Abstract
The absence of any formally licensed antiadenovirus drugs and the increasing incidence of life-threatening adenovirus infections in immunosuppressed patients warrant the development of effective antiadenovirus compounds. A detailed study was performed on the antiadenovirus activities of several classes of nucleoside and nucleotide analogues in human embryonic lung fibroblast cells. The antiadenovirus activities were evaluated by three methods, viz., evaluating the adenoviral cytopathic effect, monitoring cell viability by a colorimetric assay, and real-time PCR quantitation of viral DNA as a direct parameter for virus replication. The most active and selective compounds were the acyclic nucleoside phosphonate analogues cidofovir, its adenine analogue (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine [(S)-HPMPA], and the new derivative (S)-2,4-diamino-6-[3-hydroxy-2-(phosphonomethoxy)propoxy]pyrimidine [(S)-HPMPO-DAPy]; the N7-substituted acyclic derivative 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine (S-2242); and the 2′,3′-dideoxynucleoside analogues zalcitabine and alovudine. No antiadenovirus activity was observed for the antiviral drugs ribavirin, foscarnet, acyclovir, penciclovir, and brivudin, while ganciclovir displayed modest activity. However, in human osteosarcoma cells transfected with herpes simplex virus thymidine kinase, ganciclovir demonstrated highly potent antiadenovirus activity, suggesting that the efficacy of ganciclovir against adenovirus is limited by inefficient phosphorylation in adenovirus-infected cells, rather than by insufficient inhibition at the viral DNA polymerase level. Collectively, our antiviral data show that the adenovirus DNA polymerase exhibits sensitivity to a relatively broad spectrum of inhibitors and should be studied further as an antiviral target in antiadenovirus drug development programs.
During the past decade, the growing practice of transplantation accompanied by strong immunosuppressive therapy has led to a gradual increase in the incidence of severe adenovirus infections. Pediatric patients undergoing allogeneic stem cell transplantation are particularly prone to disseminated adenovirus infections with high morbidity and mortality (28). The wide tissue tropism of adenoviruses explains their broad disease spectrum, which includes pneumonitis, hepatitis, encephalitis, hemorrhagic cystitis, and gastroenteritis. Among the >50 human adenovirus serotypes recognized today, some variations can be seen in terms of prevalence and disease association. At this time, there is no formally approved antiviral therapy for adenovirus infections. Case studies on the nucleoside analogue ribavirin have yielded conflicting results (3, 9, 16). The nucleotide analogue cidofovir was found to be beneficial in several small-scale studies involving patients with life-threatening adenovirus infections (3, 12, 17). In a retrospective study enrolling 45 patients with adenovirus infections after allogeneic stem cell transplantation, the overall success rate for cidofovir was reported to be 69% (18). There is an increasing need for new antiviral therapeutics with potent activity against human adenoviruses and a favorable therapeutic index. Ideally, several targets should be pursued to allow suppression of the virus at different steps in its replication cycle. In the case of adenoviruses, and in contrast to herpesviruses, the number of viral proteins that have been exploited as potential antiviral targets is very limited. Most compounds reported to have antiadenovirus activity are nucleoside or nucleotide analogues that target the adenovirus DNA polymerase. The adenovirus cysteine protease was shown to be susceptible to miscellaneous protease inhibitors, albeit with rather low specificity for the viral protease (24). The adenovirus adsorption process is an attractive target, since the sulfated sialic acid derivative NMSO3 was found to inhibit cellular binding of several types of human adenovirus at concentrations that did not affect cell viability (15).
In a comparison of the available in vitro data on the activities of antiviral compounds against human adenoviruses, we were surprised by the inconsistencies in the experimental procedures used. Accurate estimation of antiviral activity obviously depends on factors, such as viral load, host cell line, and the methodology used to measure adenovirus replication. In the present study, we optimized these antiviral assays in human embryonic lung fibroblast cells. We combined indirect parameters for adenovirus replication (examining cytopathic effect and viability of infected cells) with real-time PCR analysis to directly quantify adenovirus progeny in the virus-infected cells. Using these methodologies, we now demonstrate that several nucleoside and nucleotide analogues, some of which were already known to inhibit herpesviruses and/or herpesviruses, display potent and selective antiadenovirus activity in cell culture.
MATERIALS AND METHODS
Chemical compounds.
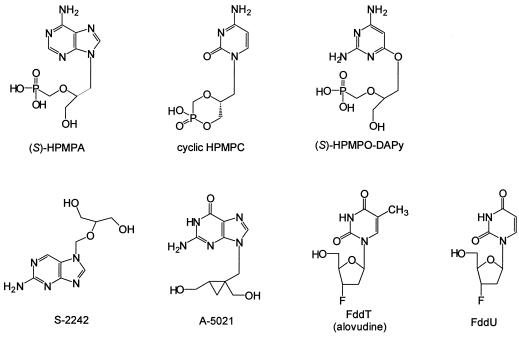
The structure and origin of all the test compounds used in this study follow. Cidofovir (CDV) [(S)-HPMPC; (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine; Vistide] and cyclic cidofovir (cCDV) {1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl] cytosine} were from Gilead Sciences, Foster City, Calif. (S)-HPMPA [(S)-9-(3-hydroxy-2-phosphonyl-methoxypropyl)adenine] and HPMPO-DAPy {2,4-diamino-6-[3-hydroxy-2-(phosphonomethoxy)-propoxy]pyrimidine} were from A. Holý, Prague, Czech Republic. Acyclovir (Zovirax), ganciclovir (Cymevene), foscarnet (Foscavir), zalcitabine (ddC; Hivid), zidovudine (AZT; Retrovir), lamivudine (3TC; Epivir), and ribavirin (Virazole) were from commercial sources. Penciclovir and S-2242 [2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine] were from I. Winkler, Hoechst, Inc., Frankfurt am Main, Germany. Brivudin {E-5-(2-bromovinyl)-2′-deoxyuridine; BVDU; Zostex}, alovudine {3′-fluoro-3′-deoxythymidine; FddT}, and 3′-fluoro-2′,3′-dideoxyuridine (FddU) were from P. Herdewijn, Leuven, Belgium. A-5021 {(1′S,2′R)-9-[1′,2′-bis(hydroxymethyl)-cycloprop-1′-yl]methyl]guanine} was from Ajinomoto, Inc., Kawasaki, Japan. Chemical structures of the investigational compounds are presented in Fig. 1.
FIG. 1.
Chemical structures of the investigational antiviral compounds.
Virus and cells.
A clinical isolate of human adenovirus type 2 (Ad2) (as determined by sequence analysis of the hexon gene) was obtained from an infant hospitalized for respiratory adenovirus infection at Hôpital St.-Pierre, Brussels, Belgium. Virus stocks were prepared in 25-cm2 flasks containing confluent human lung carcinoma A549 cells (American Type Culture Collection no. CCL-185). When ∼90% cytopathic effect (CPE) was apparent, Ad2-infected A549 cells were frozen at −80°C. After cell lysis by rapid thawing and centrifugation (10 min at 1,800 × g), the clarified culture supernatant was divided in aliquots and stored at −80°C. The Ad2 stock was titrated in A549 cells and human embryonic lung (HEL) fibroblast cells (American Type Culture Collection no. CCL-137) using a standard plaque assay (25), and the virus titers were found to be 2 × 1010 PFU/ml for A549 cells and 8 × 104 PFU/ml for HEL cells. The human osteosarcoma cell line deficient for cytosolic thymidine kinase (OST TK−) and a derived stable transfectant expressing herpes simplex virus type 1 thymidine kinase (OST TK−/HSV-1 TK+) have been previously described (8). All cell lines were subcultivated in minimal essential medium supplemented with 200 mM l-glutamine, 0.1% sodium bicarbonate, and 10% heat-inactivated fetal calf serum (fetal calf serum was reduced to a concentration of 2% for the antiviral studies). Cultures were incubated at 37°C in a humidified and CO2-controlled incubator.
Antiviral experiments in HEL cells using CPE and MTS assays or real-time PCR.
HEL cells were seeded in wells of 96-well plates at 10,000 cells per well and incubated for 4 or 5 days until confluency was reached. Fifty microliters of Ad2, diluted in medium to obtain a virus input of 5 PFU per well, was added to each well. After 2 h at 37°C, virus was aspirated and replaced by serial dilutions of the test compounds (200 μl per well). Mock-treated cultures receiving only the test compounds were included in each plate. After 10 to 12 days of incubation at 37°C, microscopy was performed to score the virus-induced CPE. The cells in the plates were then subjected to the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)-based colorimetric assay for cell viability according to the manufacturer's instructions (Promega, Leiden, The Netherlands). The A490 values, corrected for the cytotoxicity exerted by the test compounds (as determined in mock-infected cultures), were used to calculate the percent cell viability. The 50% effective concentration (EC50) was determined by extrapolation and defined as the compound concentration that produced 50% protection against the virus.
In a separate set of plates, HEL cells were infected and treated with the compounds as described above and used to measure virus replication 10 to 12 days after Ad2 infection. After removal of the culture supernatant, cells and virus particles were lysed by the addition of 70 μl of lysis buffer (10 mM Tris-HCl [pH 7.8], 0.5% sodium dodecyl sulfate [SDS], 5 mM Na2EDTA, 80 μg of proteinase K per ml) and incubated at 50°C for 1 h and then at 65°C for 20 min to inactivate proteinase K. After clarification (23,000 × g, 10 min), cell extracts were stored at −20°C until real-time PCR was performed. Extracts were diluted 100-fold in water. Two microliters of diluted extract was added to each well on optical plates containing 23 μl of SYBR green PCR master mix (Applied Biosystems, Foster City, Calif.), and the forward and reverse primers (300 μM) were added to the wells. The primers, derived from GenBank sequences, were chosen to amplify a 137-bp fragment in the conserved adenovirus hexon DNA sequence, allowing analysis of all known adenovirus types (forward primer, 5′-CGCTGGACATGACTTTTGAG-3′; reverse primer, 5′-GAACGGTGTGCGCAGGTA-3′).
Real-time PCR analysis was performed in an ABI Prism 7000 apparatus (Applied Biosystems) and consisted of 10-min activation at 95°C, followed by 40 thermal cycles, with 1 cycle consisting of 15 s at 95°C and 90 s at 60°C. A dissociation profile was taken at the end of each analysis to confirm the specificity of the PCR amplification. In each individual experiment, a standard curve (R2 >0.98 within the range of 103 to 108 copies per reaction mixture) was obtained by amplification of known amounts of a pGEM T-vector in which a 691-bp fragment of adenovirus hexon DNA was inserted using common cloning procedures. These standard curves were used to convert the cycle threshold values for the HEL extracts into the absolute number of adenovirus hexon DNA copies. The EC50was calculated by extrapolation as the compound concentration at which the number of viral DNA copies at 10 to 12 days postinfection (p.i.) was 50% compared to the value obtained for the virus control.
Cytotoxicity assays in HEL cells.
The cytotoxicities of the test compounds were determined in proliferating HEL cells, seeded at 4,000 cells per well in each well of 96-well trays, and treated with serial compound dilutions for 3 days. The cells were then counted with a Coulter Counter (1). The CC50 was defined as the compound concentration that produced 50% inhibition of cell proliferation.
Antiviral assays in A549 and OST cells using Western blot analysis.
One day prior to infection, cells were seeded in the wells of 12-well plates at a density of 400,000 cells per well for A549 cells and 200,000 cells per well for OST TK− and OST TK−/HSV-1 TK+. Ad2 was added at a high multiplicity of infection (MOI) (4 PFU/cell) and removed after 2 h of adsorption at 37°C. Medium containing serial dilutions of the test compounds was then added to the wells. After 48 h of incubation, cells were extracted with 100 μl of protein extraction buffer (20 mM Tris-HCl [pH 7.4], 0.137 M NaCl, 2 mM Na2EDTA, 1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 0.01 mg of leupeptin per ml). Samples were clarified (10 min at 23,000 × g) and analyzed by Bradford assay. Fifteen-microgram protein samples were mixed 4:1 with loading dye containing 0.25 M dithiothreitol, and the mixture was boiled for 3 min and finally loaded on 12% Tris-glycine SDS-polyacrylamide gels. After electrophoresis and electroblotting onto Hybond-P membrane (Amersham Biosciences, Buckinghamshire, United Kingdom), membranes were blocked by 5% fat-free powdered milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T) for 1 h and washed twice with PBS-T for 5 min each time. After 1-h incubation with 20 ng of primary antibody (mouse monoclonal antibody directed against human adenovirus fiber protein) (Ab-4 4D2; NeoMarkers, Fremont, Calif.) per ml and two washes with PBS-T, secondary antibody (horseradish peroxidase-linked goat anti-mouse immunoglobulin polyclonal antibody [Dako, Glostrup, Denmark]) was added for 45 min. After four final washes with PBS-T, protein bands were visualized by the ECL Plus detection system (Amersham Biosciences) and exposed to X-ray film. A single band representing the 62-kDa adenovirus fiber monomer was observed.
RESULTS
Optimal conditions for the CPE and MTS assays in HEL cells.
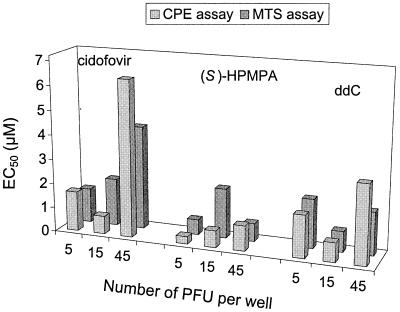
Adenovirus CPE in HEL fibroblast cells was visible after 7 to 12 days. Examining the CPE was simpler in HEL cells than in A549 cells, which are traditionally used for propagation of adenovirus. CPE in HEL cells could be monitored by microscopic examination, giving HEL cells an advantage over A549 cells for CPE-based antiviral experiments. When 90 to 100% CPE was reached, we measured cell viability by the formazan-based MTS assay. CPE and MTS assays both yielded similar antiviral EC50s for the three reference compounds, cidofovir, (S)-HPMPA, and ddC (Fig. 2). In examining the effect of MOI on antiviral activity, there was a trend toward increased EC50 as the virus input increased from 5 to 15 or 45 PFU per well. For instance, the EC50s of (S)-HPMPA were 0.3, 0.6, and 1.0 μM at virus inputs of 5, 15, and 45 PFU per well, respectively (Fig. 2). In the 45 PFU/well condition, 100% CPE was visible as early as 6 days p.i., while evaluation of the 5 PFU/well conditions took 10 to 12 days. The latter condition provided the best correlation between the EC50s obtained by the CPE and MTS assays. Therefore, we chose to perform all subsequent antiviral studies with a virus input of 5 PFU per well (which corresponds to an MOI of 0.0001 PFU per cell).
FIG. 2.
Influence of MOI on anti-Ad2 activity in HEL cells. The EC50s (in micromolar) of cidofovir, (S)-HPMPA, and zalcitabine (ddC) at a virus input of 5, 15, or 45 PFU per well were determined by CPE or MTS assay at 7 to 12 days p.i.
Antiviral assays using real-time PCR analysis.
Since the CPE and MTS assays measure indirect parameters for virus replication, we established a real-time PCR assay that allows direct quantitation of the viral progeny in adenovirus-infected HEL cells. First, we determined the optimal extraction conditions that combine easy and straightforward manipulations with high reproducibility. Adenovirus DNA was extracted from adenovirus-infected HEL cells by one of the following three procedures: total DNA extraction from the cells using a commercial DNA kit (QIAamp DNA Blood Mini kit; Qiagen); lysis of virus particles released in the culture supernatant; or lysis of intracellular virus particles (the two virus lysis methods used a buffer containing SDS). From these extracts, a 10-fold dilution series was prepared up to 1/1,000, and all dilutions were subjected to real-time PCR analysis of adenovirus hexon DNA using SYBR green. The extracts prepared by lysis of infected HEL cells proved clearly superior, since the number of adenovirus DNA copies showed an approximately 10-fold decrease with each dilution from 1/10 until 1/1,000 (data not shown). Thus, a 1/10 or 1/100 dilution is sufficient to neutralize the activity of PCR-inhibiting compounds present in these extracts. The performance of the procedure with a commercial DNA extraction kit was intermediate. Lysis of virus particles released in the culture supernatant of adenovirus-infected HEL cells was found to be inaccurate, since a 1/1,000 dilution did not reduce the activity of PCR inhibitors (probably originating from the serum fraction in the culture medium).
Next, we determined whether the extraction procedure by SDS-based lysis of infected cells was sufficiently reproducible. We first considered using an internal standard, such as β-actin genomic DNA, to correct for any inconsistencies in the extraction efficiency, but we calculated that extracts from 50,000 HEL cells, after 100-fold dilution, contained a maximum of 30 genomic β-actin DNA copies per PCR mixture, which is far below the detection limit by the SYBR green method. Therefore, we used a lysis procedure with sufficient extraction reproducibility to allow omission of an internal standard. Using the technique developed by Rasool et al. (22), we performed a triplicate extraction of Ad2-infected cells treated with different concentrations of cidofovir (Table 1). The number of adenovirus DNA copies per cell measured after 100-fold dilution and real-time PCR analysis showed good reproducibility, and an EC50of 1.58 μM for cidofovir was calculated. In the control (untreated) virus-infected cells, the number of adenovirus DNA copies measured 10 to 12 days after infection of HEL cells with 5 PFU per well was consistently 106 to 107 copies per cell.
TABLE 1.
Reproducibility of the real-time PCR method for quantitation of adenovirus DNA in HEL cellsa
| Cidofovir concn (μM) | No. of adenovirus DNA copies per cell (103)
|
% of VC | |||
|---|---|---|---|---|---|
| Extract 1 | Extract 2 | Extract 3 | Mean ± SD | ||
| 100 | 0.79 | 0.77 | 1.02 | 0.86 ± 0.14 | 0 |
| 20 | 54 | 42 | 97 | 64 ± 29 | 1 |
| 4 | 1,370 | 1,016 | 984 | 1,123 ± 214 | 13 |
| 0.8 | 7,065 | 5,594 | 6,974 | 6,544 ± 824 | 77 |
| None (VC) | 8,694 | 7,736 | 9,039 | 8,490 ± 675 | 100 |
| None (CC) | ND | ND | ND | ND | |
Ad2-infected HEL cells showing ≥90% CPE were lysed in lysis buffer (10 mM Tris-HCl [pH 7.8], 0.5% SDS, 5 mM Na2EDTA, 80 μg of proteinase K per ml) and heated (first for 1 h at 50°C and then for 20 min at 65°C). Extracts were diluted 100-fold in water and mixed with SYBR green PCR mix for real-time PCR analysis. Abbreviations: VC, virus control; CC, cell control; ND, not detected.
Antiadenovirus activities of nucleoside and nucleotide analogues.
Using the three optimized antiviral assays, we performed a detailed study on the antiadenovirus activities of several nucleoside and nucleotide analogues belonging to different chemical classes. A close correlation was observed between the EC50s obtained by the CPE, MTS, and real-time PCR assays (Table 2). The EC50s obtained for the acyclic nucleoside phosphonates cidofovir and (S)-HPMPA are close to the values reported previously, thus confirming the validity of our antiviral testing procedure (2, 15). The new derivative, (S)-HPMPO-DAPy (Fig. 1), was found to be threefold less active than cidofovir. The twofold-higher EC50obtained for the racemic mixture, (R,S)-HPMPO-DAPy, indicates that the (R)-enantiomer of HPMPO-DAPy has no appreciable antiadenovirus activity. We have previously reported on the lack of adenovirus activity for PMEO-DAPy, the 2-(phosphonomethoxy)ethoxy analogue which is identical to HPMPO-DAPy except that the acyclic chain does not contain a hydroxyl methylene group (13). This hydroxyl methylene group is present in cidofovir [(S)-HPMPC], (S)-HPMPA, and (S)-HPMPO-DAPy and appears to be a prerequisite for the antiadenovirus activity of the acyclic nucleoside phosphonates.
TABLE 2.
Activity against Ad2 in HEL cells
| Compound | EC50 (μM) for Ad2a
|
CC50 (μM)a | ||
|---|---|---|---|---|
| CPE assay | MTS assay | PCR assay | ||
| Acyclic nucleoside phosphonates | ||||
| Cidofovir | 2.0 ± 0.6 | 2.1 ± 1.1 | 3.1 ± 1.8 | 83 ± 25 |
| (S)-HPMPA | 0.66 ± 0.28 | 1.38 ± 0.67 | 0.63 ± 0.35 | 12 ± 8 |
| Cyclic HPMPC | 3.9 ± 0.6 | 4.1 ± 0.8 | 4.1 ± 1.1 | 344 ± 116 |
| (S)-HPMPO-DAPy | 5.4 ± 1.7 | 7.5 ± 2.8 | 10.0 ± 5.7 | 54 ± 34 |
| (R,S)-HPMPO-DAPy | 10 ± 0.2 | 14 ± 4 | 13 ± 4 | 170 |
| Acyclic nucleoside analogues | ||||
| Acyclovir | >1,000 | >1,000 | >500 | >750 |
| Ganciclovir | 39 ± 15 | 38 ± 17 | 35 ± 4 | 115 ± 14 |
| Penciclovir | ≥500 | >1,000 | ND | >750 |
| S-2242 | 0.57 ± 0.27 | 0.61 ± 0.35 | 1.2 ± 0.9 | >400 |
| Other nucleoside analogues | ||||
| A-5021 | 297 ± 122 | 267 ± 178 | 614 ± 321 | >175 |
| Brivudin | >1,000 | >1,000 | >500 | >500 |
| Ribavirin | >250 | >250 | >100 | 30 ± 18 |
| 2′,3′-Dideoxynucleoside analogues | ||||
| Zalcitabine (ddC) | 1.4 ± 0.4 | 2.0 ± 0.1 | 3.3 ± 1.0 | 231 ± 184 |
| Alovudine (FddT) | 0.80 ± 0.48 | 0.71 ± 0.65 | 3.2 ± 2.4 | ND |
| Lamivudine (3TC) | 125 ± 36 | 68 ± 62 | >100 | 175 ± 39 |
| Zidovudine (AZT) | >250 | >250 | >100 | 208 ± 107 |
| FddU | >100 | >100 | >100 | ND |
| Pyrophoshate analogue foscarnet | >1,000 | >1,000 | >500 | 500 |
Values are the means ± standard deviations from two to seven experiments. ND, not determined.
Among the antiherpetic compounds evaluated for antiadenovirus activity, only ganciclovir was found to be moderately active (EC50 of ∼35 μM). No inhibition of adenovirus replication was observed for acyclovir, penciclovir, brivudin, and foscarnet, while the investigational compound A-5021 (14) was effective only at concentrations exceeding 250 μM (Table 2). The most active compound in this series was the N7-substituted acyclic nucleoside analogue S-2242 (EC50of ∼1 μM), with a selectivity higher than that of cidofovir; the selectivity index, or the ratio of CC50 to EC50, of S-2242 was >400, and the selectivity index of cidofovir was 40 (Table 2). S-2242 has previously been reported to be active against all human herpesviruses and poxviruses. Its broad-spectrum anti-DNA virus activity can be explained by its activation by cellular kinases without the involvement of virus-encoded kinases (such as are present in herpesviruses but not in adenoviruses) (20, 21).
The 2′,3′-dideoxynucleoside analogues zalcitabine and alovudine displayed potent and selective activity against adenovirus; their EC50s were in the same range as those obtained for cidofovir and (S)-HPMPA (0.7 to 3 μM). The marked activity of zalcitabine and alovudine and inactivity of 5′-fluoro-2′,3′-dideoxyuridine, lamivudine, and zidovudine is in full agreement with other reports (19).
Finally, although ribavirin has been used for adenovirus infections in several case studies, this compound was found to be inactive in our in vitro studies, in line with antiviral results reported previously (2).
Antiadenovirus activity of ganciclovir in HSV TK-expressing cells.
The relatively low activity observed for ganciclovir in Ad2-infected HEL cells may be related to insufficient activation (phosphorylation) or inadequate inhibition at the level of the adenovirus DNA polymerase. Therefore, we conducted an antiviral study using conditions where ganciclovir is phosphorylated to very high levels, i.e., in human osteosarcoma cells transfected with the herpes simplex virus-encoded thymidine kinase (OST TK−/HSV TK+). The corresponding parent OST TK− cell line and A549 cells were included as controls, and cidofovir, which is not dependent on HSV TK for its activation, was included as a reference compound. Because of the short life span of the osteosarcoma cells, Ad2 infection was performed at a high MOI (4 PFU per cell) to enable determination of virus replication within 48 h after infection, using a Western blot assay for adenovirus fiber protein.
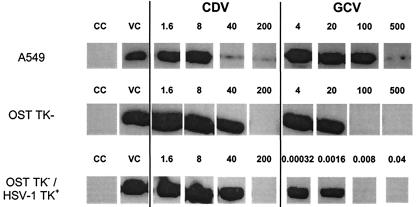
As shown in Table 3 and Fig. 3, both ganciclovir and cidofovir exerted a clear inhibition of Ad2 replication in all three cell lines. For instance, no adenovirus fiber band was detected with ganciclovir at concentrations of 100 and 0.008 μM in OST TK− and OST TK−/HSV TK+ cells, respectively. Virus was completely inhibited in both cell lines by cidofovir at a concentration of 200 μM (Fig. 3). The extremely high efficiency of ganciclovir phosphorylation in the OST TK−/HSV TK+ cells not only resulted in a 20,000-fold increase in antiviral activity but also in 5,000-fold higher cytotoxicity, thus explaining the relatively small gain in the selectivity index for the OST TK− and OST TK−/HSV TK+ cells (Table 3). The rather high EC50s are related to the high MOI used in these experiments; the difference in EC50s in the HEL assay (Table 2) and A549 assay (Table 3) were 5- and 10-fold for ganciclovir and cidofovir, respectively. In parallel, we also evaluated acyclovir and brivudin. Even at subtoxic concentrations, these two compounds produced no inhibition of adenovirus replication in A549, OST TK−, or OST TK−/HSV TK+ cells (Table 3).
TABLE 3.
Antiadenovirus activities of nucleoside analogues depending on herpesvirus thymidine kinase activation
| Compound | Anti-Ad2 activity (EC50 [μM]) by Western blottinga
|
Cytotoxicity (CC50 [μM]) by cell countinga
|
||||
|---|---|---|---|---|---|---|
| A549 | OST TK− | OST TK−/HSV-1 TK+ | A549 | OST TK− | OST TK−/HSV-1 TK+ | |
| Cidofovir | 21.0 ± 3.8 (25)b | 66.7 ± 31.0 (13) | 59.1 ± 35.9 (8) | 632 ± 273 | 868 ± 126 | 459 ± 262 |
| Ganciclovir | 206 ± 74 (10) | 53.5 ± 33.6 (3) | 0.00288 ± 0.00095 (11) | 2,030 ± 849 | 166 ± 91 | 0.0319 ± 0.0257 |
| Acyclovir | >1,000 | >1,000 | >0.2 | >5,000 | 97 | 0.195 |
| Brivudin | >1,000 | >1,000 | >0.1 | 1,795 | 1,437 | 0.142 |
Values are the means ± standard deviations from two to four independent experiments.
Selectivity index, or ratio of CC50 to EC50, is shown in parentheses.
FIG. 3.
Anti-Ad2 activities of cidofovir and ganciclovir in nontransfected or HSV-1 TK-transfected osteosarcoma cells. Western blot analysis of Ad2 fiber at 48 h after infection with 4 PFU per cell. A549 cells were included as a control. The concentrations (in micromolar) of cidofovir (CDV) and ganciclovir (GCV) are shown above the blots. Data shown are from one representative experiment. CC, uninfected cell control; VC, virus control.
DISCUSSION
Clinical reports on the impact of severe adenovirus infections in transplant recipients point to an increasing need for effective and safe antiadenovirus drugs. Small-scale studies have indicated that ribavirin is only poorly effective, particularly when therapy is initiated late in the course of infection (3, 9, 16). Cidofovir has produced an excellent antiviral response in several clinical studies and achieved a 69% success rate in a recent retrospective study in 45 bone marrow recipients (18). To overcome renal toxicity with cidofovir, it is recommended to perform coadministration of probenecid and sufficient hydration. A possible benefit of ganciclovir has been suggested by Bruno et al., who found the incidence of adenovirus infections in stem cell transplant recipients to be somewhat reduced in patients receiving ganciclovir for human cytomegalovirus (HCMV) prophylaxis (4).
While important progress in herpesvirus therapy and prophylaxis has been made during the past 10 years, adenovirus therapy has received insufficient attention in antiviral therapy or prophylaxis programs. From a survey of published studies, we concluded that the in vitro methodologies used for antiadenovirus testing are largely inconsistent. The three antiviral assays described here, which are based on a classical CPE assay, an MTS-based cell viability staining, and direct quantitation of virus progeny by real-time PCR, yielded very similar and reproducible antiviral EC50s. The real-time PCR assay can replace more-labor-intensive techniques, such as virus yield assays. Since the EC50s of cidofovir for adenovirus (∼2 μM) are in the same range as those for HCMV (23), cidofovir doses used for HCMV therapy may also be effective against adenovirus infections. The data reported here were based on experiments using a relatively low MOI with the common Ad2. We recently determined the activities of cidofovir against more than 20 adenovirus strains (either reference strains from the American Type Culture Collection or fresh clinical isolates) belonging to different subgroups (subgroup B, type 3; subgroup C, types 1, 2 and 6; and subgroup D, types 8 and 9). Cidofovir was found to be active against all serotypes studied, with EC50s ranging from 1 to 7 μM (mean, 3.5 μM) (unpublished data). These findings strongly suggest that the clinical efficacy of cidofovir is not dependent on the serotype.
We have demonstrated the marked antiadenovirus activity of the new acyclic nucleoside phosphonate analogue (S)-HPMPO-DAPy, although its activity is somewhat lower than those of cidofovir and its adenine analogue (S)-HPMPA. An intriguing finding is the inhibition of adenovirus DNA synthesis by 2′,3′-dideoxynucleoside analogues, as reported for zalcitabine (ddC) more than 20 years ago (27), and further documented by Mentel et al. (19). At this time, alovudine (FddT) is in phase II trials for human immunodeficiency virus (HIV) therapy. Since zalcitabine and alovudine have EC50s for HIV that are 5- to 50-fold lower than the EC50s for adenovirus, it is unlikely that these two compounds would adequately suppress adenovirus replication at the doses commonly used for treatment of HIV infections (7). Higher drug concentrations in plasma may be difficult to achieve safely, given the dose-dependent mitochondrial toxicity of ddC and FddT. However, the biochemical basis for their antiadenovirus effect (related to inhibition of adenovirus DNA polymerase) has not been studied in sufficient detail. The triphosphates of zalcitabine and alovudine are typical chain-terminating inhibitors of HIV reverse transcriptase, but their mode of interaction with the adenovirus DNA polymerase remains to be investigated. The same holds for ganciclovir triphosphate, since our studies in HEL cells and human osteosarcoma cells transfected with HSV TK clearly show that ganciclovir is active against adenovirus. However, this activity is minimized by the lack of a virus-encoded kinase in adenovirus-infected cells. The fairly high EC50 of ganciclovir against adenovirus (35 μM) is in the range of the maximum drug concentrations in plasma obtained after intravenous administration of ganciclovir (6). This may relate to the moderate efficacy of ganciclovir in the prophylaxis of adenovirus infections (4). Another interesting implication from our studies in HSV-1 TK+ osteosarcoma cells concerns the potential usefulness of ganciclovir in the context of gene therapy using adenovirus vectors with limited replication capacity (5). The safety of these vectors could be increased considerably by the incorporation of HSV-1 TK, since accidental recombination of the vector with a latent adenovirus in the patient would result in a replication-competent adenovirus that may be controlled by low doses of ganciclovir (29).
Momentum in the design of nucleoside analogues for antiadenovirus therapy is their ability to discriminate between the adenovirus DNA polymerase and cellular DNA polymerases. This is clearly the case for the N7-substituted purine derivative S-2242, which shows potent and selective activity against adenovirus due to its high phosphorylation efficiency by cellular kinases (20, 21). Akin to the acyclic nucleoside phosphonates and the dideoxynucleoside analogues, S-2242 points to the importance of the adenovirus DNA polymerase as an attractive target for antiadenovirus drug design and development, in addition to other possible targets, such as the adenovirus adsorption process (15).
Finally, although antiadenovirus drugs are most urgently needed for the treatment of severe systemic adenovirus infections, adenovirus keratoconjunctivitis (AKC) represents another clinical manifestation for which no antiviral drugs are available at this time. In animal models for AKC, cidofovir produced strong suppression of adenovirus replication, while ganciclovir was only marginally effective (10, 26). In patients, the inflammatory character of AKC should be taken into account when designing adequate measures to control this disease (11).
In conclusion, this study points to several new and old compounds besides cidofovir, viz., (S)-HPMPA, (S)-HPMPO-DAPy, S-2242, ddC, and FddT, as potential candidates for systemic and/or topical treatment of adenovirus infections, and these compounds should be examined further in both experimentally and clinically oriented studies.
Acknowledgments
We thank Evelyne Coussée for technical assistance.
These investigations were supported in part by grants from the Fonds voor Wetenschappelijk Onderzoek (FWO grant G.0267.04) and the Belgian (Flemish Community) Geconcerteerde Onderzoeksacties (GOA grant 2000/12). Liesbeth Lenaerts is a research assistant of the Fonds voor Wetenschappelijk Onderzoek.
REFERENCES
- 1.Andrei, G., R. Snoeck, D. Reymen, C. Liesnard, P. Goubau, J. Desmyter, and E. De Clercq. 1995. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur. J. Clin. Microbiol. Infect. Dis. 14:318-328. [DOI] [PubMed] [Google Scholar]
- 2.Baba, M., S. Mori, S. Shigeta, and E. De Clercq. 1987. Selective inhibitory effect of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and 2′-nor-cyclic GMP on adenovirus replication in vitro. Antimicrob. Agents Chemother. 31:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordigoni, P., A. S. Carret, V. Venard, F. Witz, and A. Le Faou. 2001. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 32:1290-1297. [DOI] [PubMed] [Google Scholar]
- 4.Bruno, B., T. Gooley, R. C. Hackman, C. Davis, L. Corey, and M. Boeckh. 2003. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol. Blood Marrow Transplant. 9:341-352. [DOI] [PubMed] [Google Scholar]
- 5.Chuah, M. K., D. Collen, and T. VandenDriessche. 2003. Biosafety of adenoviral vectors. Curr. Gene Ther. 3:527-543. [DOI] [PubMed] [Google Scholar]
- 6.Crumpacker, C. S. 1996. Ganciclovir. N. Engl. J. Med. 335:721-729. [DOI] [PubMed] [Google Scholar]
- 7.Daluge, S. M., D. J. Purifoy, P. M. Savina, M. H. St. Clair, N. R. Parry, I. K. Dev, P. Novak, K. M. Ayers, J. E. Reardon, G. B. Roberts, J. A. Fyfe, M. R. Blum, D. R. Averett, R. E. Dornsife, B. A. Domin, R. Ferone, D. A. Lewis, and T. A. Krenitsky. 1994. 5-Chloro-2′,3′-dideoxy-3′-fluorouridine (935U83), a selective anti-human immunodeficiency virus agent with an improved metabolic and toxicological profile. Antimicrob. Agents Chemother. 38:1590-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degrève, B., M. Johansson, E. De Clercq, A. Karlsson, and J. Balzarini. 1998. Differential intracellular compartmentalization of herpetic thymidine kinases (TKs) in TK gene-transfected tumor cells: molecular characterization of the nuclear localization signal of herpes simplex virus type 1 TK. J. Virol. 72:9535-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavin, P. J., and B. Z. Katz. 2002. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics 110:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Gordon, Y. J., E. G. Romanowski, and T. Araullo-Cruz. 1994. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Investig. Ophthalmol. Vis. Sci. 35:4135-4143. [PubMed] [Google Scholar]
- 11.Hillenkamp, J., T. Reinhard, R. S. Ross, D. Böhringer, O. Cartsburg, M. Roggendorf, E. De Clercq, E. Godehardt, and R. Sundmacher. 2002. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology 109:845-850. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman, J. A., A. J. Shah, L. A. Ross, and N. Kapoor. 2001. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 7:388-394. [DOI] [PubMed] [Google Scholar]
- 13.Holý, A., I. Votruba, M. Masojidkova, G. Andrei, R. Snoeck, L. Naesens, E. De Clercq, and J. Balzarini. 2002. 6-[2-(Phosphonomethoxy)alkoxy]pyrimidines with antiviral activity. J. Med. Chem. 45:1918-1929. [DOI] [PubMed] [Google Scholar]
- 14.Iwayama, S., N. Ono, Y. Ohmura, K. Suzuki, M. Aoki, H. Nakazawa, M. Oikawa, T. Kato, M. Okunishi, Y. Nishiyama, and K. Yamanishi. 1998. Antiherpesvirus activities of (1′S,2′R)-9-{[1′,2′-bis(hydroxymethyl)cycloprop-1′-yl]methyl}guanine (A-5021) in cell culture. Antimicrob. Agents Chemother. 42:1666-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko, H., K. Kato, S. Mori, and S. Shigeta. 2001. Antiviral activity of NMSO3 against adenovirus in vitro. Antivir. Res. 52:281-288. [DOI] [PubMed] [Google Scholar]
- 16.Lankester, A. C., B. Heemskerk, E. C. Claas, M. W. Schilham, M. F. Beersma, R. G. Bredius, M. J. van Tol, and A. C. Kroes. 2004. Effect of ribavirin on the plasma viral DNA load in patients with disseminating adenovirus infection. Clin. Infect. Dis. 38:1521-1525. [DOI] [PubMed] [Google Scholar]
- 17.Legrand, F., D. Berrebi, N. Houhou, F. Freymuth, A. Faye, M. Duval, J. F. Mougenot, M. Peuchmaur, and E. Vilmer. 2001. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 27:621-626. [DOI] [PubMed] [Google Scholar]
- 18.Ljungman, P., P. Ribaud, M. Eyrich, S. Matthes-Martin, H. Einsele, M. Bleakley, M. Machaczka, M. Bierings, A. Bosi, N. Gratecos, C. Cordonnier, and Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. 2003. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 31:481-486. [DOI] [PubMed] [Google Scholar]
- 19.Mentel, R., M. Kinder, U. Wegner, M. von Janta-Lipinski, and E. Matthes. 1997. Inhibitory activity of 3′-fluoro-2′ deoxythymidine and related nucleoside analogues against adenoviruses in vitro. Antivir. Res. 34:113-119. [DOI] [PubMed] [Google Scholar]
- 20.Neyts, J., and E. De Clercq. 2001. Efficacy of 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine for treatment of vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 45:84-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neyts, J., J. Balzarini, G. Andrei, Z. Chaoyong, R. Snoeck, A. Zimmermann, T. Mertens, A. Karlsson, and E. De Clercq. 1998. Intracellular metabolism of the N7-substituted acyclic nucleoside analog 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine, a potent inhibitor of herpesvirus replication. Mol. Pharmacol. 53:157-165. [DOI] [PubMed] [Google Scholar]
- 22.Rasool, N. B., S. S. Monroe, and R. I. Glass. 2002. Determination of a universal nucleic acid extraction procedure for PCR detection of gastroenteritis viruses in faecal specimens. J. Virol. Methods 100:1-16. [DOI] [PubMed] [Google Scholar]
- 23.Shigeta, S., K. Konno, M. Baba, T. Yokota, and E. De Clercq. 1991. Comparative inhibitory effects of nucleoside analogues on different clinical isolates of human cytomegalovirus in vitro. J. Infect. Dis. 163:270-275. [DOI] [PubMed] [Google Scholar]
- 24.Sircar, S., A. Ruzindana-Umunyana, W. Neugebauer, and J. M. Weber. 1998. Adenovirus endopeptidase and papain are inhibited by the same agents. Antivir. Res. 40:45-51. [DOI] [PubMed] [Google Scholar]
- 25.Tollefson, A. E., T. W. Hermiston, and W. S. M. Wold. 1999. Preparation and titration of CsCl-banded adenovirus stock, p. 1-9. In W. S. M. Wold (ed.), Adenovirus methods and protocols. Humana Press, Totowa, N.J.
- 26.Trousdale, M. D., P. L. Goldschmidt, and R. Nobrega. 1994. Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea 13:435-439. [DOI] [PubMed] [Google Scholar]
- 27.Van der Vliet, P. C., and M. M. Kwant. 1981. Role of DNA polymerase gamma in adenovirus DNA replication. Mechanism of inhibition by 2′,3′-dideoxynucleoside 5′-triphosphates. Biochemistry 20:2628-2632. [DOI] [PubMed] [Google Scholar]
- 28.Walls, T., A. G. Shankar, and D. Shingadia. 2003. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet. Infect. Dis. 3:79-86. [DOI] [PubMed] [Google Scholar]
- 29.Wildner, O., D. Hoffmann, C. Jogler, and K. Uberla. 2003. Comparison of HSV-1 thymidine kinase-dependent and -independent inhibition of replication-competent adenoviral vectors by a panel of drugs. Cancer Gene Ther. 10:791-802. [DOI] [PubMed] [Google Scholar]