Abstract
Nitroimidazole resistance (nim) genes were detected in 2% of 1,502 clinical Bacteroides fragilis group strains isolated from 19 European countries, and a novel nim gene was identified. High metronidazole resistance could be induced in nim-positive strains, which emphasizes the importance of acknowledging metronidazole resistance in the clinical setting.
Anaerobic infections are common and can cause diseases associated with severe morbidity but are easily overlooked in the clinical setting. Bacteroides fragilis group species are opportunistic pathogens that constitute a dominating bacterial group in the normal intestinal microflora. In particular, B. fragilis is among the most clinically relevant anaerobic species. Interspecies lateral transfer of resistance genes among anaerobes has been shown for several clinically important antimicrobial agents such as beta-lactam agents, clindamycin, tetracycline, and metronidazole (3, 17, 19, 22, 24). Metronidazole, a 5-nitroimidazole agent, is particularly useful against Bacteroides spp. that tend to be resistant to a wide range of antimicrobial agents (1, 2). Although the rate of resistance remains low, at <1%, metronidazole-resistant organisms are beginning to emerge (8, 11, 15, 16, 21). Five nitroimidazole resistance genes, nimA to -E, have been identified that confer reduced susceptibility to 5-nitroimidazole antibiotics on species of the B. fragilis group (10, 23, 26). The proposed resistance mechanism conferred by the nim genes is that they encode a 5-nitroimidazole reductase (4). Four of the nim genes have been shown to be associated with different mobile insertion sequence (IS) elements flanked by inverted repeats (10, 25). There is strong evidence that these IS elements carry regulatory signals for expression of certain resistance genes, including the nim genes (10, 13, 15, 21, 25).
A total number of 1,502 clinical B. fragilis group isolates were surveyed for the prevalence and distribution of various nim genes. The strains, originating from a previous surveillance study of antimicrobial susceptibility including 19 European countries (11), were collected during 1999 to 2001. The different Bacteroides species had previously been identified by conventional biochemical tests, and the MIC of metronidazole was determined by the agar dilution method as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (14).
The strains were screened for nim genes by PCR with specific primers (26) and cleaved with the restriction enzyme Hsp92II for determination of the nim type. In addition, HpaII and TacI were used for selected strains. One strain (HUN19) was sequenced after it was found to have unique and novel restriction fragment length polymorphism (RFLP) patterns. All nim-positive strains were further investigated by PCR for associated IS elements and the locations of the elements with respect to the nim gene (17) with specific primers newly designed or previously described (10).
Twelve nim-positive clinical strains for which the initial MICs were relatively low (0.25 to 8.0 μg/ml), representing different species and nim types, were tested for induction of metronidazole resistance by a broth microdilution technique. The nim genes, as well as the region directly upstream including the IS element-borne promoter, were sequenced both pre- and postinduction.
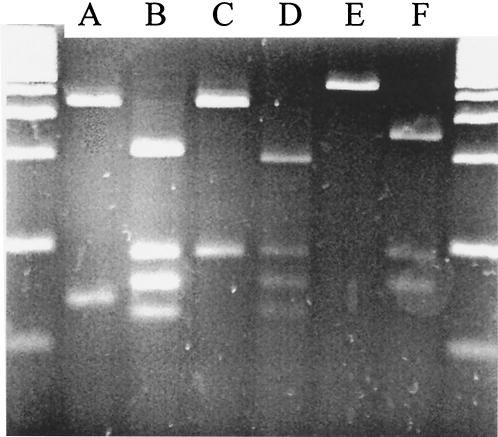
Thirty (2%) of the 1,502 B. fragilis strains studied turned out to be positive for nim genes. The RFLP analyses of the different nim genes resulted in unique fragment patterns (Fig. 1). All five previously described nim genes were detected, and a novel nim gene (nimF) was detected that showed a significant difference in its DNA sequence (EMBL nucleotide sequence database accession no. AJ515145). Pairwise alignment and comparison by ClustalW/EMBL revealed 69 to 78% identity between the different nim genes. The highest identity to the novel nimF gene was shown by nimD at 78%, followed by nimB (75%), nimA and -C (71%), and nimE (69%).
FIG. 1.
PCR-RFLP patterns of the nimA to -F genes cleaved with restriction enzyme Hsp92II. A 100-bp marker was used on the agarose gel, which was stained with ethidium bromide. The expected and obtained fragment sizes were 347 and 69 bp for nimA; 297, 94, 80, and 77 bp for nimB; 364 and 94 bp for nimC; 193, 94, 80, and 70 bp for nimD; 441 bp for nimE; and 250, 94, and 80 bp for nimF.
Twenty-three of the 30 nim-positive strains each harbored one detectable IS element (Table 1). It was also demonstrated that in 11 of the 12 nim-positive strains tested that were susceptible or had reduced susceptibility to metronidazole (MIC, 0.25 to 8.0 μg/ml) a high level of resistance (MIC, ≥256 μg/ml) could be induced after three passages in subinhibitory concentrations of metronidazole, whereas this phenomenon could not be induced in strains lacking nim genes or in the nimF-positive strain.
TABLE 1.
MICs of metronidazole, nim loci, and presence of IS elements in nim-positive Bacteroides sp. strains
| nim-positive strain | MIC (μg/ml) | Locus | Species | IS element | IS- nima |
|---|---|---|---|---|---|
| Reference strains | |||||
| 638R/pIP417 | 24 | nimA | B. fragilis | IS1168 | |
| BF8 | >32 | nimB | B. fragilis | IS1168 | |
| 638R/pIP419 | 6.0 | nimC | B. fragilis | IS1170 | |
| 638R/pIP421 | 16 | nimD | B. fragilis | IS1169 | |
| R6881 | >32 | nimE | B. fragilis | ||
| Clinical strains | |||||
| GBR7 | >64 | nimA | B. fragilis | IS1168 | + |
| FRA55 | 16 | nimA | B. ovatus | IS1168 | + |
| FRA35 | 8.0 | nimA | B. fragilis | IS1168 | + |
| ITA63 | 8.0 | nimA | B. vulgatus | IS1168 | + |
| AUT85 | 4.0 | nimA | B. ovatus | ||
| FRA6 | 4.0 | nimA | B. thetaiotaomicron | IS1168 | + |
| FRA42 | 4.0 | nimA | B. fragilis | IS1168 | + |
| DEU35 | 2.0 | nimA | B. fragilis | IS1169 | + |
| DEU50 | 2.0 | nimA | B. fragilis | IS1168 | + |
| FIN40 | 2.0 | nimA | B. thetaiotaomicron | IS1168 | + |
| FRA56 | 2.0 | nimA | B. vulgatus | ||
| NLD5 | 2.0 | nimA | B. fragilis | IS1168 | + |
| CZE92 | 1.0 | nimA | B. vulgatus | ||
| FRA57 | 1.0 | nimA | B. thetaiotaomicron | IS1168 | + |
| HUN31 | 1.0 | nimA | B. fragilis | IS1168 | + |
| ITA64 | 1.0 | nimA | B. vulgatus | IS1168 | |
| AUT91 | 2.0 | nimB | B. fragilis | IS1168 | + |
| CZE16 | 1.0 | nimB | B. fragilis | IS1168 | + |
| GBR32 | 4.0 | nimB | B. fragilis | IS1168 | + |
| FRA100 | 4.0 | nimC | B. fragilis | IS1170 | + |
| CZE89 | 2.0 | nimC | B. fragilis | ||
| FRA2 | 8.0 | nimD | B. fragilis | IS1169 | + |
| DNK23 | 8.0 | nimD | B. fragilis | IS1169 | + |
| SWE94 | 4.0 | nimD | B. thetaiotaomicron | IS1169 | + |
| AUT1 | 2.0 | nimD | B. fragilis | IS1169 | + |
| FIN68 | 1.0 | nimD | B. fragilis | IS1169 | |
| FRA90 | 0.25 | nimD | B. thetaiotaomicron | IS1169 | + |
| GBR13 | 64 | nimE | B. fragilis | ||
| DNK5 | 8.0 | nimE | B. fragilis | ||
| HUN19 | 1.0 | nimF | B. vulgatus |
IS-nim indicates that the IS element is located directly upstream of the nim gene.
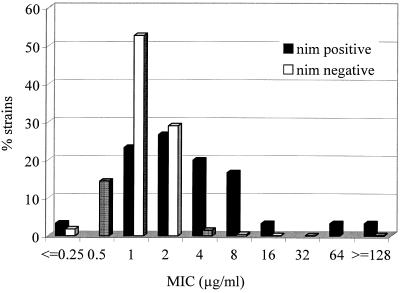
Less than 1% of the strains (6 of 1,502), of which 2 carried a nim gene, were resistant to metronidazole according to the breakpoint recommended by the NCCLS. The distribution of the MICs for nim-negative and nim-positive strains indicates that these two groups constitute two distinct populations (Fig. 2). Although the relative risk of metronidazole resistance for strains harboring a nim gene was significant (odds ratio of 26 [95% confidence interval, 4.6 to 149]), the relative risk for reduced susceptibility with a MIC of at least 8.0 μg/ml, a clinically relevant breakpoint, was even higher (odds ratio of 53 [95% confidence interval, 19 to 147]), further emphasizing the relevance of nim genes in conferring resistance to metronidazole. These results are supported by a previous study by Stubbs et al. (23). The finding of four highly metronidazole-resistant strains (MIC, >64 μg/ml) and one strain with borderline resistance (MIC, 16 μg/ml), all lacking nim genes, indicates the importance of additional mechanisms of resistance.
FIG. 2.
Distribution of MICs for nim-positive (n = 30) and nim-negative (n = 1,472) clinical B. fragilis group strains isolated from 19 European countries (1999 to 2001).
The most common nim identity in the present study was nimA, which was detected in 53% of the strains (Table 1), which is in agreement with the findings of Stubbs et al. (23). According to Lubbe et al., there is an indication of a potential nimA gene source in anaerobic environments (12). In 20 of the 23 isolates harboring IS elements, the transposable genes were shown to be situated directly upstream of the corresponding nim gene. None of the susceptible nim-negative type strains harbored any of the IS elements investigated. No correlation was shown between the species or MICs and the presence of IS elements among the 30 nim-positive strains. One highly resistant strain, GBR13, lacked known IS elements, suggesting the possible presence of an unknown IS element, involvement of other ways of activating the nim gene, or other resistance mechanisms.
Of the 12 strains tested for susceptibility to induction, high-level resistance was induced in 9 carrying nim genes and IS elements (Table 2), irreversibly in 2 of them. High-level resistance (MIC, 256 μg/ml) was reversibly induced in two strains, AUT85 and DNK5, in which no IS element was detected. This either indicates the presence of an unknown IS element not detected by the primers used or puts into question the notion that IS elements are mandatory for induction. No alterations of IS element position or mutational events in the nim genes or promoter region of the sequence were recorded in the strains with the induced high-resistance phenotypes compared to their preinduction state.
TABLE 2.
MICs of metronidazole before and after exposure to subinhibitory concentrations of metronidazole for three passages and after three additional passages without metronidazole
| Strain | Species | Locus | MIC (μg/ml) preinduction | Induced-resistance MIC (μg/ml) after 3 passages with metronidazole | Stability of MIC (μg/ml) after 3 additional passages without metronidazole | IS element upstream of nim gene |
|---|---|---|---|---|---|---|
| ATCC 25285 | B. fragilis | 0.5 | 2.0 | 2.0 | ||
| CCGU 29741 | B. thetaiotaomicron | 1.0 | 2.0 | 1.0 | ||
| AUT41 | B. fragilis | 0.064 | 0.064 | 0.064 | ||
| FRA01 | B. ovatus | 0.064 | 0.064 | 0.064 | ||
| CZE58 | B. thetaiotaomicron | 1.0 | 1.0 | 1.0 | ||
| HUN31 | B. fragilis | nimA | 1.0 | >256 | 32 | + |
| FRA35 | B. fragilis | nimA | 8.0 | 256 | 256 | + |
| CZE16 | B. fragilis | nimB | 1.0 | >256 | 8.0 | + |
| AUT91 | B. fragilis | nimB | 2.0 | >256 | 16 | + |
| GBR32 | B. fragilis | nimB | 4.0 | >256 | 256 | + |
| FRA100 | B. fragilis | nimC | 4.0 | >256 | 4.0 | + |
| FRA2 | B. fragilis | nimD | 8.0 | 64 | 8.0 | + |
| DNK5 | B. fragilis | nimE | 8.0 | 256 | 8.0 | |
| AUT85 | B. ovatus | nimA | 4.0 | 256 | 8.0 | |
| FRA90 | B. thetaiotaomicron | nimD | 0.25 | 256 | 16 | + |
| SWE94 | B. thetaiotaomicron | nimD | 4.0 | 256 | 16 | + |
| HUN19 | B. vulgatus | nimF | 1.0 | 1.0 | 1.0 |
The fact that a high level of resistance could be reversibly induced in susceptible strains or strains with slightly reduced susceptibility indicates that under clinical conditions, metronidazole treatment could select for a nim-positive subpopulation with potential resistance to the drug. Similar observations were recently reported by Gal and Brazier (9). Together with the reported transferability of the nim genes (17, 24), this indicates the clinical importance of the B. fragilis group in the human colon as a reservoir of metronidazole resistance. Metronidazole treatment may also lead to other unwanted effects in addition to selection for resistance, such as increased virulence, as demonstrated by Diniz et al. (5).
It is important to acknowledge the emerging resistance to metronidazole within the B. fragilis group. Clinical failures following metronidazole treatment due to Bacteroides strains with reduced susceptibility have been reported (7, 18, 20). Different mechanisms of resistance have been proposed (4, 6), and the importance of nim genes has been reported by several groups (3, 10, 25). Knowledge of the resistance status and the mechanisms of resistance is critical for the choice of antimicrobial therapy and the design of new antimicrobial agents, as well as in prevention of dissemination of resistant strains in clinical medicine. The reliance upon the metronidazole susceptibility tests used in many clinical bacteriology laboratories for detection of anaerobes in primary cultures may have contributed to an underreporting of metronidazole-resistant strains (2, 23). The NCCLS-recommended breakpoint of 32 μg/ml is high, which means that strains with reduced susceptibility are easily overlooked by conventional methods.
In this large surveillance study including 1,502 clinical B. fragilis group strains, we have shown that only a low number of strains were judged resistant but 2% of the strains constituted a subpopulation of nim-positive strains with decreased susceptibility to metronidazole. The fact that high levels of resistance were easily induced in nim-positive strains is of great clinical concern and emphasizes the importance of acknowledging metronidazole resistance in the clinical setting.
Acknowledgments
We are grateful to G. Reysset for the reference strains for nimA to -D (B. fragilis 638R/pIP417, BF8, 638R/pIP419, and 638R/pIP421, respectively) and to J. Stubbs and J. S. Brazier for the reference strain for nimE (B. fragilis R6881).
REFERENCES
- 1.Aldridge, K. E. 1995. The occurrence, virulence, and antimicrobial resistance of anaerobes in polymicrobial infections. Am. J. Surg. 169:2S-7S. [PubMed] [Google Scholar]
- 2.Brazier, J. S., S. L. Stubbs, and B. I. Duerden. 1999. Metronidazole resistance among clinical isolates belonging to the Bacteroides fragilis group: time to be concerned? J. Antimicrob. Chemother. 44:580-581. [DOI] [PubMed] [Google Scholar]
- 3.Breuil, J., A. Dublanchet, N. Truffaut, and M. Sebald. 1989. Transferable 5-nitroimidazole resistance in the Bacteroides fragilis group. Plasmid 21:151-154. [DOI] [PubMed] [Google Scholar]
- 4.Carlier, J. P., N. Sellier, M. N. Rager, and G. Reysset. 1997. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob. Agents Chemother. 41:1495-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diniz, C. G., R. M. Arantes, D. C. Cara, F. L. Lima, J. R. Nicoli, M. A. Carvalho, and L. M. Farias. 2003. Enhanced pathogenicity of susceptible strains of the Bacteroides fragilis group subjected to low doses of metronidazole. Microbes Infect. 5:19-26. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, D. I. 1993. Nitroimidazole drugs—action and resistance mechanisms. II. Mechanisms of resistance. J. Antimicrob. Chemother. 31:201-210. [DOI] [PubMed] [Google Scholar]
- 7.Elsaghier, A. A., J. S. Brazier, and E. A. James. 2003. Bacteraemia due to Bacteroides fragilis with reduced susceptibility to metronidazole. J. Antimicrob. Chemother. 51:1436-1437. [DOI] [PubMed] [Google Scholar]
- 8.Fang, H., C. Edlund, G. Zhang, and M. Hedberg. 1999. Detection of imipenem-resistant and metronidazole-resistant Bacteroides fragilis group strains in fecal samples. Clin. Microbiol. Infect. 5:753-758. [Google Scholar]
- 9.Gal, M., and J. S. Brazier. 2004. Metronidazole resistance in Bacteroides spp. carrying nim genes and the selection of slow-growing metronidazole-resistant mutants. J. Antimicrob. Chemother. 54:109-116. [DOI] [PubMed] [Google Scholar]
- 10.Haggoud, A., G. Reysset, H. Azeddoug, and M. Sebald. 1994. Nucleotide sequence analysis of two 5-nitroimidazole resistance determinants from Bacteroides strains and of a new insertion sequence upstream of the two genes. Antimicrob. Agents Chemother. 38:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedberg, M., and C. E. Nord. 2003. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe. Clin. Microbiol. Infect. 9:475-488. [DOI] [PubMed] [Google Scholar]
- 12.Lubbe, M. M., K. Stanley, and L. J. Chalkley. 1999. Prevalence of nim genes in anaerobic/facultative anaerobic bacteria isolated in South Africa. FEMS Microbiol. Lett. 172:79-83. [DOI] [PubMed] [Google Scholar]
- 13.Nagy, E., J. Soki, E. Urban, I. Szoke, E. Fodor, and R. Edwards. 2001. Occurrence of metronidazole and imipenem resistance among Bacteroides fragilis group clinical isolates in Hungary. Acta Biol. Hung. 52:271-280. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Methods for antimicrobial susceptibility testing of anaerobic bacteria—5th edition: approved standards M11-A5. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 15.Podglajen, I., J. Breuil, A. Rohaut, C. Monsempes, and E. Collatz. 2001. Multiple mobile promoter regions for the rare carbapenem resistance gene of Bacteroides fragilis. J. Bacteriol. 183:3531-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen, B. A., K. Bush, and F. P. Tally. 1997. Antimicrobial resistance in anaerobes. Clin. Infect. Dis. 24(Suppl. 1):S110-S120. [DOI] [PubMed] [Google Scholar]
- 17.Reysset, G. 1996. Genetics of 5-nitroimidazole resistance in Bacteroides species. Anaerobe 2:59-69. [DOI] [PubMed] [Google Scholar]
- 18.Rotimi, V. O., M. Khoursheed, J. S. Brazier, W. Y. Jamal, and F. B. Khodakhast. 1999. Bacteroides species highly resistant to metronidazole: an emerging clinical problem? Clin. Microbiol. Infect. 5:166-169. [DOI] [PubMed] [Google Scholar]
- 19.Salyers, A. A., and N. B. Shoemaker. 1996. Resistance gene transfer in anaerobes: new insights, new problems. Clin. Infect. Dis. 23(Suppl. 1):S36-S43. [DOI] [PubMed] [Google Scholar]
- 20.Schapiro, J. M., R. Gupta, E. Stefansson, F. C. Fang, and A. P. Limaye. 2004. Isolation of metronidazole-resistant Bacteroides fragilis carrying the nimA nitroreductase gene from a patient in Washington State. J. Clin. Microbiol. 42:4127-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebald, M. 1994. Genetic basis for antibiotic resistance in anaerobes. Clin. Infect. Dis. 18(Suppl.)4:S297-S304. [DOI] [PubMed] [Google Scholar]
- 22.Shoemaker, N. B., H. Vlamakis, K. Hayes, and A. A. Salyers. 2001. Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl. Environ. Microbiol. 67:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stubbs, S. L., J. S. Brazier, P. R. Talbot, and B. I. Duerden. 2000. PCR-restriction fragment length polymorphism analysis for identification of Bacteroides spp. and characterization of nitroimidazole resistance genes. J. Clin. Microbiol. 38:3209-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinh, S., A. Haggoud, and G. Reysset. 1996. Conjugal transfer of the 5-nitroimidazole resistance plasmid pIP417 from Bacteroides vulgatus BV-17: characterization and nucleotide sequence analysis of the mobilization region. J. Bacteriol. 178:6671-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinh, S., A. Haggoud, G. Reysset, and M. Sebald. 1995. Plasmids pIP419 and pIP421 from Bacteroides: 5-nitroimidazole resistance genes and their upstream insertion sequence elements. Microbiology 141(Pt. 4):927-935. [DOI] [PubMed] [Google Scholar]
- 26.Trinh, S., and G. Reysset. 1996. Detection by PCR of the nim genes encoding 5-nitroimidazole resistance in Bacteroides spp. J. Clin. Microbiol. 34:2078-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]