Abstract
The in vitro activity of the histatin derivative P-113, alone or combined with eight antibiotics, was investigated against multidrug-resistant strains isolated from clinical specimens of immunocompromised patients with pneumonia. The gram-negative isolates were susceptible to P-113. S. aureus showed less susceptibility. Synergy was demonstrated when P-113 was combined with beta-lactams against gram-negative organisms.
Respiratory infections due to multiresistant organisms such as Pseudomonas aeruginosa, Staphylococcus aureus, and Stenotrophomonas maltophilia have become increasingly significant as a consequence of the growing population of immunocompromised patients (1, 13, 21). The search for novel antimicrobial agents continues because of the emergence of bacterial pathogens resistant to nearly all the clinically used antibiotics (3, 14, 17). Among the candidates for new classes of antimicrobial drugs are antimicrobial peptides (9).
Antimicrobial polycationic peptides are an emerging class of antibiotic with a unique mechanism of action. They are part of an innate immune system widely distributed in nature and have been found in many different organisms, including animal, plant, and bacterial species (2, 10, 11). Their mechanism of action is being actively studied, and the available information continues to grow.
Histatins are a group of small, histidine-rich antimicrobial peptides secreted into saliva by human parotid and submandibular-sublingual glands. Histatin 1 and histatin 3 are encoded by distinct genes, while the other 10 histatin peptides isolated from saliva are believed to arise from them by proteolytic processing (16, 22). They have found to have antimicrobial activity against a broad spectrum of bacteria and fungi (18, 19). The antimicrobial activity of histatins appears to be a distinctive multistep mechanism involving depletion of the microrganism intracellular ATP content as a result of nonlytic ATP efflux (18, 19, 22). Other activities are ascribed to these peptides, such as inhibition of hemagglutination, coaggregation, protease activity, and neutralization of lipopolysaccharide by binding to lipid A (5, 18, 20). The derivative P-113 is composed of a sequence of 12 amino acid residues contained within the 24 amino acid residues of histatin 5 and is amidated at its C terminus. Like the other histatins, it is active against clinically important microorganisms such as streptococci, staphylococci, Pseudomonas spp., and Candida albicans (18, 19, 20).
In this study, we investigated the in vitro activity of P-113 alone and in combination with eight clinically used antimicrobial agents, was investigated against several multidrug-resistant strains of P. aeruginosa, S. aureus, and S. maltophilia isolated from sputum, bronchoalveolar lavage, or blood of immunocompromised patients with pneumonia.
Organisms.
Clinical isolates of P. aeruginosa, methicillin-resistant S. aureus, and S. maltophilia (20 strains for each species) cultured from sputum, bronchoalveolar lavage, or blood derived from immunocompromised patients with pneumonia were tested. All isolates were considered responsible for nosocomially acquired infections since the positive specimen cultures were obtained more than 48 h after admission and there was no evidence of infection at the time of admission. The isolates were obtained from distinct patients coming from central Italy with unrelated sources of infection and admitted to the Hospital Umberto I, Ancona, Italy, from January 2001 to December 2003. Identification of the strains was performed according to standard procedures. The identification was confirmed by means of the API 20 systems (bioMérieux Italia, Italy). The different API codes and susceptibility patterns stated the independence of all isolates. S. aureus ATCC 43300 and P. aeruginosa ATCC 27853 were used as quality control strains.
Synthetic peptides and antimicrobial agents.
P-113 (AKRHHGYKRKFH-NH2) was synthesized by 9-fluorenylmethoxycarbonyl (Fmoc) solid-phase chemistry according to the following procedure. (i) Five- and 15-min deprotection steps were performed with 20% piperidine in the mixture N,N-dimethylformamide (DMF)-N-methyl-2-pyrrolidone (NMP)(1:1 [vol/vol]) in the presence of 1% Triton. (ii) The coupling reactions were carried out with the protected amino acid diluted in a mixture DMF-NMP (1:1 [vol/vol]) in the presence of 1% Triton, using N,N′-diisopropylocarbodiimide (DIC) as the coupling reagent in the presence of 1-hydroxybenzotriazole (HOBt) (Fmoc-AA-DIC-HOBt at 1:1:1) for 1 h. The completeness of each coupling reaction was monitored by the chloranil test (4, 7). The protected peptidyl resin was treated with a mixture of 95% trifluoroacetic acid, 2.5% water, and 2.5% triisopropylsilane (TIS) for 1 h. After cleavage, the solid support was removed by filtration, and the filtrate was concentrated under reduced pressure. The cleaved peptide was precipitated with diethyl ether and lyophilized. P-113 was purified by solid-phase extraction (SPE) on Kromasil sorbent (C8, 5 μm, 100 Å) by the protocol described in reference 12. The resulting fractions with purity greater than 97 to 98% were tested by high-performance liquid chromatography. The peptide was analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF). The peptide was solubilized in phosphate-buffered saline (pH 7.2), yielding a 1-mg/ml stock solution. Solutions of drugs were made fresh on the day of assay or stored at −80°C in the dark for short periods.
For comparison, the in vitro activities of the following antibiotics were evaluated: amikacin, vancomycin, and colistin sulfate from Sigma-Aldrich; clarithromycin from Abbott, Rome, Italy; ciprofloxacin from Bayer, Milan, Italy; ceftazidime from GlaxoSmithKline, Verona, Italy; imipenem from Merck, Sharp & Dohme, Milan, Italy; and piperacillin-tazobactam from Wyeth-Lederle, Aprilia, Italy. Laboratory standard powders were diluted in accordance with the manufacturers' recommendations, yielding a 1-mg/ml stock solution. Stock solutions of these antimicrobial drugs were stored at −80°C until they were used. The concentration range assayed for each antibiotic was 0.125 to 128 μg/ml.
MIC and MBC determinations.
The MIC was determined by a broth microdilution method with Mueller-Hinton (MH) broth (Becton Dickinson Italia, Milan, Italy) and an initial inoculum of 5 × 105 CFU/ml, according to the procedures outlined by the National Committee for Clinical Laboratory Standards (15). Polystyrene 96-well plates (Becton Dickinson and Co., Franklin Lakes, N.J.) were incubated for 18 h at 37°C in air. When peptides were tested, since they have a tendency to precipitate, plates were shaken throughout the study. The MIC was taken as the lowest drug concentration at which observable growth was inhibited. The minimal bactericidal concentration (MBC) was taken as the lowest concentration of each drug that resulted in more than 99.9% reduction of the initial inoculum. Experiments were performed in triplicate.
Bacterial killing assay.
All organisms were grown at 37°C in MH broth. Aliquots of exponentially growing bacteria were resuspended in fresh MH broth at approximately 107 cells/ml and separately exposed to each peptide at two times the MIC for 0, 2, 5, 10, 20, 30, 40, 50, and 60 min at 37°C. After these times, samples were serially diluted and plated onto MH agar plates to obtain viable colonies. The limit of detection for this method was approximately 10 CFU/ml.
Synergy studies.
In interaction studies, 18 strains (six for each species) were used to test the antibiotic combinations by a checkerboard titration method using 96-well polypropylene microtiter plates. The ranges of drug dilutions used were 0.125 to 64 μg/ml for P-113 and 0.25 to 256 μg/ml for clinically used antibiotics. The fractionary inhibitory concentration (FIC) index for combinations of two antimicrobials was calculated according to the equation FIC index = FICA + FICB = A/MICA + B/MICB, where A and B are the MICs of drugs A and B in the combination, MICA and MICB are the MICs of drug A and drug B alone, and FICA and FICB are the FICs of drug A and drug B. The FIC indices were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifferent; and >4.0, antagonism (6).
MIC and MBC results.
All gram-negative organisms were inhibited by P-113 at concentrations of 0.5 to 8 μg/ml. In particular, S. maltophilia was more susceptible than P. aeruginosa, for which the MIC ranges were 0.5 to 4 and 1 to 8 μg/ml, respectively. In contrast, against the S. aureus clinical isolates, the peptide exhibited a MIC range of 2 to 16 μg/ml.
Overall, high rates of resistance to the clinically used antibiotics were demonstrated. The results are summarized in Table 1.
TABLE 1.
MICs and MBCs of P-113 and other clinically used antibiotics for 60 clinical isolates
| Strain (no.) and agent | MIC (μg/ml)a
|
MBC (μg/ml)a
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| S. aureus (20) | ||||||
| P-113 | 2-16 | 4 | 16 | 2-32 | 8 | 32 |
| TZPb | 8-256 | 64 | 256 | 8->256 | 64 | >256 |
| Imipenem | 0.25-64 | 16 | 64 | 1-128 | 16 | 64 |
| Amikacin | 4-128 | 16 | 32 | 4-128 | 32 | 32 |
| Ciprofloxacin | 0.50-16 | 4 | 8 | 1-16 | 8 | 16 |
| Clarithromycin | 0.50-16 | 4 | 16 | 1-128 | 32 | 128 |
| Vancomycin | 0.25-4 | 0.5 | 2 | 0.50-4 | 1 | 4 |
| P. aeruginosa (20) | ||||||
| P-113 | 1-8 | 4 | 8 | 1-32 | 8 | 32 |
| Colistin | 0.5-8 | 2 | 8 | 0.5-16 | 4 | 16 |
| TZP | 4->256 | 16 | 128 | 8-256 | 32 | >256 |
| Ceftazidime | 1-256 | 16 | 64 | 8-256 | 32 | 128 |
| Imipenem | 0.50-64 | 4 | 16 | 1-128 | 16 | 64 |
| Amikacin | 1-64 | 2 | 16 | 1-128 | 4 | 32 |
| Ciprofloxacin | 0.50-32 | 4 | 8 | 1-32 | 8 | 32 |
| S. maltophilia (20) | ||||||
| P-113 | 0.5-4 | 2 | 4 | 1-16 | 4 | 16 |
| Colistin | 0.5-4 | 1 | 4 | 0.5-8 | 2 | 8 |
| TZP | 4-128 | 16 | 64 | 8-256 | 32 | 256 |
| Ceftazidime | 1-128 | 8 | 64 | 4-256 | 32 | 128 |
| Imipenem | 16-128 | 32 | 128 | 64->256 | 128 | >256 |
| Amikacin | 0.5-32 | 2 | 8 | 1-64 | 4 | 16 |
| Ciprofloxacin | 0.50-16 | 2 | 8 | 1-32 | 4 | 16 |
50% and 90%, MIC (or MBC) at which 50% and 90% of isolates tested are inhibited (or killed), respectively.
TZP, tazobactam-piperacillin.
Bacterial killing assay.
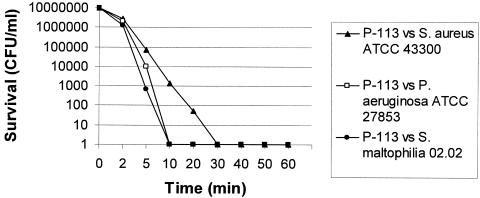
Killing by P-113 was shown to be very rapid: its activity against the gram-negative organisms was complete after a 10-min exposure period at a concentration of two times the MIC. On the other hand, the peptide exhibited a slower bactericidal effect on S. aureus. Figure 1 summarizes the results obtained from the quality control strains S. aureus ATCC 43300 and P. aeruginosa ATCC 27853 and the multiresistant clinical isolate S. maltophilia 02.02. These results were similar for all other strains.
FIG. 1.
Time-kill kinetics of P-113 against the quality control strains S. aureus ATCC 43300 and P. aeruginosa ATCC 27853 and the multiresistant clinical isolate S. maltophilia 02.02. The results were similar for all other strains.
Synergy studies.
In the combination studies, relevant differences were detected between the two control strains. In detail, when S. aureus ATCC 43300 was tested, synergy was never observed, even if indifference was demonstrated between P-113 and clarithromycin. In contrast, FIC indices of 0.312 were observed by testing P-113 combined with ceftazidime, tazobactam-piperacillin, and imipenem against P. aeruginosa ATCC 27853, while the combination between the two peptides P-113 and colistin showed an indifferent effect. The results are summarized in Table 2.
TABLE 2.
Results of studies of the interaction between P-113 and other drugs
| Agent | FIC index for P113 interactiona
|
||
|---|---|---|---|
| S. aureus | P. aeruginosa | S. maltophilia | |
| Colistin | NT | 0.927 (0.750-1.250) | 0.917 (0.750-1.250) |
| TZPb | 1.292 (0.750-2.00) | 0.312 (0.187-0.50) | 0.385 (0.187-0.50) |
| Ceftazidime | NT | 0.458 (0.312-0.50) | 0.312 (0.187-0.50) |
| Imipenem | 1.50 (1.00-2.00) | 0.385 (0.312-0.50) | 0.312 (0.187-0.50) |
| Amikacin | 1.167 (0.750-1.50) | 1.458 (1.250-2.00) | 1.292 (1.00-2.00) |
| Ciprofloxacin | 1.250 (0.750-2.00) | 1.167 (0.750-2.00) | 1.50 (1.00-2.00) |
| Clarithromycin | 0.917 (0.750-1.250) | NT | NT |
| Vancomycin | 1.833 (1.250-2.00) | NT | NT |
The ranges of concentrations tested were 0.125 to 64 μg/ml for P-113 and 0.25 to 256 μg/ml for the other antimicrobial agents. The FIC indices were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifferent; and >4.0, antagonism. Values in parentheses represent the mean FICs of six bacterial strains. NT, not tested.
TZP, tazobactam-piperacillin.
Our data demonstrate that P-113 has a powerful bactericidal effect on multiresistant clinical isolates of P. aeruginosa and S. maltophilia: against these pathogens, its activity is comparable to that of colistin sulfate, a well-characterized antimicrobial peptide. Nevertheless, different from colistin, P-113 exhibited good activity against methicillin-resistant strains of S. aureus too. Although the differences in the antimicrobial spectra between the two peptides cannot be explained on the basis of current knowledge, our results point out two potential therapeutic uses of P-113.
First, data from synergy studies suggest that it could be usefully administered in combinations with beta-lactam antibiotics to treat severe gram-negative infections. As mentioned above, the cationic peptides allow maximal entry of several substrates inside the cell: the synergistic interaction with beta-lactam antibiotics could be due to their increased passage through the outer bacterial membrane. On the other hand, peptides and beta-lactams may have a common target: it had been hypothesized that cationic peptides might render bacteria nonviable by activating their autolytic wall enzymes, such as muramidases (8).
Second, like colistin, P-113 could have potential for use in inhalant antibacterial treatment because of its strong activity against the most important multiresistant pathogens responsible for severe respiratory infections in immunocompromised patients. Since the worldwide emergence of antimicrobial resistance is an increasing problem in human medicine, the antimicrobial peptides are promising compounds: several studies have shown that peptide-resistant mutants emerge rarely because of their mechanism of action, which primarily involves the chemical and physical structure of the biological membrane. For this reason, they represent a conserved theme in host antimicrobial defenses throughout nature. Today the increasing amount of data concerning the antibacterial activity of polycationic peptides makes these agents potentially valuable as an adjuvant for antimicrobial chemotherapy.
Acknowledgments
This work was supported by Italian Ministry of Education, University and Research (PRIN 2003).
REFERENCES
- 1.Baughman, R. P. 1999. The lung in the immunocompromised patient. Infectious complications. Part 1. Respiration 66:95-109. [DOI] [PubMed] [Google Scholar]
- 2.Cannon, M. 1987. A family of wound healers. Nature 328:478. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli, Y., N. Troillet, G. M. Eliopoulos, and M. H. Samore. 1999. Emergence of antibiotic-resistant Pseudomona aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 43:1379-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen, T. 1979. Qualitative test for monitoring coupling completeness in solid phase peptide synthesis using chloranil. Acta Chem. Scand. Ser. B 33:763-766. [Google Scholar]
- 5.Cirioni, O., A. Giacometti, R. Ghiselli, F. Orlando, W. Kamysz, G. D'Amato, F. Mocchegiani, J. Łukasiak, C. Silvestri, V. Saba, and G. Scalise. 2004. Potential therapeutic role of histatins derivative P-113D in experimental rat models of Pseudomonas sepsis. J. Infect. Dis. 190:356-364. [DOI] [PubMed] [Google Scholar]
- 6.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-393. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 7.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Peptide Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 8.Ginsburg, I. 2004. Bactericidal cationic peptides can also function as bacteriolysis-inducing agents mimicking beta-lactam antibiotics? It is enigmatic why this concept is consistently disregarded. Med. Hypotheses 62:367-374. [DOI] [PubMed] [Google Scholar]
- 9.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, R. E. W., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamysz, W., M. Okrój, E. Øempicka, T. Ossowski, and J. Øukasiak. 2004. Fast and efficient purification of synthetic peptides by solid-phase extraction. Acta Chromatogr. 14:180-186. [Google Scholar]
- 13.Lyczac, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouton, J. W., J. G. den Hollander, and A. M. Horrevorts. 1993. Emergence of antibiotic resistance amongst Pseudomonas aeruginosa isolates from cystic fibrosis patients J. Antimicrob. Chemother. 31:919-926. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Oppenheim, F. G., T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, G. D. Offner, and R. F. Troxler. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472-7477. [PubMed] [Google Scholar]
- 17.Raad, I., A. Alrahwan, and K. Rolston. 1998. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin. Infect. Dis. 26:1182-1187. [DOI] [PubMed] [Google Scholar]
- 18.Rothstein, D. M., P. Spacciapoli, L. T. Tran, T. Xu, F. D. Roberts, M. Dalla Serra, D. K. Buxton, F. G. Oppenheim, and P. Friden. 2001. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 45:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajjan, U. S., L. T. Tran, N. Sole, C. Rovaldi, A. Akiyama, P. M. Friden, J. F. Forstner, and D. M. Rothstein. 2001. P-113d, an antimicrobial peptide active against Pseudomonas aeruginosa, retains activity in the presence of sputum from cystic fibrosis patients. Antimicrob. Agents Chemother. 45:3437-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyama, K. 1993. Anti-lipopolysaccharide activity of histatins, peptides from human saliva. Experientia 49:1095-1097. [DOI] [PubMed] [Google Scholar]
- 21.Tablan, O. C., L. J. Anderson, R. Besser, C. Bridges, R. Hajjeh et al. 2004. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. Morb. Mortal. Wkly. Rep. 53(RR-3):1-36. [PubMed] [Google Scholar]
- 22.Tsai, H., and L. A. Bobek. 1998. Human salivary histatins: promising antifungal therapeutic agents. Crit. Rev. Oral Biol. Med. 9:480-497. [DOI] [PubMed] [Google Scholar]