Abstract
The antileishmanial efficacy of four novel quinoline derivatives was determined in vitro against Leishmania chagasi, using extracellular and intracellular parasite models. When tested against L. chagasi-infected macrophages, compound 3b demonstrated 8.3-fold greater activity than did the standard pentavalent antimony. No significant activity was found for compounds 3a, 4a, and 4b. The antilesihmanial effect of compound 3b was independent of host cell activation, as demonstrated by nitric oxide production. Ultrastructural studies of promastigotes treated with compound 3b showed mainly enlarged mitochondria, with matrix swelling and reduction in the number of cristae. Synthetic analogues based on the quinoline ring structure, already an established template for antiparasitic drugs, could provide further useful compounds.
Protozoan parasites affect 3 billion people, with malaria and trypanosomatid parasites causing the greatest morbidity (10, 28). Leishmaniasis is estimated to afflict 12 million people worldwide, causing disease ranging from skin lesions in cutaneous leishmaniasis to a progressive and fatal hepatosplenomegaly in visceral leishmaniasis (9).
Treatment of leishmaniasis suffers from problems of drug resistance and severe toxicity (9) and requires parenteral administration (14). Despite the great advances in many scientific fields, the 92-year-old antimonials (29) are still the main treatment in the majority of developing countries. In addition, problems have occurred with the manufacture and supply of antimonials in developing countries (20) and high levels of arsenic contaminants have also been described (22). Second-line drugs, such as pentamidine and amphotericin B, are important in combined therapy or in cases of antimony treatment failures (3). Experimental studies have identified the anticancer drug miltefosine as an effective antileishmanial agent (8). However, clinical trials in India have identified gastrointestinal toxicity and teratogenicity in association with this drug (9). Therefore, the development of new antiprotozoal compounds with improved pharmacological properties is imperative.
Several drugs based on the quinoline structure have improved the therapy of protozoal diseases, especially malaria (5). These have also shown considerable in vivo efficacy against Leishmania parasites; this is exemplified by the drug WR 6026, which is currently undergoing clinical trials for its effectiveness in treating visceral leishmaniasis (11). In this paper, we describe the synthesis of four novel 3-substituted quinolines with allyl and cinnamyl motifs covalently attached to the quinoline entity. In vitro parasite models are used to evaluate their antileishmanial activity. Possible mechanisms of action for the most active compound were investigated through ultrastructural studies and the potential activation of macrophages.
MATERIALS AND METHODS
Materials.
Sodium dodecyl sulfate was purchased from Merck. Lipopolysacharide (LPS), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (Thiazol blue; MTT), M-199 and RPMI-PR− 1640 medium (without phenol red) were purchased from Sigma. Pentavalent antimony (Aventis-Pharma) and pentamidine (Eurofarma) were used as the standard drugs. Sodium nitrite, sulfanilamide, and N-1-naphthylethylenediamine dihydrochloride (NED) were purchased from Merck.
Animals and parasites.
Animals were supplied by the Animal Breeding Facility at the Faculty of Medicine of São Paulo University. They were maintained in sterilized cages under a controlled environment, receiving water and food ad libitum. Animal procedures were performed under the approval of the Research Ethics Commission, according to the Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences (http://www.nap.edu). Leishmania chagasi (MHOM/BR/1972/LD) was maintained in golden hamsters. Approximately 60 to 70 days postinfection, amastigotes were obtained from the hamster spleen by differential centrifugation and the parasite burden was determined using the Stauber equation (24). Promastigotes were maintained in M-199 medium supplemented with 10% calf serum and 0.25% hemin at 24°C.
Determination of the 50% inhibitory concentration (IC50).
The antileishmanial activity against promastigotes was determined as described elsewhere (27), using pentamidine as the standard drug. Parasite viability was determined using the MTT assay (25). The antileishmanial activity against intracellular amastigotes was determined with infected macrophages (26), using pentavalent antimony as the standard drug. The parasite burden was defined as the number of infected macrophages in a total of 400 cells. Each assay was performed in triplicate.
Cytotoxicity assay.
RAW 264.7 cells (ATCC TIB-71) were seeded (4 × 104/well) in 96-wells microplates and incubated with different drug concentrations for 48 h at 37°C in a humidified mixture of 5% CO2 and 95% air in an incubator. The cell viability was determined using the MTT assay. The selectivity index (S.I.) was calculated using the following equation: S.I. = IC50 (RAW 264.7 cells)/IC50 (Leishmania amastigotes).
Ultrastructural studies.
L. chagasi promastigotes were incubated with compound 3b at 10 μg ml−1 for both 30 and 60 min at 24°C in 24-well plates. They were then processed (12) and observed under a transmission electron microscope (JEOL).
Nitric oxide production.
The nitric oxide production by macrophages was measured in the presence of compound 3b by the Griess reaction (19). Peritoneal macrophages were incubated for 24 h at 37°C with compound 3b (10 μg ml−1). LPS (10 μg ml−1) was used to induce NO up-regulation and was incubated alone with compound 3b (10 μg mL−1) under the same conditions. The absorbance was determined at 540 nm using a Multiskan MS (Uniscience) microplate reader.
Drug synthesis.
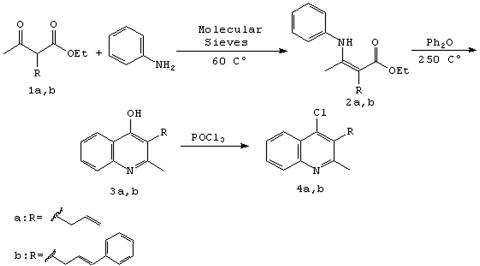
Ethyl-2-acetylpent-4-enoate (compound 1a) and ethyl-(4E)-2-acetyl-5-phenylpent-4-enoate (compound 1b) were obtained by a previously described method (1, 2). Ethyl-(2Z)-2-(1-anilinoethylidene)pent-4-enoate (compound 2a) and ethyl-(2Z,4E)-2-(1-anilinoethylidene)-5-phenylpent-4-enoate (compound 2b) were obtained from ketoester 1a or 1b and excess of aniline in the presence of molecular sieves: 5 Å (0.1 g/mmol) at 60°C for 24 h (16). 3-Substituted 4-hydroxyquinolines (compounds 3a and 3b) and 3-substituted 4-chloroquinolines (compounds 4a and 4b) were obtained by a previously described method (6, 7, 15).
(i) 3-Allyl-2-methylquinolin-4-ol (compound 3a).
The yield was 1.494 g (75%) of a pale yellow solid with a melting point of 244°C to 245°C. 1H nuclear magnetic resonance (NMR) (DMSO-d6): 2.25 (s, 3H), 3.18 (d, 2H, J = 6.0 Hz), 4.81 (d, 1H, J = 9.3 Hz), 4.86 (d, 1H, J = 15.2 Hz), 5.73 (ddt, 1H, J = 15.2, J = 9.3, and J = 6.0 Hz), 7.16 (t, 1H, J = 7.8 Hz), 7.37 (d, 1H, J = 7.8 Hz), 7.48 (t, 1H, J = 7.8 Hz), 7.95 (d, 1H, J = 7.8 Hz), 11.37 (br s, 1H). 13C NMR (DMSO-d6): 17.3, 28.7, 114.0, 116.1, 117.5, 122.4, 123.3, 125.1, 131.0, 136.4, 139.1, 146.7, 175.1. MS (m/z): 199 [M]+, 184, 92, 77. Analysis calculated for C13H13NO (199.25): C, 78.37; H, 6.58; N, 7.03. Found: C, 78.17; H, 6.39; N, 7.11.
(ii) 2-Methyl-3-[(2E)-3-phenylprop-2-enyl]quinolin-4-ol (compound 3b).
The yield was 1.542 g (56 %) of a yellow solid with a melting point of 238°C to 240°C. 1H NMR (DMSO-d6): 2.33 (s, 3H), 3.35 (d, 2H, J = 5.6 Hz), 6.18 (dt, 1H, J = 16.0 and J = 5.7 Hz), 6.29 (d, 1H, J = 16.0 Hz), 7.07 (t, 1H, J = 7.1 Hz), 7.10 to 7.15 (m, 4H), 7.25 (d, 1H, J = 7.2 Hz), 7.40 (d, 1H, J = 8.0 Hz), 7.50 (t, 1H, J = 7.5 Hz), 7.99 (d, 1H, J = 7.9 Hz), 11.43 (br s, 1H). 13C NMR (DMSO-d6): 17.5, 27.8, 116.3, 117.5, 122.4, 123.4, 125.1, 125.4, 125.7, 126.7, 128.4, 128.9, 131.0, 137.2, 139.1, 146.8, 175.2. MS (m/z): 275 [M]+, 184, 92, 77. Analysis calculated for C19H17NO (275.35): C, 82.88; H, 6.22; N, 5.09. Found: C, 82.57; H, 6.29; N, 5.01.
(iii) 3-Allyl-4-chloro-2-methylquinoline (compound 4a).
The yield was 2.068 g (95%) as a brown solid with a melting point of 140°C. 1H NMR (CDCl3): 3.23 (s, 3H), 3.86 (d, 2H, J = 7.0 Hz), 4.98 (d, 1H, J = 17.1 Hz), 5.23 (d, 1H, J = 10.2 Hz), 5.93 (m, 1H), 7.89 (t, 1H, J = 7.4 Hz), 8.02 (t, 1H, J = 8.4 Hz), 8.39 (d, 1H, J = 8.5 Hz), 9.06 (d, 1H, J = 8.5 Hz). 13C NMR (CDCl3): 19.2, 33.6, 118.2, 122.2, 125.0, 125.9, 130.3, 130.8, 131.6, 134.1, 137.7, 150.6, 157.0. MS (m/z): 219, 217 [M]+, 184, 182, 169, 167, 77. Analysis calculated for C13H13NCl (217.69): C, 71.73; H, 5.55; N, 6.43, Cl, 16.29. Found: C, 71.28; H, 5.39; N, 6.61, Cl, 16.59.
(iv) 4-Chloro-2-methyl-3-[(2E)-3-phenylprop-2-enyl]quinoline (compound 4b).
The yield was 2.203 g (75%) as a brown solid with a melting point of 108°C. 1H NMR (CDCl3): 2.84 (s, 3H), 3.93 (d, 2H, J = 3.4 Hz), 6.26 to 6.39 (m, 1H), 6.30 (d, 1H, J = 15.9 Hz), 7.19 to 7.29 (m, 5H), 7.61 (t, 1H, J = 8.3 Hz), 7.74 (t, 1H, J = 8.3 Hz), 8.11 (d, 1H, J = 8.3 Hz), 8.23 (d, 1H, J = 8.3 Hz). 13C NMR (CDCl3): 23.6, 33.63, 124.4, 124.7, 125.6, 125.9, 126.1, 127.3, 127.5, 128.0, 129.8, 130.2, 131.7, 136.7, 145.8, 158.7. MS (m/z): 295, 293 [M]+, 260, 258, 182, 180, 77. Analysis calculated for C19H16NCl (293.799): C, 77.68; H, 5.49; N, 4.77; Cl, 12.06. Found: C, 77.38; H, 5.31; N, 4.62; Cl, 11.59.
Statistical analysis.
Data represent the mean and standard deviation of duplicate or triplicate samples from two or three independent assays. The IC50s were calculated using sigmoid dose-response curves in Graph Pad Prism 3.0 software, and the 95% confidence intervals are included in parentheses.
RESULTS
Determination of the IC50. (i) Promastigotes.
The antileishmanial activity of the four quinoline compounds was initially determined against L. chagasi promastigotes. Table 1 shows that three of these compounds killed promastigotes at low concentrations, in a dose-dependent manner. A strong antiparasitic effect was observed for compounds 3b and 3a (IC50 < 0.8 μg ml−1) compared to the standard drug, pentamidine, which had an IC50 of 2.02 μg ml−1. All compounds killed 100% of parasites at the maximal concentration of 50 μg ml−1; however, compound 4a was the least potent (IC50 = 18.78 μg ml−1) and the most toxic against mammalian cells. Compound 4b showed a significant antiparasitic activity with an IC50 of 1.79 μg ml−1, being at least 8.5-fold less toxic for mammalian cells than the standard drug pentamidine.
TABLE 1.
Effect of novel quinoline compounds on L. chagasi and mammalian cytotoxicitya
| Test agent | IC50 (μg ml−1) (95% C.I.)b for:
|
Cytotoxicity; IC50 (μg ml−1) (95% C.I.) | S.I. | |
|---|---|---|---|---|
| Promastigotes | Amastigotes | |||
| 3a | 0.79 (0.45-1.37) | >15 | 31.60 (19.82-50.38) | |
| 3b | 0.091 (0.06-0.13) | 3.55 (3.34-3.78) | 26.89 (20.47-35.34) | 7.57 |
| 4a | 18.78 (8.02-44.00) | >15 | 13.77 (10.75-17.62) | |
| 4b | 1.79 (0.73-4.23) | >15 | 22.00 (16.24-29.80) | |
| Sb Vc | 29.55 (28.09-31.09) | >100 | >3.38 | |
| Pentamidine | 2.02 (1.75-2.35) | 2.57 (2.23-2.96) | ||
Promastigotes were incubated with compounds and standard drugs for 24 h at 24°C. RAW 264.7 cells were incubated for 48 h at 37°C, and the viability of both parasite and RAW cells was determined using the MTT assay.
C.I., confidence interval.
Sb V, pentavalent antimony.
(ii) Intracellular amastigotes.
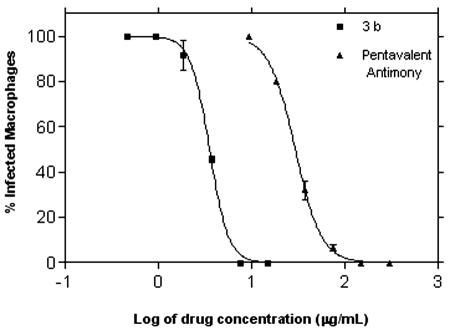
Peritoneal macrophages were infected with L. chagasi amastigotes and treated with the test compounds over the concentration range of 15 to 0.117 μg ml−1 (twofold dilution) for 120 h. Only compound 3b showed a significant reduction in the number of intracellular amastigotes, with an IC50 of 3.55 μg ml−1. The sigmoid dose-response curve showed 100% parasite inhibition for macrophages treated with a drug concentration of less than 8.0 μg ml−1 (Fig. 1). Despite the susceptibility of mammalian cells (RAW 264.7) to the quinoline compounds in a range of 13 to 32 μg ml−1, the S.I. of compound 3b was 7.5 (Table 1). Compound 3b was not shown to be toxic to macrophages by light microscopy, as confirmed by normal morphology and attachment to plates.
FIG. 1.
Determination of the IC50 of compound 3b against L. chagasi-infected macrophages. Pentavalent antimony was used as a standard drug. Macrophages were treated for 120 h at 37°C with drugs, and the number of infected macrophages in Giemsa-stained glass coverslips was determined by light microscopy. Dose-response curves were obtained in GraphPad Prism 3.0 software. Data are mean and standard deviation.
Nitric oxide production.
Peritoneal macrophages, both LPS activated and nonactivated, were incubated for 24 h in the presence of compound 3b. The nitrite content was evaluated by the Griess reaction. Our results showed that compound 3b did not produce significantly more nitrite than untreated controls and consequently did not up-regulate NO production by macrophages at 10 μg ml−1 (data not shown).
Ultrastructural studies.
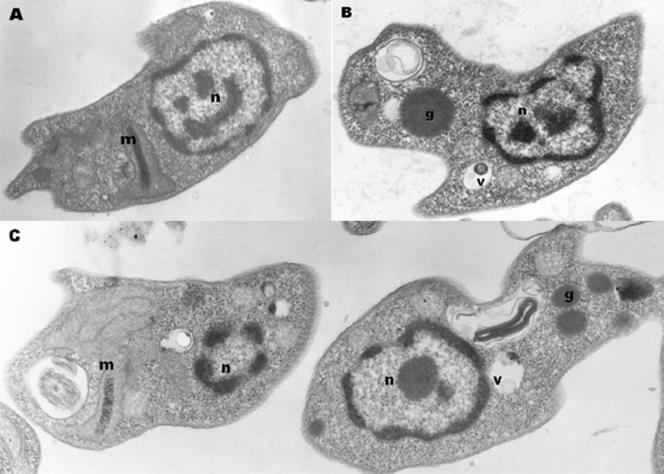
To evaluate the intracellular damage caused in Leishmania promastigotes by compound 3b, the ultrastructural modifications were investigated by transmission electron microscopy after a short-term incubation (Fig. 2). The images clearly demonstrate time-dependent damage, with most significant changes at 60 min of incubation. After 60 min of incubation (Fig. 2C), compound 3b induced an enlargement of the kinetoplast and a considerable enhancement in the number of lipid granules and vacuoles. After 30 min of incubation (Fig. 2B), initial changes in promastigotes were observed as an increase in the number of vacuoles and lipid granules, which were much more pronounced at 60 min incubation.
FIG. 2.
Ultrastructural effects of compound 3b on Leishmania promastigotes. (B and C) Promastigotes were incubated with compound 3b at 10 μg ml−1 for 30 (B) and 60 min (C) at 24°C and subsequently observed under a transmission electron microscope. (A) Untreated cells were used as parasite controls. m, mitochondria; n, nucleus; g, granules; v, vacuoles.
Drug synthesis.
The synthesis of chloroquinolines (compounds 4a and 4b) and quinoline (compounds 3a and 3b) derivatives is shown in Fig. 3. Among the methods for generating β-enaminones, condensation of amines and β-dicarbonyl compounds is a classical method, in which the azeotropic removal of water is achieved by refluxing in an aromatic solvent. In this paper, β-enamine esters (compounds 2a and 2b) were prepared by the reaction of the dicarbonyl compounds with aniline in the presence of the molecular sieve (5 Å) and the products were easily separated from the remaining aniline. The resulting derivatives were then cyclized using phenyl ether to yield 4-hydroxyquinolines (compounds 3a and 3b), which then gave the 4-chloroquinolines derivatives (compounds 4a and 4b) after treatment with phosphorus oxychloride.
FIG. 3.
Synthesis of quinolines 3a through 4b.
DISCUSSION
Previous studies using quinoline compounds have shown potent in vitro and in vivo antileishmanial activity (23), and recent clinical trials have also been undertaken using these compounds (30). In this work, we have demonstrated the efficacy of novel quinoline derivatives against L. chagasi parasites. The synthesis of these quinoline compounds, in addition to being relatively simple, resulted in large amounts of pure materials, as confirmed by physical and spectroscopic data. Our results showed antileishmanial activity for all compounds tested against promastigotes, with most IC50s being lower than for the standard drug pentamidine. The chemical structure of quinolines and their biological activity relationship showed that the substitution of the Cl− (compound 4b) by OH− (compound 3b) in the quinoline ring caused a 19-fold increase in the antiparasitic effect. Likewise, the same substitution for compounds 4a and 3a showed similar improvements in antiparasitic effect, suggesting that the OH− at position 4 might be essential for the antileishmanial activity. Based on these results, we suggest that the substitution of allyl by cinnamyl groups in the quinoline ring might have contributed to a better antileishmanial effect. Furthermore, the four quinoline compounds had less toxicity than pentamidine when incubated with mammalian cells. The introduction of the OH− group resulted in a decrease in cytotoxicity, which could be an improvement if we also consider the improvement of its antiparasitic effect.
Compound 3b had the lowest IC50 of any compounds tested against L. chagasi promastigotes, with an IC50 of 0.091 μg ml−1. It was at least 22-fold more effective than the standard drug, pentamidine. However, antileishmanial activity against promastigotes does not guarantee activity against intracellular amastigotes, the clinically more relevant form of the parasite. The intracellular amastigotes have also been shown to express different enzymatic patterns, which could improve the parasite defense (21). Incubation of L. chagasi-infected macrophages with quinoline compounds demonstrated no significant antileishmanial activity for compounds 3a, 4a, and 4b at the highest concentration. Only compound 3b showed efficacy, with an IC50 of 3.55 μg/ml, which was 8.3-fold more active than the standard pentavalent antimony (IC50 = 29.55 μg/ml). Furthermore, this in vitro activity was similar to that of other antileishmanial quinolines, such as WR 6026, which had an IC50 of 1.6 μg/ml against Leishmania tropica-infected macrophages (6). The relationship between mammalian toxicity and antiparasitic effect, given by the S.I., demonstrated that compound 3b was at least 7.6-fold more harmful to intracellular amastigotes than to mammalian cells (RAW 264.7). These results also suggest that the introduction of OH− into the quinoline ring might have contributed to the improved antileishmanial activity, compared to compound 4b, which had a Cl− instead of OH− in position 4. Furthermore, the potential in vivo antileishmanial activity of novel 2-substituted quinolines, originally isolated from medicinal plants, has been described previously (13).
The inefficacy of the other quinoline compounds against intracellular amastigotes might have been due to one of the following: poor drug uptake by macrophages and subsequently low drug concentration reaching the parasitophorous vacuole; inactivation of the quinoline compounds inside macrophages; or metabolic differences of amastigotes, such as the enzymatic antioxidant system (catalase or superoxide dismutase) (27). Compound 3b was chosen for further investigations since it had the lowest IC50 against promastigotes, the lowest deviation (95% confidence interval), and efficacy against intracellular amastigotes.
Macrophages, the target cells in therapy of leishmaniasis, play an important role in the immunological control of intracellular parasites through the production of cytokines and oxygen metabolites (4). One of the main mechanisms is the up-regulation of nitric oxide inside the cell, which is an effective mediator of amastigote killing (18). We have investigated the possible activation of macrophages induced by compound 3b by incubation of this compound with both LPS-activated and nonactivated macrophages. No up-regulation of nitric oxide was seen when compound 3b was incubated in the presence of nonactivated macrophages. These results indicate that compound 3b might have a specific antiparasitic activity rather than causing activation of NO production by macrophages. In addition, efficacy against extracellular promastigotes supports the hypothesis of a specific antiparasitic activity for compound 3b. Ultrastructural studies of promastigotes treated with compound 3b (10 μg ml−1) suggested damage to Leishmania mitochondria, showing an enlargement of the matrix and a reduction in the number of cristae clearly observed after 1 h of incubation. Furthermore, an increase in the numbers of electron-dense granules and vacuoles within the parasite cytoplasm was observed, suggesting a time-dependent damage to intracellular organelles. The naphthoquinones have previously been shown to alter the mitochondrial membrane potential in Leishmania spp. (17), causing extensive and irreversible damage, which dramatically affected parasite survival.
In conclusion, compound 3b had the most potent antileishmanial activity and was not toxic to mammalian cells at concentrations required to kill parasites. Experimental in vivo studies are under investigation in our laboratory, using both free drug and drug entrapped in phosphatidylserine-rich liposomes. Liposomes have been described as an effective drug-targeting tool in leishmaniasis (26). Further investigations of antiparasitics using quinolines as the lead structure for the design and synthesis of novel pharmacological compounds represent an important and cost-effective strategy for addressing neglected diseases as leishmaniasis.
Acknowledgments
We thank Tracy Garnier (London School of Hygiene and Tropical Medicine) for manuscript revision and Cleuza Takakura for microscopy assistance.
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 99/08491-4), LIM-49 HCFMUSP and CNPq.
REFERENCES
- 1.Amougay, A., O. Letsch, and J. P. Pete. 1996. Photorearrangement of N- alkanoyl β-enaminones. Application to the synthesis of α-amino-β,γ-unsaturated acid derivatives. Tetrahedron 52:2405-2420. [Google Scholar]
- 2.Antonioletti, R., F. Bonadies, L. R. Orelli, and A. Scettri. 1992. Selective C- alkylation of 1,3-dicarbonyl compounds. Gazz. Chim. Ital. 122:237-238. [Google Scholar]
- 3.Balaña-Fouce, R., R. M. Reguera, C. Cubria, and D. Ordóñez. 1998. The pharmacology of leishmaniasis. Gen. Pharmacol. 30:435-443. [DOI] [PubMed] [Google Scholar]
- 4.Balaraman, S., P. Tewary, V. K. Singh, and R. Madhubala. 2004. Leishmania donovani induces interferon regulatory factor in murine macrophages: a host defense response. Biochem. Biophys. Res. Commun. 30:639-647. [DOI] [PubMed] [Google Scholar]
- 5.Basco, L. K., O. Dechy-Cabaret, M. Ndounga, F. S. Meche, A. Robert, and B. Meunier. 2001. In vitro activities of DU-1102, a new trioxaquine derivative, against Plasmodium falciparum isolates. Antimicrob. Agents Chemother. 45:1886-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman, J. D., and J. V. Gallalee. 1985. Semiautomated assessment of in vitro activity of potential antileishmanial drugs. Antimicrob. Agents Chemother. 28:723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt, C. A., H. M. C. Ferraz, E. O. Oliveira, and M. E. Payret-Arrua. 1995. A new and efficient approach to cyclic β-enamino esters and β-enamino ketones by iodine-promote cyclization. J. Org. Chem. 60:7357-7359. [Google Scholar]
- 8.Croft, S. L., D. Snowdon, and V. Yardley. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 38:1041-1047. [DOI] [PubMed] [Google Scholar]
- 9.Davies, C. R., P. Kaye, S. L. Croft, and S. Sundar. 2003. Leishmaniasis: new approaches to disease control. Br. Med. J. 15:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjeux, P. 1992. Human leishmaniasis: epidemiology and public health aspects. World Health Stat. Q. 45:267-275. [PubMed] [Google Scholar]
- 11.Dietze, R, S. F. Carvalho, L. C. Valli, J. Berman, T. Brewer, W. Milhous, J. Sanchez, B. Schuster, and M. Grogl. 2001. Phase 2 trial of WR6026, an orally administered 8-aminoquinoline, in the treatment of visceral leishmaniasis caused by Leishmania chagasi. Am. J. Trop. Med. Hyg. 65:685-689. [DOI] [PubMed] [Google Scholar]
- 12.Duarte, M. I., O. N. Mariano, C. F. Takakura, D. Everson, and C. E Corbett. 1992. A fast method for processing biologic material for electron microscopic diagnosis in infectious disease. Ultrastruct. Pathol. 16:475-482. [DOI] [PubMed] [Google Scholar]
- 13.Fournet, A., M. E. Ferreira, A. De Arias Rojas, S. de Ortiz Torres, S. Fuentes, H. Nakayama, A. Schinini, and R. Hocquemiller. 1996. In vivo efficacy of oral and intralesional administration of 2-substituted quinolines in experimental treatment of new world cutaneous leishmaniasis caused by Leishmania amazonensis. Antimicrob. Agents Chemother. 40:2447-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnier T., and S. L. Croft. 2002. Topical treatment for cutaneous leishmaniasis. Curr. Opin. Investig. Drugs 3:538-544. [PubMed] [Google Scholar]
- 15.Hauser, C. R., and G. A. Reynolds. 1948. Reactions of β-keto esters with aromatic amines. Synthesis of 2-and-4-hydroxyquinoline derivatives. J. Am. Chem. Soc. 70:2402-2404. [Google Scholar]
- 16.Holtzclaw, H. F., J. P. Collman, and R. M. Alire. 1958. Synthesis and infrared spectra of α,β-unsaturated-β-ketoamines and their copper chelates. J. Am. Chem. Soc. 80:1100-1102. [Google Scholar]
- 17.Luque-Ortega J. R., O. M. Rivero-Lezcano, S. L. Croft, and L. Rivas. 2001. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob. Agents Chemother. 45:1121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauel, J., and A. Ransijn. 1997. Leishmania spp. mechanisms of toxicity of nitrogen oxidation products. Exp. Parasitol. 87:98-111. [DOI] [PubMed] [Google Scholar]
- 19.Panaro, M. A., A. Acquafredda, S. Lisi, D. D. Lofrumento, T. Trotta, R. Satalino, M. Saccia, V. Mitolo, and O. Brandonisio. 1999. Inducible nitric oxide synthase and nitric oxide production in Leishmania infantum-infected human macrophages stimulated with interferon-gamma and bacterial lipopolysaccharide. Int. J. Clin. Lab. Res. 29:122-127. [DOI] [PubMed] [Google Scholar]
- 20.Pecoul, B., P. Chirac, P. Trouller, and J. Pinel. 1999. Access to essential drugs in poor countries: a lost battle? JAMA 281:361-367. [DOI] [PubMed] [Google Scholar]
- 21.Rabinovitch, M., and S. C. Alfieri. 1987. From lysosomes to cells, from cells to Leishmania: amino acid esters as potential chemotherapeutic agents. Braz. J. Med. Biol. Res. 20:665-674. [PubMed] [Google Scholar]
- 22.Romero, G. A., M. Flores, R. M. Noronha, and E. F. Macedo. 2003. High frequency of skin reactions in patients with leishmaniasis treated with meglumine antimoniate contaminated with heavy metals: a comparative approach using historical controls. Mem. Inst. Oswaldo Cruz 98:145-149. [DOI] [PubMed] [Google Scholar]
- 23.Sahu, N. P., C. Pal, N. B. Mandal, S. Banerjee, M. Raha, A. P. Kundu, A. Basu, M. Ghosh, K. Roy, and M. Bandyopadhyay. 2002. Synthesis of a novel quinoline derivative, 2-(2-methylquinolin-4-ylamino)-N-phenylacetamide—a potential antileishmanial agent. Bioorg. Med. Chem. 10:1687-1693. [DOI] [PubMed] [Google Scholar]
- 24.Stauber, L. A., E. M. Franchino, and J. Grun. 1958. An eight-day method for screening compounds against Leishmania donovani in golden hamsters. J. Protozool. 5:269-273. [Google Scholar]
- 25.Tada, H., O. Shiho, K. Kuroshima, M. Koyama, and M. Tsukamoto. 1986. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 93:157-165. [DOI] [PubMed] [Google Scholar]
- 26.Tempone A. G., D. Perez, S. Rath, A. L. Vilarinho, R. A. Mortara, and H. F. Jr. Andrade. 2004. Targeting Leishmania (L.) chagasi amastigotes through macrophage scavenger receptors: the use of drugs entrapped in liposomes containing phosphatidylserine. J. Antimicrob. Chemother. 54:60-68. [DOI] [PubMed] [Google Scholar]
- 27.Tempone, A. G., H. F. Jr. Andrade, P. J. Spencer, C. O. Lourenço, J. R. Rogero, and N. Nascimento. 2001. Bothrops moojeni venom kills Leishmania spp. with hydrogen peroxide generated by its l-amino acid oxidase. Biochem. Biophys. Res. Commun. 26:620-624. [DOI] [PubMed] [Google Scholar]
- 28.Trouiller, P., E. Torreele, P. Olliaro, N. White, S. Foster, D. Wirth, and B. Pécoul. 2001. Drugs for neglected diseases: a failure of the market and public health failure? Trop. Med. Int. Health. 6:945-951. [DOI] [PubMed] [Google Scholar]
- 29.Vianna, G. O. 1912. Tratamento da Leishmaniose tegumentar por injeções intravenosas de tártaro emético. An. 7° Congr. Bras. Med. Cirurg. 4:426-428.7 [Google Scholar]
- 30.Yeates, C. 2002. Sitamaquine (GlaxoSmithKline/Walter Reed Army Institute). Curr. Opin. Investig. Drugs 3:1446-1452. [PubMed] [Google Scholar]