to the action of P-gp (36). Recently, vinblastine was discovered to be a substrate for MRP1 also (2). As for indinavir, the results of the present study for vinblastine are consistent with the action of P-gp predominating over that of MRP1 in limiting maternal-to-fetal transfer.
Antipyrine diffuses rapidly across the placenta, and its transfer is flow sensitive (33). For these reasons, it was chosen as the marker of passive diffusion in the present study (8). In contrast to both indinavir and vinblastine, there was no significant difference between the maternal-to-fetal and fetal-to-maternal transfer clearances of antipyrine, verifying that the placental transfer of antipyrine occurs exclusively by passive diffusion in both directions. In addition, the antipyrine clearance observed in this study was similar to values reported previously (8).
The role of P-gp in the placental transfer of PIs has been investigated by using the in vivo mouse model. Maternal-to-fetal transfer of saquinavir was sevenfold higher in P-gp knockout mice than in wild-type mice. In addition, pharmacological inhibition of P-gp in wild-type mice was shown to increase the placental transfer of saquinavir to values similar to those observed in P-gp knockout mice (34). This indicates that placental P-gp limits fetal exposure to saquinavir, and possibly other PIs, in mice. Evidence from the present study, conducted with intact human placentae, and from cultured placenta cells (36) suggests that placental P-gp in humans has a similar role in retarding PI transfer from mother to fetus. Thus, the action of placental P-gp may create the fetus as a pharmacological sanctuary to which xenobiotics, including PIs, have limited access (15).
Amazingly, the transfer clearance index for indinavir in the maternal-to-fetal direction in the present study was identical to that reported for amprenavir (0.39 ± 0.09) (4). Both of these studies were conducted using a perfusate without added proteins. In contrast, in studies involving ritonavir (6) and saquinavir (11) in the presence of human serum albumin in the perfusate, the clearance indices in the maternal-to-fetal direction were substantially lower (0.05 ± 0.05 for saquinavir and 0.09 ± 0.05 for ritonavir). Saquinavir and ritonavir are both highly protein bound (∼98%) (25), and the lower maternal-to-fetal clearance indices for these drugs may result from protein binding in the maternal perfusate. Unfortunately, in the studies with ritonavir (6), saquinavir (11), and amprenavir (4), the transfer in the fetal-to-maternal direction was not determined. Therefore, it is not possible to conclude whether the lower maternal-to-fetal clearance indices for ritonavir and saquinavir, relative to those for amprenavir and indinavir, result from greater affinity for placental transport systems pumping the compounds in the fetal-to-maternal direction (e.g., via P-gp) or from binding of these PIs to protein added to the perfusate.
There is only a limited amount of information on the placental transfer of PIs in vivo. The concentrations of PIs in umbilical cord plasma or serum at the time of delivery for women receiving these drugs have been reported to be very low (21, 23), and the ratio of concentrations in umbilical cord plasma to those in matched maternal plasma is also low (21). The authors concluded that the extensive protein binding and relatively high molecular weight of the PIs limited their placental transfer (21, 23). Marzolini et al. also implicated placental P-gp as a possible contributor to the low maternal-to-fetal placental transfer (21); however, they did not provide evidence to support this suggestion. Taken together, the low maternal-to-fetal transfer clearance indices in perfused placentae for indinavir in this study, and reported for other PIs (4, 6, 11), and the low concentrations of PIs found in umbilical cord plasma from in vivo studies are consistent with low transfer from mother to fetus. In our study, we measured the transplacental clearance of indinavir in both directions within each placenta. As a result, we were able to demonstrate that an important contributor to the low maternal-to-fetal clearance index for indinavir was a placental transport system, possibly P-gp, that pumps the PI in the fetal-to-maternal direction.
The limited maternal-to-fetal transfer of indinavir demonstrated in this study may have implications for the management of HIV-infected pregnant women. Preloading the fetus peripartum with antiretroviral agents has the potential to reduce MTCT if transplacental passage of these drugs results in therapeutic concentrations in the fetus (15). Since PIs have low placental transfer, fetal PI concentrations may be ineffective against potential HIV infection and may encourage the rapid development of resistant strains (3). If placental P-gp is proven to restrict the maternal-to-fetal transfer of indinavir and other PIs, coadministration of P-gp inhibitors should be investigated. One approach could be to utilize dual PI therapy with ritonavir (a P-gp inhibitor) (14) in the peripartum period to increase maternal-to-fetal transfer and fetal PI concentrations. While this approach may increase protection against MTCT of HIV, concentration-related toxicities in the fetus must be considered. Indinavir has been associated with dose-related hyperbilirubinemia, which could lead to kernicterus in the neonate (32). Further consideration must also be given to the possibility that P-gp inhibitors such as ritonavir may induce P-gp expression upon chronic dosing (31). The application of possible preloading strategies in the peripartum period would therefore require careful consideration of these factors in addition to the implications for the overall management of the maternal HIV infection.
This study revealed relatively low maternal-to-fetal transfer of indinavir, a result that is consistent with findings for other PIs in the isolated perfused human placenta (4, 6, 11). More importantly, a differential bidirectional transfer was observed whereby the maternal-to-fetal transfer clearance of indinavir was substantially and significantly lower than the corresponding fetal-to-maternal transfer clearance. These results are consistent with the role of a membrane transporter, possibly P-gp, serving to restrict the movement of indinavir in the maternal-to-fetal direction. Further investigation is necessary to determine the clinical impact of these findings. Information from this study may assist in designing strategies for more-effective delivery of PIs to the fetus in order to further reduce MTCT of HIV.
Acknowledgments
Financial support for this project was provided by a Monash Research Fund project grant (3656037) and a Melbourne Research Grant Scheme (406). S. Sudhakaran is the recipient of a Monash University, Faculty of Pharmacy, Ph.D. scholarship.
We are grateful to Merck Research Laboratories for the generous supply of indinavir. Finally, we thank Sue Nisbert of the Department of Perinatal Medicine, and the obstetric and midwifery staff at the Royal Women's Hospital, for assistance in the recruitment of patients.
Footnotes
This research is dedicated to Lori Esch, HIV pharmacotherapy expert, who recently passed away. She was a true inspiration to all who knew her.
REFERENCES
- 1.Anderson, P. L., R. C. Brundage, L. Bushman, T. N. Kakuda, R. P. Remmel, and C. V. Fletcher. 2000. Indinavir plasma protein binding in HIV-1-infected adults. AIDS 14:2293-2297. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, D., S. Greenwood, C. Sibley, J. Glazier, and L. Fairbairn. 2003. Role of MDR1 and MRP1 in trophoblast cells; elucidated using retroviral gene transfer. Am. J. Physiol. Cell Physiol. 285:C584-C591. [DOI] [PubMed] [Google Scholar]
- 3.Back, D. J., S. H. Khoo, S. E. Gibbons, and C. Merry. 2001. The role of therapeutic drug monitoring in treatment of HIV infection. Br. J. Clin. Pharmacol. 51:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bawdon, R. E. 1998. The ex vivo human placental transfer of the anti-HIV nucleoside inhibitor abacavir and the protease inhibitor amprenavir. Infect. Dis. Obstet. Gynecol. 6:244-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourget, P., C. Roulot, and H. Fernandez. 1995. Models for placental transfer studies of drugs. Clin. Pharmacokinet. 28:161-180. [DOI] [PubMed] [Google Scholar]
- 6.Casey, B. M., and R. E. Bawdon. 1998. Placental transfer of ritonavir with zidovudine in the ex vivo placental perfusion model. Am. J. Obstet. Gynecol. 179:758-761. [DOI] [PubMed] [Google Scholar]
- 7.Ching, M. S., M. A. Czuba, G. W. Mihaly, D. J. Morgan, K. M. Hyman, J. D. Paull, and R. A. Smallwood. 1988. Mechanism of triamterene transfer across the human placenta. J. Pharmacol. Exp. Ther. 246:1093-1097. [PubMed] [Google Scholar]
- 8.Ching, M. S., G. W. Mihaly, D. J. Morgan, N. M. Date, K. J. Hardy, and R. A. Smallwood. 1987. Low clearance of cimetidine across the human placenta. J. Pharmacol. Exp. Ther. 241:1006-1009. [PubMed] [Google Scholar]
- 9.Cooper, E. R., M. Charurat, L. Mofenson, I. C. Hanson, J. Pitt, C. Diaz, K. Hayani, E. Handelsman, V. Smeriglio, R. Hoff, W. Blattner, et al. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J. Acquir. Immune Defic. Syndr. 29:484-494. [DOI] [PubMed] [Google Scholar]
- 10.Csajka, C., C. Marzolini, K. Fattinger, L. A. Decosterd, A. Telenti, J. Biollaz, and T. Buclin. 2004. Population pharmacokinetics of indinavir in patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 48:3226-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forestier, F., P. de Renty, G. Peytavin, E. Dohin, R. Farinotti, and L. Mandelbrot. 2001. Maternal-fetal transfer of saquinavir studied in the ex vivo placental perfusion model. Am. J. Obstet. Gynecol. 185:178-181. [DOI] [PubMed] [Google Scholar]
- 12.Garcia, P. M., L. A. Kalish, J. Pitt, H. Minkoff, T. C. Quinn, S. K. Burchett, J. Kornegay, B. Jackson, J. Moye, C. Hanson, C. Zorrilla, and J. F. Lew. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N. Engl. J. Med. 341:394-402. [DOI] [PubMed] [Google Scholar]
- 13.Ghabrial, H., M. A. Czuba, C. K. Stead, R. A. Smallwood, and D. J. Morgan. 1991. Transfer of acipimox across the isolated perfused human placenta. Placenta 12:653-661. [DOI] [PubMed] [Google Scholar]
- 14.Gutmann, H., G. Fricker, J. Drewe, M. Toeroek, and D. S. Miller. 1999. Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Mol. Pharmacol. 56:383-389. [DOI] [PubMed] [Google Scholar]
- 15.Huisman, M. T., J. W. Smit, and A. H. Schinkel. 2000. Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS 14:237-242. [DOI] [PubMed] [Google Scholar]
- 16.Ioannidis, J. P., E. J. Abrams, A. Ammann, M. Bulterys, J. J. Goedert, L. Gray, B. T. Korber, M. J. Mayaux, L. M. Mofenson, M. L. Newell, D. E. Shapiro, J. P. Teglas, and C. M. Wilfert. 2001. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads <1000 copies/ml. J. Infect. Dis. 183:539-545. [DOI] [PubMed] [Google Scholar]
- 17.Joint United Nations Program on HIV/AIDS and World Health Organization. 2002. AIDS epidemic update, December 2002. [Online.] http://www.unaids.org/wad2004/EPI_1204_pdf_en/EpiUpdate04_en.pdf.
- 18.Lallemant, M., G. Jourdain, S. Le Coeur, S. Kim, S. Koetsawang, A. M. Comeau, W. Phoolcharoen, M. Essex, K. McIntosh, and V. Vithayasai. 2000. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 343:982-991. [DOI] [PubMed] [Google Scholar]
- 19.Lee, C. G., M. M. Gottesman, C. O. Cardarelli, M. Ramachandra, K. T. Jeang, S. V. Ambudkar, I. Pastan, and S. Dey. 1998. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37:3594-3601. [DOI] [PubMed] [Google Scholar]
- 20.Lin, J. H., and M. Yamazaki. 2003. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin. Pharmacokinet. 42:59-98. [DOI] [PubMed] [Google Scholar]
- 21.Marzolini, C., C. Rudin, L. A. Decosterd, A. Telenti, A. Schreyer, J. Biollaz, T. Buclin, and the Swiss Mother + Child HIV Cohort Study. 2002. Transplacental passage of protease inhibitors at delivery. AIDS 16:889-893. [DOI] [PubMed] [Google Scholar]
- 22.Merck & Co. 1999. Crixivan (indinavir sulphate) product information. Merck & Co., Inc., Rahway, N.J.
- 23.Mirochnick, M., A. Dorenbaum, D. Holland, B. Cunningham-Schrader, C. Cunningham, R. Gelber, L. Mofenson, M. Culnane, J. Connor, and J. L. Sullivan. 2002. Concentrations of protease inhibitors in cord blood after in utero exposure. Pediatr. Infect. Dis. J. 21:835-838. [DOI] [PubMed] [Google Scholar]
- 24.Mofenson, L. M., Centers for Disease Control and Prevention, and U.S. Public Health Service Task Force. 2002. U.S. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. Morb. Mortal. Wkly. Rep. 51(RR-18):1-38; quiz CE1-4. [PubMed] [Google Scholar]
- 25.Molla, A., G. R. Granneman, E. Sun, and D. J. Kempf. 1998. Recent developments in HIV protease inhibitor therapy. Antivir. Res. 39:1-23. [DOI] [PubMed] [Google Scholar]
- 26.Morris, A. B., S. Cu-Uvin, J. I. Harwell, J. Garb, C. Zorrilla, M. Vajaranant, A. R. Dobles, T. B. Jones, S. Carlan, and D. Y. Allen. 2000. Multicenter review of protease inhibitors in 89 pregnancies. J. Acquir. Immune Defic. Syndr. 25:306-311. [DOI] [PubMed] [Google Scholar]
- 27.Pacifici, G. M., and R. Nottoli. 1995. Placental transfer of drugs administered to the mother. Clin. Pharmacokinet. 28:235-269. [DOI] [PubMed] [Google Scholar]
- 28.Pasanen, M. 1999. The expression and regulation of drug metabolism in human placenta. Adv. Drug Deliv. Rev. 38:81-97. [DOI] [PubMed] [Google Scholar]
- 29.Peckham, C., and D. Gibb. 1995. Mother-to-child transmission of the human immunodeficiency virus. N. Engl. J. Med. 333:298-302. [DOI] [PubMed] [Google Scholar]
- 30.Penfold, P., L. Drury, R. Simmonds, and F. E. Hytten. 1981. Studies of a single placental cotyledon in vitro. I. The preparation and its viability. Placenta 2:149-154. [DOI] [PubMed] [Google Scholar]
- 31.Perloff, M. D., L. L. von Moltke, J. M. Fahey, J. P. Daily, and D. J. Greenblatt. 2000. Induction of P-glycoprotein expression by HIV protease inhibitors in cell culture. AIDS 14:1287-1289. [DOI] [PubMed] [Google Scholar]
- 32.Rayner, C. R., L. D. Esch, H. E. Wynn, and R. Eales. 2001. Symptomatic hyperbilirubinemia with indinavir/ritonavir-containing regimen. Ann. Pharmacother. 35:1391-1395. [DOI] [PubMed] [Google Scholar]
- 33.Schneider, H., M. Panigel, and J. Dancis. 1972. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am. J. Obstet. Gynecol. 114:822-828. [DOI] [PubMed] [Google Scholar]
- 34.Smit, J. W., M. T. Huisman, O. van Tellingen, H. R. Wiltshire, and A. H. Schinkel. 1999. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J. Clin. Investig. 104:1441-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivas, R. V., D. Middlemas, P. Flynn, and A. Fridland. 1998. Human immunodeficiency virus protease inhibitors serve as substrates for multidrug transporter proteins MDR1 and MRP1 but retain antiviral efficacy in cell lines expressing these transporters. Antimicrob. Agents Chemother. 42:3157-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ushigome, F., H. Takanaga, H. Matsuo, S. Yanai, K. Tsukimori, H. Nakano, T. Uchiumi, T. Nakamura, M. Kuwano, H. Ohtani, and Y. Sawada. 2000. Human placental transport of vinblastine, vincristine, digoxin and progesterone: contribution of P-glycoprotein. Eur. J. Pharmacol. 408:1-10. [DOI] [PubMed] [Google Scholar]
- 37.Young, A. M., C. E. Allen, and K. L. Audus. 2003. Efflux transporters of the human placenta. Adv. Drug Deliv. Rev. 55:125-132. [DOI] [PubMed] [Google Scholar]