Abstract
Human immunodeficiency virus type 1 (HIV-1) with a lysine-to-arginine substitution at codon 65 (HIV-165R) of reverse transcriptase (RT) can rapidly emerge in patients being treated with specific combinations of nucleoside analog RT inhibitors (NRTIs). A better understanding of the activity of approved and investigational NRTIs against HIV-165R is needed to select optimal therapy for patients infected with this mutant and to devise strategies to prevent its emergence. Therefore, we tested a broad panel of NRTIs that differed by enantiomer, pseudosugar, and base component against HIV-165R to determine how NRTI structure affects activity. Drug susceptibilities of recombinant wild-type (HIV-165K) or mutant HIV-165R were determined using a single-replication-cycle susceptibility assay with P4/R5 cells and/or a multiple-replication-cycle susceptibility assay with MT-2 cells. All d, l, and acyclic NRTIs were significantly less active against HIV-165R than against HIV-165K except for analogs containing a 3′-azido moiety. Pseudosugar structure and base component but not enantiomer influenced NRTI activity against HIV-165R. These findings support the inclusion of 3′-azido-3′-deoxythymidine in drug combinations to treat patients having HIV-165R and to prevent its emergence.
The lysine-to-arginine (AAA to AGA) mutation at codon 65 (K65R) in human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) has been selected in vitro by several nucleoside analog RT inhibitors (NRTIs), including zalcitabine (21), adefovir (3), tenofovir (18), (−)β-d-dioxolane-guanosine (d-DXG) (2), (+/-)-2′-deoxy-3′-oxa-4′-thio-5-fluorocytidine [(+/-)dOTFC] (13), (−)-2′-deoxy-3′-oxa-4′-thiocytidine [(−)DOTC], β-d-2′,3′-didehydro-3′-deoxycytidine (d-d4C), β-d-2′-fluoro-2′,3′-didehydro-2′3′-dideoxyadenine (d-2′F-d4A), β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (d-d4FC) (6, 10), and stavudine (5).
Paradoxically, the 65R substitution had been rarely observed in patients failing combination antiretroviral therapy. In recent clinical trials, however, HIV-165R has emerged in 24 to 92% of patients with treatment failure on regimens containing tenofovir and lamivudine combined with a third NRTI (abacavir or didanosine) or the nonnucleoside RT inhibitor efavirenz (C. Farthing, H. Khanlou, and V. Yeh, Abstr. 2nd IAS Conf. HIV Pathogenesis and Treatment, abstr. 43, 2003; M. D. Miller, N. A. Margot, D. J. McColl, T. Wrin, D. F. Coakley, and A. K. Cheng, Abstr. XII Int. HIV Drug Resist. Workshop, abstr. 135, 2003; J. Jemsek, P. Hutcherson, and E. Harper, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 51, 2004; R. Landman, G. Peytavin, D. Descamps, F. Brun Vezinet, H. Benech, A. Benalisherif, A. Trylesinski, C. Katlama, P. M. Girard, F. Raffi, P. Yeni, M. Bentata, B. Jarrousse, C. Michelet, P. Flandre, and the Tonus Study Group, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 52, 2004). The reason for the high frequency of 65R in these trials is not known but may be related to the exclusion of 3′-azido-3′-deoxythymidine (AZT) from the treatment regimens studied (19, 20).
The mechanism by which 65R causes reduced susceptibility to dideoxy NRTIs has recently been proposed. The wild-type lysine at residue 65 lies in the fingers subdomain of HIV-1 RT and interacts with the γ-phosphate of the deoxynucleotide triphosphate (dNTP) substrate, properly aligning it for incorporation into the nascent DNA chain. The larger arginine residue of 65R likely alters the position of the dNTP, decreasing its incorporation relative to wild-type RT (17). A hydrogen bond between the 3′ hydroxyl and one of the β-phosphate oxygen of the incoming dNTP may maintain proper positioning so that catalysis occurs, albeit at a lower rate than for wild-type RT. In the case of dideoxynucleoside triphosphates (ddNTP), which lack the 3′ hydroxyl, the hydrogen bond between the 3′ hydroxyl and the oxygen of the β-phosphate is absent such that proper positioning of the ddNTP is not maintained and catalysis is decreased further (15). As a consequence, 65R RT is likely to incorporate ddNTP less efficiently than dNTP, resulting in effective discrimination against ddNTP and resistance to these analogs (15, 17). Other studies have shown that RT with the 65R mutation is less susceptible to 5′-triphosphorylated 2′,3′-dideoxyinosine (ddI) and 2′,3′-dideoxycytidine (ddC) than to thymidine and guanosine analog triphosphates, but the influence of NRTI structure on activity against 65R RT is not well defined (1, 7, 8). Therefore, we investigated the structural features of NRTIs that affect their activity against HIV-165R. This is the first detailed analysis of the activity of NRTIs against HIV-165R by the use of a large panel of approved and investigational NRTI analogs that differ by base, enantiomer, and pseudosugar structure.
MATERIALS AND METHODS
Chemicals.
The following analogs were provided by Raymond Schinazi: β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (d-d4FC), β-l-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (l-d4FC), β-d-2′-fluoro, 2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (d-2′F-d4FC), β-l-2′-fluoro, 2′,3′-didehydro-2′,3′-dideoxy-5-cytidine (l-2′F-d4C), β-d-2′-fluoro-2′,3′-didehydro-2′3′-dideoxyadenine (d-2′F-d4A), β-(+)-2′,3′-dideoxy-3′-thiacytidine [(+)-BCH-189 or (+)-3TC], (−)-β-2′,3′-dideoxy-3′-thiacytidine [(−)-3TC], β-d-2′,3′-dideoxy-5-fluoro-3′-thiacytidine [(+)-FTC], β-l-2′,3′-dideoxy-5-fluoro-3′-thiacytidine [(−)-FTC], β-d-dioxolanefluorocytidine (d-DOFC), β-l-dioxolane-5-fluorocytidine (l-DOFC), β-d-dioxolanecytidine (d-DOC), β-l-dioxolanecytidine (l-DOC), β-d-dioxolaneguanosine (DXG), β-d-2′,3′-didehydro-2′3′-dideoxycytidine (d-d4C), β-d-3′-deoxy-2′,3′-didehydrothymidine (d4T), and 3′-azido-2′,3′-dideoxycytidine (AZC). 9-[2-(Phosphonomethoxy)]ethyladenine (PMEA [adefovir]) and 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA [tenofovir]) were provided by Gilead Sciences (Foster City, Calif.). 3′Fluoro-3′-deoxythymidine (FLT) and 3′fluoro-3′-deoxyguanosine (FLG) were obtained from Medivir AB (Huddinge, Sweden). 3′-azido-2′,3′-dideoxyadenine (AZA) and 3′-azido-2′,3′-dideoxyguanosine (AZG) were obtained from Trilink (San Diego, Calif.). 3′-Azido-3′-deoxythymidine (AZT) was obtained from Trilink and Sigma Chemical Corporation (St. Louis, Mo.). 2′,3′-Dideoxyinosine (ddI) and 2′,3′-dideoxycytidine (ddC) were also obtained from Sigma Chemical Corporation. 2′,3′-Dideoxythymidine (ddT) was obtained from Calbiochem (La Jolla, Calif.). β-d-2′-deoxy-3′-oxa-4′-thiocytidine [(−)-dOTC] and β-l-2′-deoxy-3′-oxa-4′-thiocytidine [(+)-dOTC] were obtained from Biochem Pharma (Laval, Quebec, Canada). (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1) (abacavir) was obtained from GlaxoWellcome (Research Triangle Park, N.C.). 2′,3′-Dideoxyguanosine (ddG) was obtained from ICN Biomedicals, Inc. (Irvine, Calif.).
The compounds were dissolved in dimethyl sulfoxide or sterile water as 10 mM or 30 mM stock solutions and stored at −20°C. Compounds were diluted immediately before use to the desired concentration in RPMI 1640 culture medium (Whittaker MA Bioproducts, Walkersville, Md.) or Dulbecco's modified Eagle medium, Phenol Red Free (DMEM-PRF: Gibco-BRL, Grand Island, N.Y.). Pseudosugar structures were drawn using ChemDraw Ultra 7.0.
Cells.
MT-2 cells (AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, Utah), 10 mM HEPES buffer, 50 IU of penicillin/ml, and 50 μg of streptomycin/ml. The P4/R5 reporter cell line (provided by Ned Landau, Salk Institute, La Jolla, Calif.) is a HeLa cell line stably transfected with a Tat-activated β-galactosidase gene under the control of an HIV-long terminal repeat promoter. P4/R5 cells were cultured in DMEM-PRF supplemented with 10% FBS, 50 IU of penicillin/ml, 50 μg of streptomycin/ml, and 0.5 μg of puromycin (Clontech, Palo Alto, Calif.)/ml. 293T cells (provided by John Julias and Steven Hughes, National Cancer Institure, Frederick, Md.) were maintained in DMEM supplemented with 5% FBS, 5% fetal calf serum (HyClone), 50 IU of penicillin/ml, and 50 μg of streptomycin/ml.
Generation of mutant recombinant HIV-1.
Mutations in RT were introduced by site-directed mutagenesis using an Altered Sites II kit (Promega, Madison, Wis.). Silent 5′XmaI and 3′XbaI restriction sites in the xxRT clone (16) facilitated subcloning of the mutated RT fragment into the pxxHIV-1LAI clone, which was then used to generate infectious virus.
Viruses.
Stock viruses were prepared using the xxHIV-1LAI clones with a 65K or 65R mutation by electroporating (Gene Pulser; Bio-Rad, Hercules, Calif.) 5 to 10 μg of plasmid DNA into 1.3 × 107 MT-2 cells. At 7 days posttransfection, cell-free supernatant was harvested and stored at −80°C. Alternatively, 5 to 10 μg of plasmid DNA was transfected into 293T cells by the use of a calcium phosphate solution (2 M CaCl2, 1.37 M NaCl, 0.05 M KCl, 0.007 M Na2HPO4·7H2O, 0.06 M dextrose, 1 M HEPES). Transfected cells were washed with phosphate-buffered saline at 8, 24, and 32 h posttransfection. Cell-free supernatant was harvested 48 to 52 h posttransfection, and aliquots were stored at −80°C. The genotype of stock viruses was confirmed by extracting RNA from virions (QIAamp kit; QIAGEN, Valencia, Calif.), treating the extract with DNase I (Roche, Indianapolis, Ind.), amplifying the full-length coding region (amino acids 1 to 550) of RT by RT-PCR, purifying the PCR product (Wizard PCR purification system; Promega), and sequencing the PCR product using a Big Dye terminator kit (v. 3.1) on an ABI 3100 automated DNA sequencer (Applied Biosystems, Foster City, Calif.). The 50% tissue culture infective dose (TCID50) for the virus stock was determined for MT-2 cells or P4/R5 cells by threefold endpoint dilution assays (six wells per dilution) and calculated using the Reed and Muench equation (12).
Single-replication-cycle drug susceptibility assay.
In a 96-well plate, two- or threefold serial dilutions of an inhibitor were added to P4/R5 cells in triplicate. The cells were then infected at a multiplicity of infection of 0.05 as determined by endpoint dilution in P4/R5 cells. Alternatively, cells were infected with the amount of virus that would yield a relative light unit value of 100 in the no-drug, virus-infected control wells. At 48 h postinfection, a cell lysis buffer and luminescent substrate (Gal-Screen; Tropix/Applied Biosystems) was added to each well, and relative light unit values were determined using a luminometer (ThermoLabSystems, Waltham, Mass.). Inhibition of virus replication was calculated as the concentration of compound required to inhibit virus replication by 50% (IC50). Fold resistance values were determined by dividing the IC50 for mutant HIV-1 by the IC50 for wild-type HIV-1.
Multiple-replication-cycle drug susceptibility assay.
In a 96-well plate, threefold serial dilutions of an inhibitor were added to MT-2 cells in triplicate. The cells were infected at a multiplicity of infection of 0.01 as determined by endpoint dilution in MT-2 cells. At 7 days postinfection, culture supernatants were harvested and treated with 0.5% Triton X-100. The p24 antigen concentration in the supernatants was then determined using a commercial enzyme-linked immunosorbent assay (DuPont, NEN Products, Wilmington, Del.). IC50 and severalfold-resistance values were calculated as described above.
Statistical analysis.
IC50 values from at least three independent experiments were log10 transformed and compared using a two-sample Student's t test. P values less than 0.05 were considered to be statistically significant.
RESULTS
Activity of FDA-approved NRTIs against HIV-165R.
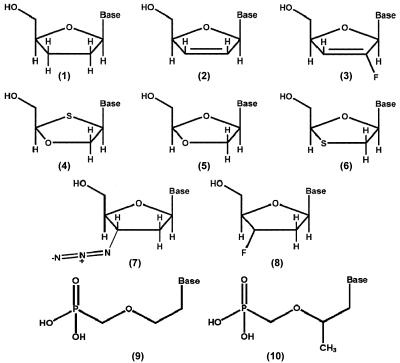
We first studied the activity of the eight Food and Drug Administration (FDA)-approved NRTIs against HIV-165R; among these NRTIs was PMEA (adefovir), which is approved for treatment of hepatitis B virus infection but also has activity against HIV-1. In the single-replication-cycle susceptibility assay using P4/R5 cells, each of the approved NRTIs showed significantly reduced activity against HIV-165R except for AZT (Table 1). Fold decreases in activity against HIV-165R ranged from 2.5-fold for tenofovir to 77-fold for 3TC. AZT was similarly active against HIV-165R and wild-type HIV-165K (1.1-fold decrease). Similar results were obtained with the multiple-replication-cycle susceptibility assay using MT-2 cells (Table 1). The approved NRTIs studied differ by stereochemistry (3TC and FTC have the l enantiomeric configuration; all others have the d configuration), by base (AZT and d4T are thymidine analogs, 3TC and ddC are cytidine analogs, ddI and tenofovir are adenine analogs, and abacavir (ABC) is a guanosine analog), and by pseudosugar structure. PMPA and PMEA both have acyclic pseudosugar structures (Fig. 1).
TABLE 1.
Activity of FDA-approved NRTIs against HIV-1 RT with the K65R mutationc
| Compound | IC50 by single-cycle replication assaya
|
IC50 by multiple cycle replication assayb
|
||||
|---|---|---|---|---|---|---|
| HIV-1WT | HIV-165Rd | P | HIV-1WT | HIV-165Rd | P | |
| AZT (zidovudine) | 0.20 ± 0.12 | 0.22 ± 0.16 (1.1) | 0.77 | 0.031 ± 0.024 | 0.028 ± 0.009 (0.9) | 0.58 |
| d4T (stavudine) | 5.72 ± 3.34 | 21.8 ± 23.8 (3.8) | <0.001 | 1.87 ± 0.76 | 3.93 ± 1.34 (2.1) | <0.05 |
| ddC (zalcitabine) | 1.35 ± 0.70 | 6.79 ± 3.98 (5.0) | <0.0001 | 0.13 ± 0.18 | 0.29 ± 0.23 (2.3) | 0.01 |
| ddI (didanosine) | 2.32 ± 0.98 | 6.26 ± 2.92 (2.7) | <0.0001 | 0.49 ± 0.32 | 2.37 ± 1.80 (4.8) | <0.0001 |
| ABC (abacavir) | 6.21 ± 1.66 | 26.3 ± 4.18 (4.2) | <0.0001 | 0.076 ± 0.010 | 0.60 ± 0.11 (7.9) | <0.0001 |
| 3TC (lamivudine) | 0.78 ± 0.48 | 60.5 ± 66.8 (77) | <0.0001 | 0.60 ± 0.46 | 15.3 ± 10.8 (25) | <0.0001 |
| FTC (emtricitabine) | 0.17 ± 0.07 | 3.75 ± 1.75 (22) | <0.0001 | 0.044 ± 0.036 | 0.90 ± 0.42 (20) | <0.001 |
| PMPA (tenofovir) | 4.67 ± 2.31 | 11.4 ± 3.8 (2.5) | <0.0001 | 0.54 ± 0.61 | 3.82 ± 1.91 (7.1) | <0.005 |
| PMEA (adefovir)e | 6.21 ± 0.91 | 23.3 ± 7.9 (3.7) | <0.001 | Not tested | Not tested | Not tested |
IC50 values determined by measuring inhibition of luminescence in P4/R5 cells.
IC50 values determined by measuring inhibition of p24 production in MT-2 cells.
Means ± standard deviations from at least three independent experiments.
Fold increase in IC50 relative to wild-type results are shown in parentheses.
Approved for treatment of hepatitis B virus but not HIV-1.
FIG. 1.
Pseudosugar structures of NRTIs tested. (1) 2′,3′-Dideoxynucleosides (ddC, ddT, ddI, ddG); (2) 2′,3′-didehydro-2′3′-dideoxynucleosides (d4C, d4T); (3) 2′-fluoro-2′,3′-didehydro-2′3′-dideoxynucleosides (2′-F-d4FC, 2′-F-d4A); (4) 2′-deoxy-3′-oxa-4′-thiocytidines (dOTC); (5) dioxolane-nucleosides (DOC, DXG); (6) 2′,3′-dideoxy-3′-thiacytidines (3TC, FTC); (7) 3′-azido-3′-deoxynucleosides (AZT, AZC, AZG, AZA); (8) 3′-fluoro-3′deoxynucleosides (FLT, FLG); (9) 9-[2-(phosphonomethoxy)ethyl nucleotide (PMEA); (10) 9-[(R)-2-(phosphonomethoxy)propyl] nucleotide (PMPA).
Activity of investigational NRTIs against HIV-165R.
The reduced activity of the approved NRTIs against HIV-165R led us to further explore the structural features of NRTIs that influence activity against this mutant. A panel of 30 NRTIs that differed by stereochemistry, base, and pseudosugar structure were tested against HIV-165R and wild-type HIV-165K. All compounds were tested in the single-cycle susceptibility assay; compounds that were not active in P4/R5 cells were tested in the multiple-cycle assay using MT-2 cells.
Figure 1 shows the pseudosugar structures of the investigational NRTIs studied. All lack a 3′ OH group (structures 1 to 11); the 3′ OH has been replaced by a hydrogen (structure 1), a double bond (structures 2 and 3), an azido group (structure 7), or a fluorine (structure 8). In structures 4 to 6, the 3′ carbon of the pseudosugar ring has been replaced by an oxygen (4 and 5) or a sulfur (structure 6). DOTC (structure 4) also has a sulfur replacing the normal oxygen of the pentose ring. The enantiomeric configurations of the analogs (d versus l) and the base components linked to the pseudosugar also differ; bases include adenine, cytosine, guanine, thymine, inosine, 5-fluorocytosine (FC), and 2-amino-6-(cyclopropylamino)-purine (abacavir).
Activity of NRTIs differing by base component against HIV-165R.
HIV-165R was tested against dideoxy compounds that differed only by base component: ddC, ddG, ddI, and ddT. The activity of ddC, ddG, ddI, and ddT against HIV-165R was decreased 5.0-, 4.0-, 2.7-, and 2.2-fold, respectively, compared to wild-type HIV-165K results (Table 2), indicating a moderate influence of base component on NRTI activity. Several compounds differing only by cytosine fluorination, including both d-enantiomers (d-d4C versus d-d4FC and d-DOC versus d-DOFC) and l-enantiomers (l-DOC versus l-DOFC and (−)-3TC versus (−)-FTC), were tested. Fluorination of cytosine did not significantly affect activity against HIV-165R (P > 0.05) (Table 1, Table 2, and Table 3).
TABLE 2.
In vitro activity of analogs with dideoxy and D4 pseudosugars against HIV-1 RT with the K65R mutation
| Compounda | Mean IC50 (μM) ± SDb
|
P | ||
|---|---|---|---|---|
| HIV-1WT | HIV-165R | Fold increasec | ||
| ddC | 1.35 ± 0.70 | 6.79 ± 3.98 | 5.0 | <0.0001 |
| ddG | 3.11 ± 0.64 | 12.5 ± 1.5 | 4.0 | <0.0001 |
| ddI | 2.32 ± 0.98 | 6.26 ± 2.92 | 2.7 | <0.0001 |
| ddTd | 10.2 ± 4.8 | 22.3 ± 9.3 | 2.2 | 0.01 |
| d4C | 2.63 ± 1.20 | 17.9 ± 6.4 | 6.8 | <0.0001 |
| d4T | 5.72 ± 3.34 | 21.8 ± 23.8 | 3.8 | <0.001 |
| d4FC | 1.26 ± 0.51 | 14.6 ± 7.1 | 12 | <0.0001 |
| 2′-F-d4A | 2.19 ± 0.91 | 4.35 ± 1.4 | 2.1 | <0.01 |
| 2′-F-d4FCd | 7.35 ± 5.6 | 84.5 ± 9.6 | 11.5 | 0.001 |
| L-d4FC | 0.23 ± 0.14 | 1.20 ± 0.17 | 5.2 | 0.005 |
Compounds are d-enantiomers except for L-d4FC.
Means ± standard deviations from at least three independent experiments. All assays were done with P4/R5 cells and a single-replication-cycle assay unless otherwise noted.
Fold increase in IC50 relative to wild-type results.
Assay done with MT-2 cells (see Materials and Methods).
TABLE 3.
In vitro activity of d- and l-enantiomers of analogs with 3′ oxygen or 3′ sulfur substitutions against HIV RT with the K65R mutation
| Compound | Mean IC50 (μM) ± SDa
|
P | ||
|---|---|---|---|---|
| HIV-1WT | HIV-165R | Fold increaseb | ||
| d-enantiomers | ||||
| (+)-3TC | 1.20 ± 0.56 | 32.9 ± 10 | 27 | <0.0001 |
| (+)-FTC | 11.6 ± 3.4 | >90 | 7.7 | <0.0001 |
| D-DOC | 0.34 ± 0.07 | 3.57 ± 1.4 | 11 | <0.0001 |
| D-DOFC | 0.89 ± 0.56 | 5.41 ± 1.6 | 6.1 | <0.0001 |
| (−)-DOTCc | 6.34 ± 1.74 | 65.5 ± 37 | 10 | <0.01 |
| DXG | 3.60 ± 0.31 | 21.8 ± 8.2 | 6.0 | <0.005 |
| FLG | 1.02 ± 0.41 | 4.05 ± 0.89 | 4.0 | <0.005 |
| FLT | 0.18 ± 0.05 | 0.41 ± 0.03 | 2.3 | <0.005 |
| l-enantiomers | ||||
| (−)3TC | 0.78 ± 0.48 | 60.5 ± 66.8 | 77 | <0.0001 |
| (−)-FTC | 0.17 ± 0.07 | 3.75 ± 1.75 | 22 | <0.0001 |
| L-DOC | 0.082 ± 0.04 | 0.66 ± 0.18 | 8.1 | <0.001 |
| L-DOFC | 0.032 ± 0.01 | 0.34 ± 0.21 | 10 | <0.0005 |
| (+)-DOTCc | 11.2 ± 4.4 | >90 | 8.1 | <0.001 |
Means ± standard deviations from at least three independent experiments.
Fold increase in IC50 relative to wild-type results.
Assay done with MT-2 cells (see Materials and Methods).
Activity of NRTIs differing by enantiomer against HIV-165R.
All l-enantiomeric NRTIs tested had lower activity against HIV-165R than against the wild type; these NRTIs included (−)-3TC (77-fold), (−)-FTC (22-fold), l-DOFC (10-fold), (+)-DOTC (8.1-fold), l-DOC (8.1-fold) and l-d4FC (5.2-fold). The corresponding d-enantiomeric compounds (+)-3TC, (+)-FTC, d-DOFC, (−)-DOTC, d-DOC, and d-d4FC all had reductions in activity similar to those seen with their l-enantiomer counterparts (Table 2 and 3), indicating that the enantiomer did not affect activity against HIV-165R.
Activity against HIV-165R of NRTIs differing by pseudosugar component.
The dideoxy compounds differ from natural nucleosides only by a replacement of the 3′ hydroxyl by a 3′ hydrogen. Dideoxy compounds were 3.4-fold less active on average against HIV-165R compared with wild-type HIV-165K results. Replacing the 3′ hydrogen with a double bond in d4 compounds further reduced activity against HIV-165R by an average of 6.7-fold. Adding a 2′ fluorine to d-d4FC did not significantly affect activity against HIV-165R (d-d4FC, 12-fold decrease; d-2′F-d4FC, 11.5-fold decrease). However, altering the base from a cytosine to an adenine (d-2′F-d4FC versus d-2′F-d4A) significantly (P < 0.0005) increased activity against HIV-165R; d-2′F-d4FC was 11.5-fold less active against HIV-165R, whereas d-2′F-d4A was only 2.1-fold less active. This provides further evidence that the base component can affect NRTI activity against HIV-165R.
Dioxolanes contain an oxygen in place of the 3′ carbon in the pseudosugar ring. 3TC and FTC are oxathiolane analogs that contain a 3′ sulfur in the pseudosugar ring structure. Compounds with a substitution of oxygen or sulfur for the 3′ carbon showed the greatest loss of activity against HIV-165R. Both enantiomeric forms of 3TC showed a >20-fold loss of activity (Table 3). (−)-FTC showed a >20-fold loss of activity against HIV-165R, and (+)-FTC had no activity against HIV-165R at the highest concentration tested (90 μM) The dioxolanes also showed decreased activity: d-DOC (11-fold decrease), (−)-DOTC (10-fold), d-DOFC (6.1-fold), and d-DXG (6-fold).
FLT and FLG have a 3′ fluorine in place of the 3′ hydroxyl. FLG and FLT were 4.0-fold and 2.3-fold less active, respectively, against HIV-165R than against wild-type HIV-165K (Table 3). The 3′ fluorines in FLT and FLG have positions and negative charges similar to those of the natural 3′ hydroxyl group, which may explain the relative preservation of their activity against HIV-165R.
Activity of 3′-azido analogs against HIV-165R.
3′-azido-containing analogs were the only compounds that did not show consistent loss of activity against HIV-165R (Table 4). AZT and AZA showed very little loss of activity against HIV-165R (1.1- and 0.9-fold, respectively). AZG and AZC were 2.0- and 2.5-fold less active, respectively, against HIV-165R than against HIV-165K, but this reduction in activity was not significant (P = 0.09 for AZG) or showed borderline significance (P = 0.05 for AZC).
TABLE 4.
In vitro activity of 3′-azido analogs against HIV-1 RT with the K65R mutation
| Compound | Mean IC50 (μM) ± SDa
|
P | ||
|---|---|---|---|---|
| HIV-1WT | HIV-165R | Fold changeb | ||
| AZA | 3.92 ± 1.1 | 3.72 ± 0.30 | 0.9 | 0.89 |
| AZGc | 7.90 ± 4.63 | 15.8 ± 9.8 | 2.0 | 0.09 |
| AZCc | 15.0 ± 10.7 | 37.8 ± 28.5 | 2.5 | 0.05 |
| AZT | 0.20 ± 0.12 | 0.22 ± 0.16 | 1.1 | 0.77 |
Means ± standard deviations from at least three independent experiments.
Fold change relative to wild-type results.
Assay done using MT-2 cells.
DISCUSSION
Prior studies have shown that the K65R mutation in HIV-1 RT decreases susceptibility to various NRTIs, including ddC, PMEA, PMPA, d-DXG, (+/-)dOTC, d-d4FC, and d4T (2, 3, 5, 6, 13, 18, 21). Our study was the first to systemically analyze the structural components of NRTIs that influence activity against HIV-165R by the use of the same susceptibility assays. We found that all d and l and acyclic NRTIs tested had significantly reduced activity against HIV-165R except those containing a 3′-azido moiety (AZT and AZA). In addition, the structural features of NRTIs that influence activity were identified.
The pseudosugar structure had the greatest impact on activity against HIV-165R. Comparing cytidine analogs that differed only by pseudosugar structure, the greatest loss of activity was seen with (+)-3TC (77-fold), whereas the least was seen with ddC (5.0-fold). The rank order of greatest to least loss of activity against HIV-165R for d or (+) cytidine pseudosugars is as follows: 3TC ≅ FTC > dOTC > DOC > d4C > ddC. The fold resistance for 3TC was significantly (P ≤ 0.05) different from DOC, d4C and ddC results, with a trend towards significance for dOTC (P = 0.06). The fold resistance results for dOTC and DOC were also significantly (P < 0.05) different from ddC results. A similar pattern was seen for different pseudosugars with other base components, although the differences in severalfold resistance were not significant. For example, DXG showed greater loss of activity than ddG (6.0-fold versus 4.0-fold), as did d4T (3.8-fold) compared with ddT (2.2-fold). A conclusion cannot be made for DXT (data not shown), because this compound was poorly active in both P4/R5 cells and MT-2 cells.
The role of HIV-165R in resistance to d4T has not been well defined. In our study, HIV-165R did exhibit significantly reduced susceptibility to d4T in P4/R5 cells (3.8-fold; P < 0.001) and in MT-2 cells (2.1-fold; P < 0.05). The recent report by J. G. Garcia-Lerma indicates that 65R can be selected by D4T in vitro (5). In addition, 65R has been reported to be selected in two clinical trials of d4T-containing regimens that excluded tenofovir. In the Gilead 903 study, two patients (0.7%) who failed d4T/3TC/efavirenz therapy had virus with a 65R mutation (4), and in a Danish study, five out of eight patients who failed d4T/ddI/ABC therapy had virus with a 65R mutation (14). Collectively, these data support the view that the 65R mutation reduces susceptibility to D4T and that this reduction in susceptibility can be clinically relevant.
As noted above, (+)- or (−)-3TC and (+)- or (−)-FTC showed the greatest loss of activity against HIV-165R. In addition, a greater than 10-fold loss of activity was also observed for most of the dioxolane compounds and the dioxathiolane dOTC. This suggests that the 65R mutation in RT preferentially interferes with incorporation of analogs containing pseudosugars with 3′ oxygen or sulfur components. The 3′ oxygen or sulfur, which is larger and more negatively charged than the normal 3′ carbon, may distort the positioning of the analog triphosphates to a greater extent than for other pseudosugars. Further, the 3′ hydroxyl of the incoming dNTP makes a stabilizing intramolecular hydrogen bond with one oxygen of the β-phosphate (15). The lack of the 3′ hydroxyl group and the negatively charged oxygen or sulfur at the 3′ position is likely to further distort the configuration of the analog triphosphate. As a consequence, natural dNTPs are likely to be much more efficiently incorporated by HIV65R RT than 3′ oxygen- or sulfur-substitution analogs, leading to high-level resistance to these analogs.
Our results show that the base component has a moderate effect on NRTI activity against HIV-165R. Comparing compounds that were structurally identical except for base component shows that the rank order of greatest to least loss of activity is C > G > A > T. For example, the rank order of activity loss for the dideoxy compounds is ddC > ddG > ddI > ddT. However, only the differences in activity of ddC versus ddI or ddT are statistically significant (P = 0.02). Although not all the possible variations of base component for other pseudosugar structures are active against HIV-1, a similar pattern is apparent for the active analogs tested. Specifically, the loss of activity was greater for d-DOC than for d-DXG (P = 0.02), for FLG than for FLT (P = 0.04), and for d4C than for d4T (P = 0.13). Biochemical studies have showed a similar trend with HIV65R RT, showing greater discrimination against ddCTP than against ddTTP (15, 17). The influence of base component on activity against HIV-165R is unexplained but may be mediated through differences in binding affinity or position of the analog in the substrate binding site.
Stereochemical differences (l- versus d-enantiomer) did not influence NRTI activity against HIV-165R. Six sets of compounds that differed only by stereochemistry were tested: (+)- and (−)-3TC, (+)- and (−)-FTC, l- and d-DOFC, l- and d-dOTC, l- and d-DOC, and l- and d-d4FC. The loss of activity was not significantly (P > 0.5) different for any enantiomeric pair. One hypothesis to explain this is that altering the enantiomer from d to l does not further affect the position of the 5′ triphosphates of the analog, which are linked to the pseudosugar by flexible single bonds.
AZT and AZA showed no loss of activity against HIV-165R, whereas AZG and AZC showed a trend toward a significant loss of activity (2.0- and 2.5-fold decrease, respectively). Thus, the base component has a role in the activity of the 3′-azido-containing compounds, with C and G analogs showing a greater loss of activity than A or T analogs, as was observed with other pseudosugar structures. The relative preservation of activity of 3′-azido analogs may be explained by interaction of the 3′-azido with the β- and γ-phosphates of the analog triphosphate, restoring the correct configuration of the analog for incorporation. RT with a 65R mutation has reduced excision capability compared to the wild type, allowing AZT to remain incorporated (11; U. Parikh, D. Koontz, N. Sluis-Cremer, J. Hammond, L. Bacheler, R. Schinazi, and J. Mellors, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 54, 2004). The decreased excision capacity of 65R may help explain the preservation of activity of compounds with 3′-azido groups against HIV-165R. The use of AZT in combination with other NRTIs is likely to prevent the emergence of HIV-165R, because this substitution does not confer a selective advantage for the virus in the presence of AZT. Recent clinical data support this hypothesis (9, 19, 20; R. Elion, C. Cohen, E. DeJesus, R. Redfield, J. Gathe, R. Hsu, L. Yau, L. Ross, B. Ha, R. Lanier, T. Scott, and the COL40263 Study Team, Abst. 11th Conf. Retrovir. Opportunistic Infect., abstr. 53, 2004).
In summary, the NRTI structures that retain activity against HIV-165R are those having a thymine or adenine base and a 3′-azido component. NRTIs with the 3′ carbon replaced by a sulfur or oxygen have the least activity in either the d or l conformation. The data presented provide evidence that 65R is a multi-NRTI-resistance mutation and support the use of AZT in drug combinations to treat patients with HIV-165R and to prevent its emergence.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (U01AI38858) and from the National Cancer Institute (SAIC contract 20XS190A). R.F.S. is supported by National Institutes of Health grants R37-AI-41980 and 2P30-AI-50409 and by the Department of Veterans Affairs. R.F.S. and C.K.C. are also supported by National Institutes of Health grant R01-AI-32351.
R.F.S. is the principal founder and shareholder in Pharmasset, Inc., and will receive future royalties from sales of D-d4FC (Reverset). J.W.M. is a consultant to and holds share options in Pharmasset, Inc.
REFERENCES
- 1.Arion, D., G. Borkow, Z. Gu, M. A. Wainberg, and M. A. Parniak. 1996. The K65R mutation confers increased DNA polymerase processivity to HIV-1 reverse transcriptase. J. Biol. Chem. 271:19860-19864. [DOI] [PubMed] [Google Scholar]
- 2.Bazmi, H. Z., J. L. Hammond, S. C. Cavalcanti, C. K. Chu, R. F. Schinazi, and J. W. Mellors. 2000. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (−)-β-d-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob. Agents Chemother. 44:1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foli, A., K. M. Sogocio, B. Anderson, M. Kavlick, M. W. Saville, M. A. Wainberg, Z. Gu, J. M. Cherrington, H. Mitsuya, and R. Yarchoan. 1996. In vitro selection and molecular characterization of human immunodeficiency virus type 1 with reduced sensitivity to 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA). Antivir. Res. 32:91-98. [DOI] [PubMed] [Google Scholar]
- 4.Gallant, J. E., S. Staszewski, A. L. Pozniak, E. DeJesus, J. M. Suleiman, M. D. Miller, D. F. Coakley, B. Lu, J. J. Toole, A. K. Cheng, and the 903 Study Group. 2004. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191-201. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lerma, J. G., H. MacInnes, D. Bennett, P. Reid, S. Nidtha, H. Weinstock, J. E. Kaplan, and W. Heneine. 2003. A novel genetic pathway of human immunodeficiency virus type 1 resistance to stavudine mediated by the K65R mutation. J. Virol. 77:5685-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geleziunas, R., K. Gallagher, H. Zhang, L. Bacheler, S. Garber, J. T. Wu, G. Shi, M. J. Otto, R. F. Schinazi, and S. Erickson-Viitanen. 2003. HIV-1 resistance profile of the novel nucleoside reverse transcriptase inhibitor beta-d-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine (Reverset). Antivir. Chem. Chemother. 14:49-59. [DOI] [PubMed] [Google Scholar]
- 7.Gu, Z., E. J. Arts, M. A. Parniak, and M. A. Wainberg. 1995. Mutated K65R recombinant reverse transcriptase of human immunodeficiency virus type 1 shows diminished chain termination in the presence of 2′,3′-dideoxycytidine 5′-triphosphate and other drugs. Proc. Natl. Acad. Sci. USA 92:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu, Z., R. S. Fletcher, E. J. Arts, M. A. Wainberg, and M. A. Parniak. 1994. The K65R mutant reverse transcriptase of HIV-1 cross-resistant to 2′,3′-dideoxycytidine, 2′,3′-dideoxy-3′-thiacytidine, and 2′,3′-dideoxyinosine shows reduced sensitivity to specific dideoxynucleoside triphosphate inhibitors in vitro. J. Biol. Chem. 269:28118-28122. [PubMed] [Google Scholar]
- 9.Gulick, R. M., H. J. Ribaudo, C. M. Shikuma, S. Lustgarten, K. E. Squires, W. A. Meyer III, E. P. Acosta, B. R. Schackman, C. D. Pilcher, R. L. Murphy, W. E. Maher, M. D. Witt, R. C. Reichman, S. Snyder, K. L. Klingman, and D. R. Kuritzkes. 2004. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N. Engl. J. Med. 350:1850-1861. [DOI] [PubMed] [Google Scholar]
- 10.Hammond, J. 2000. Investigation of the structural features of human immunodeficiency virus reverse transcriptase inhibitors that influence the selection of resistance mutations. Ph.D. thesis. University of Pittsburgh, Pittsburgh, Pa.
- 11.Meyer, P. R., S. E. Matsuura, D. Zonarich, R. R. Chopra, E. Pendarvis, H. Z. Bazmi, J. W. Mellors, and W. A. Scott. 2003. Relationship between 3′-azido-3′-deoxythymidine resistance and primer unblocking activity in foscarnet resistant mutants of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 77:6127-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 13.Richard, N., H. Salomon, M. Oliveira, R. Rando, T. Mansour, Z. Gu, and M. A. Wainberg. 2000. Selection of resistance-conferring mutations in HIV-1 by the nucleoside reverse transcriptase inhibitors (+/-)dOTC and (+/-)dOTFC. Antivir. Chem. Chemother. 11:359-365. [DOI] [PubMed] [Google Scholar]
- 14.Roge, B. T., T. L. Katzenstein, N. Obel, H. Nielsen, O. Kirk, C. Pedersen, L. Mathiesen, J. Lundgren, and J. Gerstoft. 2003. K65R with and without S68: a new resistance profile in vivo detected in most patients failing abacavir, didanosine and stavudine. Antivir. Ther. 8:173-182. [PubMed] [Google Scholar]
- 15.Selmi, B., J. Boretto, S. R. Sarfati, C. Guerreiro, and B. Canard. 2001. Mechanism-based suppression of dideoxynucleotide resistance by K65R human immunodeficiency virus reverse transcriptase using an alpha-boranophosphate nucleoside analogue. J. Biol. Chem. 276:48466-48472. [DOI] [PubMed] [Google Scholar]
- 16.Shi, C., and J. W. Mellors. 1997. A recombinant retroviral system for rapid in vivo analysis of human immunodeficiency virus type 1 susceptibility to reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 41:2781-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sluis-Cremer, N., D. Arion, N. Kaushik, H. Lim, and M. A. Parniak. 2000. Mutational analysis of Lys65 of HIV-1 reverse transcriptase. Biochem. J. 348(Pt. 1):77-82. [PMC free article] [PubMed] [Google Scholar]
- 18.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 19.Winston, A., S. Mandalia, D. Pillay, B. Gazzard, and A. Pozniak. 2002. The prevalence and determinants of the K65R mutation in HIV-1 reverse transcriptase in tenofovir-naive patients. AIDS 16:2087-2089. [DOI] [PubMed] [Google Scholar]
- 20.Winston, A., A. Pozniak, S. Mandalia, B. Gazzard, D. Pillay, and M. Nelson. 2004. Which nucleoside and nucleotide backbone combinations select for the K65R mutation in HIV-1 reverse transcriptase. AIDS 18:949-957. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, D., A. M. Caliendo, J. J. Eron, K. M. DeVore, J. C. Kaplan, M. S. Hirsch, and R. T. D'Aquila. 1994. Resistance to 2′,3′-Dideoxycytidine conferred by a mutation in codon 65 of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 38:282-287. [DOI] [PMC free article] [PubMed] [Google Scholar]