Abstract
Systemic inflammatory response, which represents the presence of cachexia, is observed often in patients with lung cancer. To evaluate the prognostic significance of the presence of a systemic inflammatory response in small cell lung cancer (SCLC) patients, a retrospective study using modified Glasgow prognostic Score (mGPS) was performed. This score is composed of serum albumin and C-reactive protein levels. All the patients with SCLC who were diagnosed in Tsukuba University Hospital, Tsukuba Medical Center Hospital and Mito Medical Center between April 1999 and July 2016 were included in this study. During the study period, 332 patients with SCLC were consecutively admitted to these hospitals. Among them, 54 (16.9%) had mGPS=1, and 73 (22.9%) had mGPS=2. Male sex, advanced stage, poor performance status and no chemotherapy were unfavorable prognostic factors in uni- and multivariate-analysis. In addition, the presence of a systemic inflammatory response was confirmed as an unfavorable prognostic factor. In patients with SCLC, an existing systemic inflammatory response adversely affected the outcome. The patient's extent of disease as well as medical conditions including systemic inflammatory response must be taken into consideration when deciding whether to offer a standard therapy that may increase treatment-associated mortality.
Keywords: modified Glasgow prognostic score, small cell lung-cancer, serum albumin, C-reactive protein
Introduction
In patients with cancer, a systemic inflammatory response leads to increased protein breakdown and progressive nutritional decline by a direct catabolic effect on skeletal muscle and other host tissue (1–4). Such progressive nutritional decline leads to poorer survival (1–4). The measurement of the systemic inflammatory response has been subsequently refined using a selective combination of serum albumin and C-reactive protein (CRP) (termed the modified Glasgow Prognostic Score, mGPS) and has been revealed to have prognostic value, independent of tumor stage, in non-small cell lung, gastrointestinal and renal cancer (5,6).
Lung cancer remains one of the most common and fatal malignant diseases. The overall survival in patients with lung cancer, particularly those with small cell lung cancer (SCLC), remains poor and has not improved to a satisfactory level, despite the progress made in various therapeutic modalities (7). The treatment for patients with SCLC with a systemic inflammatory response may be complex due to a high level of post-therapeutic pulmonary complications and mortality. However, the clinic pathological features of patients with SCLC with a systemic inflammatory response have not been clarified, and the influence of the existence of the systemic inflammatory response on survival in these patients has rarely been examined (8). In the present study, the prognostic significance of coexistent systemic inflammatory response has been examined in patients with SCLC.
Patients and methods
All the patients with pathologically or cytologically proven SCLC who were admitted to Tsukuba University Hospital, Tsukuba Medical Center Hospital ((Tsukuba, Japan) and Mito Medical Center, University of Tsukuba (Mito, Japan) between April 1999 and July 2016 were analyzed retrospectively. Patients with SCLC were divided into two groups according to the staging system of the Veterans Administration Lung Cancer Study Groups: Limited stage (LD) or extensive stage (ED). Patients with LD-SCLC have involvement of the ipsilateral hemithorax within a single radiation port. ED-SCLC is defined as the presence of apparent metastatic disease. This classification has an important role in the indication of treatment and outcome. The present study was approved by the institutional review committee of Mito Medical Center, University of Tsukuba (Mito, Japan) (no. 16–20). Informed consent was obtained from all the patients for use of their data.
Serum albumin and CRP were those measured at the time of the diagnosis of SCLC. The mGPS was calculated according to the method by Forrest et al (9): Patients with a normal albumin level (3.5 g/dl) and CRP (1.0 mg/dl) were allocated a score of 0, those with both low albumin (<3.5 g/dl) and high CRP (>1.0 mg/dl) were scored 2. Patients with only an elevated CRP (>1.0 mg/dl) were assigned a score of 1.
The Mann-Whitney U test and the χ2 test were used to determine statistically significant differences between the two groups. To assess survival curves, the Kaplan-Meier method was used. To evaluate the statistical significance of differences, the log-rank test was used. The length of survival was defined as the interval in months from the date of the initial therapy or supportive care until the date of last follow-up or the date of mortality. For multivariate analysis of the effect of clinicopathological factors on survival, the Cox proportional hazards model was used. All statistical analysis was conducted using SPSS 10.1 for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
During the study period, 319 patients were diagnosed pathologically or cytologically as having SCLC. Table I presents the characteristics of these patients. They were 273 (85.6%) males and 46 females. The median patient age was 71 (range, 49–94) years. In total, 109 (34.1%) patients were ≥75 years old. There were 192 (78.3%) patients with good performance status (PS) (Eastern Cooperative Oncology Group 0–1) (10), and 103 (32.3%) patients with LD-SCLC. Among all the patients, 97 (30.4%) had serum albumin <3.5 g/dl, and 127 (39.8%) of them had serum C-reactive protein (CRP) >1.0 mg/dl. In total, 192 (60.2%) patients had mGPS=0, 54 (16.9%) had mGPS=1 and 73 (22.9%) had mGPS=2. Table II presents the association between mGPS and various patient characteristics. The results indicated that aged patients (≥75 years), those with poor PS (PS, 2–4), extensive disease and those receiving supportive care only had a higher mGPS. For male patients, smokers did not have a higher mGPS.
Table I.
Characteristics of 319 patients with lung cancer.
| Variables | N (%) |
|---|---|
| Age (years) | Median, 71 |
| Range, 49–94 | |
| ≥75 | 109 (34.1) |
| Sex | |
| Male | 273 (85.6) |
| Female | 46 (14.4) |
| Smoking habit | |
| Ex- or current smoker | 304 (95.3) |
| Never smoker | 15 (4.7) |
| Performance status | |
| 0–2 | 192 (78.3) |
| 3–4 | 127 (21.7) |
| Clinical stage | |
| Limited disease | 103 (32.3) |
| Extensive disease | 216 (67.7) |
| Initial treatment | |
| Chemotherapy | 276 (86.5) |
| Supportive care | 43 (13.5) |
Table II.
Patient characteristics and mGPS.
| mGPS score, N (%) | ||||
|---|---|---|---|---|
| Variable | 0 | 1 | 2 | P-value |
| No. of patients | 192 | 54 | 73 | |
| Age (≥75 years) | 54 (28.1) | 19 (35.2) | 36 (49.3) | 0.005 |
| Sex (male) | 161 (83.9) | 49 (90.4) | 63 (86.3) | 0.741 |
| Smoking habit (present) | 182 (94.8) | 52 (96.3) | 70 (95.9) | 0.866 |
| PS (2–4) | 56 (29.2) | 21 (38.9) | 50 (68.5) | 0.001 |
| Disease (extensive disease) | 119 (62.5) | 35 (64.8) | 62 (84.9) | 0.002 |
| Treatment (supportive care) | 18 (9.4) | 5 (9.3) | 20 (27.4) | 0.004 |
P-values were calculated using the χ2 test. mGPS, modified Glasgow prognostic score; PS, performance status.
Fig. 1 reveals the survival curves of the patients with mGPS=0, 1 and 2. The median survival time of patients was 13.1, 10.0 and 5.9 month, respectively. There was a statistically significant difference in survival among these patient groups (P=0.0001). Table II presents the results of uni- and multivariate analysis of prognostic factors in these patients. In univariate analysis, aged 75 years old [median survival time (MST) in patients ≥75 years, 8.7 months; MST in those ≤74 years, 12.7 months), poor PS (MST in patients with PS 2–4, 6.2 months; MST in those with PS 0–1, 14.6 months), extensive disease (MST in patients with ED, 8.0 months; MST in those with LD, 19.2 months), supportive care only (MST in patients with supportive care only, 1.5 months; MST in those with chemotherapy, 12.7 months) and higher mGPS (MST in patients with mGPS=2, 5.9 months; MST in those with mGPS=1, 10.0 months; MST in those with mGPS=2, 13.1 months) were unfavorable prognostic factors. In the multivariate analysis, extensive disease and supportive care were unfavorable prognostic factors. In addition, mGPS=2 was confirmed as an unfavorable prognostic factor in the multivariate analysis (Table III).
Figure 1.
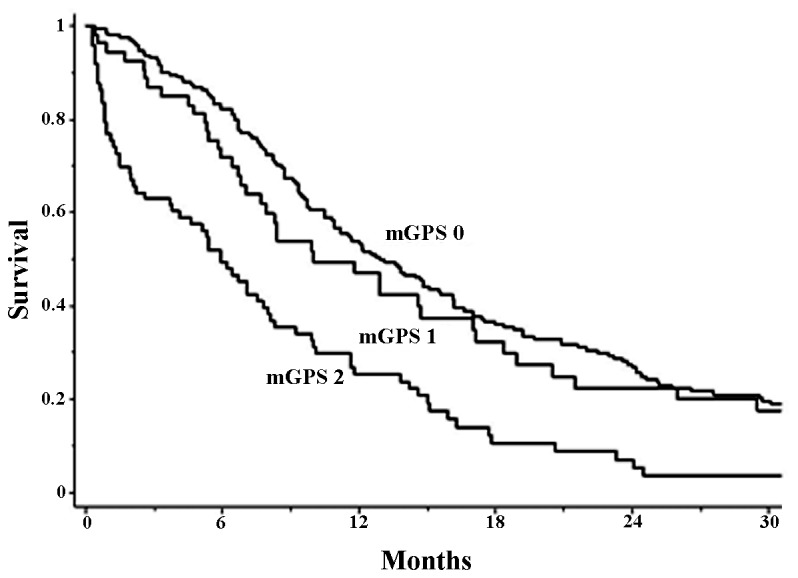
Survival curves of the patients with mGPS=0, 1 and 2. The median patient survival time was 13.1, 10.0 and 5.9 months, respectively. There was statistical significant difference in survival among them (P=0.0001). mGPS, modified Glasgow prognostic score.
Table III.
Univariate and multivariate analyses of unfavorable prognostic factors in 319 patients with small cell lung cancer.
| Multivariate analysisb | |||||
|---|---|---|---|---|---|
| Factors | Univariate analysisa MST | P-value | Hazard ratio | 95% CI | P-value |
| Age (>75 vs. <75) | 8.7 vs. 12.7 | 0.001 | 1.25 | 0.95–1.63 | 0.111 |
| Sex (male vs. female) | 10.5 vs. 13.8 | 0.346 | |||
| Smoking (present vs. absent) | 10.8 vs. 12.5 | 0.571 | |||
| PS (2–4 vs. 0–1) | 6.2 vs. 14.6 | 0.001 | 1.34 | 0.99–1.81 | 0.055 |
| Disease extent (ED vs. LD) | 8.0 vs. 19.2 | 0.001 | 2.43 | 1.82–3.24 | 0.001 |
| 1st-line Tx (SC vs. chemo) | 1.5 vs. 12.7 | 0.001 | 5.95 | 3.76–9.11 | 0.001 |
| mGPS (2 vs. 1 vs. 0) | 5.9 vs. 13.1 | 0.001 | |||
| mGPS (mGPS=1) | 1.23 | 0.86–1.74 | 0.247 | ||
| mGPS (mGPS=2) | 2.04 | 1.51–2.78 | 0.001 | ||
Log-rank test
Cox proportional hazards model. 95% CI, 95% confidence interval; MST, median survival time; PS, performance status; ED, extensive disease; LD, limited stage; Tx, therapy; SC, supportive care only; mGPS, modified Glasgow prognostic score.
Discussion
McMillan et al (5) proposed a prognostic score as a measure of systemic inflammation and nutritional status determined by serum albumin values and the CRP value (5). This prognostic score is now believed to reflect the condition of cachexia in malignant disease. In various cancer types such as gastrointestinal cancer, it is evaluated as an independent prognostic factor (11,12). Its clinical significance has been also evaluated in NSCLC (13,14), but has scarcely evaluated in patients with SCLC (8). At present, there is only one previous study that evaluates the clinical significance of mGPS as a prognostic factor (8). Due to the lack of data on CRP and albumin in this previous study, mGPS in 359/460 consecutive patients was evaluated. There were only 18 (5.0%) patients with PS 2, 11 (3.1%) patients with mGPS=2, and 20 (5.6%) patients with albumin <3.5 g/dl. These data revealed that the proportion of patients with prognostic values, independent of tumor stage, was too low to evaluate. In addition, median age of the patients was 60 years. Therefore, the results from this previous study may differ from those of a typical population of patients with SCLC. The present study was performed to evaluate the prognostic significance of the presence of a systemic inflammatory response in patients with SCLC. The majority of patients with lung cancer are aged and have comorbid wasting diseases such as chronic obstructive pulmonary disease and chronic heart disease; therefore, they potentially have a high risk of developing cachexia. For patients with NSCLC, the clinical usefulness of anamorelin, an anti-cachexia drug in global phase III and domestic phase II studies in Japan have recently been reported (15,16). For patients with SCLC, early intervention such as prescription of anti-cachexia and nutritional support may improve prognosis.
In the present study, the prognostic significance of a systemic inflammatory response was evaluated using mGPS in unselected patients with SCLC. As has been reported in a previous study (8), it was demonstrated that male sex, extensive disease, poor PS and no chemotherapy were unfavorable prognostic factors. In addition to these factors, the present study revealed that systemic inflammatory response (mGPS=2) was unfavorable prognostic factor. These results suggested that patients with SCLC with a systemic inflammatory response may have a poor prognosis.
Many clinical trials have not shown the outcome of treatment of patients with SCLC patients with a systemic inflammatory response, as these studies excluded patients with impairment of organ function and active inflammation. Therefore, there is scarce published information with regard to the results of unfavorable prognostic factors in unselected patients with SCLC. Therefore, the present study included those with systemic inflammatory response, and revealed that a systemic inflammatory response (mGPS=2) was unfavorable prognostic factor. The results suggested that patients with SCLC with a systemic inflammatory response may have a poor prognosis. Based on the results, it is suggested that clinicians must take the patient's medical condition, including systemic inflammatory response, into consideration when deciding whether to offer a standard therapy that may increase treatment-associated mortality.
Due to the design of clinical trials in which eligibility criteria preclude the involvement of patients with impairment of organ function, numerous published studies have not revealed the outcome of treatment of patients with SCLC with a systemic inflammatory response. As a result, there is little published information regarding the results of treatment and prognostic factors in unselected groups of patients with SCLC, including those with SCLC and a systemic inflammatory response. Therefore, the results of treatment and prognostic factors in unselected patients with SCLC who were admitted to the three hospitals were evaluated. In the present series of patients, female sex, LD-SCLC and good PS were favorable prognostic factors for SCLC, as has been reported in a previous study (8). Additionally, it was revealed that patients with a systemic inflammatory response had poorer overall survival than those without, and the existence of a systemic inflammatory response was one of the unfavorable prognostic factors for survival in patients with SCLC. It is very important to understand why a systemic inflammatory response worsened the prognosis of SCLC in these patients. Therefore, standard therapy or adequate palliative care may be essential to provide prolonged quality survival, which is the primary goal of therapy for patients with SCLC with systemic inflammatory responses.
In conclusion, the prognostic significance of the presence of a systemic inflammatory response, as determined using mGPS, in SCLC was demonstrated in the current study. A well-planned large sized prospective study is required to support these results.
References
- 1.Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: Inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. 1999;23:584–588. doi: 10.1007/PL00012351. [DOI] [PubMed] [Google Scholar]
- 2.McMillan DC, Scott HR, Watson WS, Preston T, Milroy R, McArdle CS. Longitudinal study of body cell mass depletion and the inflammatory response in cancer patients. Nutr Cancer. 1998;31:101–105. doi: 10.1080/01635589809514687. [DOI] [PubMed] [Google Scholar]
- 3.McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39:210–213. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 4.Meek CL, Wallace AM, Forrest LM, McMillan DC. The relationship between the insulin-like growth factor-1 axis, weight loss, an inflammation-based score and survival in patients with inoperable non-small cell lung cancer. Clin Nutr. 2010;29:206–209. doi: 10.1016/j.clnu.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 5.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer; Proc Nutr Soc; 2008; pp. 257–262. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 7.Dowell JE. Small cell lung cancer: Are we making progress? Am J Med Sci. 2010;339:68–76. doi: 10.1097/MAJ.0b013e3181bccef5. [DOI] [PubMed] [Google Scholar]
- 8.Zhou T, Hong S, Hu Z, Hou X, Huang Y, Zhao H, Liang W, Zhao Y, Fang W, Wu X, et al. A systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancer. Tumour Biol. 2015;36:337–343. doi: 10.1007/s13277-014-2623-4. [DOI] [PubMed] [Google Scholar]
- 9.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Crumley AB, Stuart RC, McKernan M, Going JJ, Shearer CJ, McMillan DC. Comparison of pre-treatment clinical prognostic factors in patients with gastro-oesophageal cancer and proposal of a new staging system. J Gastrointest Surg. 2010;14:781–787. doi: 10.1007/s11605-010-1162-6. [DOI] [PubMed] [Google Scholar]
- 12.Roxburgh CS, Platt JJ, Leitch EF, Kinsella J, Horgan PG, McMillan DC. Relationship between preoperative comorbidity, systemic inflammatory response, and survival in patients undergoing curative resection for colorectal cancer. Ann Surg Oncol. 2011;18:997–1005. doi: 10.1245/s10434-010-1410-8. [DOI] [PubMed] [Google Scholar]
- 13.Fan H, Shao ZY, Xiao YY, Xie ZH, Chen W, Xie H, Qin GY, Zhao NQ. Comparison of the glasgow prognostic score (GPS) and the modified glasgow prognostic score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142:1285–1297. doi: 10.1007/s00432-015-2113-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Chen S, Ma S, Zhang S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: A meta-analysis. Springerplus. 2016;5:439. doi: 10.1186/s40064-016-2093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–531. doi: 10.1016/S1470-2045(15)00558-6. [DOI] [PubMed] [Google Scholar]
- 16.Takayama K, Katakami N, Yokoyama T, Atagi S, Yoshimori K, Kagamu H, Saito H, Takiguchi Y, Aoe K, Koyama A, et al. Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: Results of a randomized phase 2 trial. Support Care Cancer. 2016;24:3495–3505. doi: 10.1007/s00520-016-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


