Abstract
Ceftobiprole is a novel broad-spectrum cephalosporin that binds with high affinity to PBP 2a, the methicillin-resistance determinant of staphylococci, and is active against methicillin- and vancomycin-resistant Staphylococcus aureus. Ceftobiprole was compared to vancomycin in a rabbit model of methicillin-resistant S. aureus aortic valve endocarditis. Ceftobiprole and vancomycin were equally effective against endocarditis caused by methicillin-resistant S. aureus strain 76, whereas ceftobiprole was more effective than vancomycin against the vancomycin-intermediate S. aureus strain HIP5836. The activity of ceftobiprole against drug-resistant strains of S. aureus warrants its further clinical development.
Ceftobiprole (abbreviation, BPR; formerly BAL9141) (Fig. 1) is a novel, broad-spectrum, bactericidal cephalosporin with MICs of ≤ 4 μg/ml for clinical isolates of methicillin-resistant staphylococci (MRS), including vancomycin-intermediate Staphylococcus aureus (VISA) and vancomycin-resistant S. aureus (3). The anti-MRS activity of ceftobiprole stems from its high affinity for PBP 2a, the penicillin binding protein chiefly responsible for the methicillin-resistant phenotype of staphylococci. Ceftobiprole is stable to class A penicillinases produced by S. aureus and enteric gram-negative organisms and is relatively stable to some class C beta-lactamases produced by enteric gram-negative organisms (10). In mouse septicemia models, ceftobiprole proved efficacious against infections caused by methicillin-susceptible S. aureus, methicillin-resistant S. aureus (MRSA), streptococci, and gram-negative bacilli, including Escherichia coli (10). These properties make ceftobiprole an attractive candidate as an empirical monotherapy for a variety of infections while culture results are pending, as well as for definitive therapy of infections caused by MRS. To improve solubility, ceftobiprole is administered as its dioxolenylmethyl carbamate prodrug, ceftobiprole medocaril (formerly BAL5788) (Fig. 1). The purpose of the present study was to compare the efficacy of ceftobiprole to that of vancomycin against antibiotic-resistant S. aureus in a rabbit model of aortic valve endocarditis.
FIG. 1.
Structures of ceftobiprole and its dioxolenylmethyl carbamate prodrug, ceftobiprole medocaril.
MATERIALS AND METHODS
Bacterial strains.
S. aureus strain 76 is a homogeneous, β-lactamase-producing, highly methicillin-resistant clinical isolate (nafcillin MIC = 256 μg/ml) (5). S. aureus strain HIP5836 is a methicillin-resistant (nafcillin MIC = 16 μg/ml), β-lactamase-producing VISA clinical isolate from a patient in New Jersey (originally designated strain 992) (2, 7, 21).
Susceptibility testing.
MICs were determined by the standard microdilution method with Mueller-Hinton broth, using an inoculum of 2 × 106 CFU/ml. MICs were read after incubating microtiter plates for 24 h at 37°C.
Time-kill studies were performed with 50-ml centrifuge tubes containing 10 ml of Mueller-Hinton broth inoculated with 107 CFU of MRSA strain 76 or VISA strain HIP5836 per ml. Samples were incubated at 37°C on a reciprocating shaker at 200 rpm. Sample volumes (100 μl) were removed at 0, 4, and 24 h for quantitative culture to determine viable counts (numbers of CFU per milliliter). The threshold of detection for this method is 1 log10 CFU/ml.
Rabbit endocarditis model.
To establish endocarditis, a cut-down over the right carotid artery was performed and a polyethylene catheter was positioned across the aortic valve of a 2.4- to 2.6-kg New Zealand White rabbit; the catheter was secured in place for the duration of the experiment. Forty-eight hours after the positioning of the catheter, 1 ml of 0.9% saline containing approximately 106 CFU of either strain 76 or strain HIP5836 was injected intravenously into each rabbit. Antibiotic treatment was commenced 16 to 18 h after infection. Rabbits were randomized to one of three groups: (i) an untreated control group, which was euthanized at initiation of therapy to determine baseline bacterial burdens in aortic valve vegetations, spleens, and kidneys; (ii) a treated control group given vancomycin at a dose of 30 mg/kg of body weight intravenously every 12 h for 4 days; and (iii) a group treated with ceftobiprole medocaril at a dose of 25 mg/kg (equivalent to 19 mg of active drug [ceftobiprole] per kg) intramuscularly every 8 h for 4 days.
Concentrations of vancomycin in sera were determined from blood samples obtained 1 h after dosing. Vancomycin was assayed by agar diffusion with antibiotic medium 8 and Bacillus subtilis ATCC 6633 as the indicator strain. Serum ceftobiprole concentrations were determined from blood samples obtained 1 h after dosing. The assays were performed by the manufacturer by high-pressure liquid chromatography and mass spectrometry as described previously (1). The half-life of ceftobiprole was determined for one uninfected rabbit from blood samples obtained at 1, 2, and 4 h after dosing.
Rabbits that died any time after initiation of therapy were scored for mortality, but only those surviving beyond the first 24 h of treatment were included in an analysis of tissue bacterial titers. Surviving rabbits were euthanized 16 h after the last dose of drug. Aortic valve vegetations, spleens, and kidneys were removed. Tissues were homogenized in 0.5 ml of 0.9% saline, and 100-μl volumes were quantitatively cultured on blood agar to determine the number of bacteria present. The limit of detection of this method is 5 CFU per vegetation, or approximately 1.70 log10CFU/g. Emergence of resistance to ceftobiprole was assayed by inoculation of 100 μl of homogenized vegetations from control and ceftobiprole-treated rabbits onto Trypticase soy agar plates containing 5 μg of ceftobiprole per ml.
Data analysis.
The bacterial titers in the aortic valve vegetation, kidney, and spleen of each rabbit was expressed as log10 numbers of CFU per gram of tissue. Cultures yielding no growth were scored as sterile and were assigned a value of 1.70 log10CFU/g when means were calculated. Differences between mean titers of the treatment groups were tested for statistical significance (defined as a P of <0.05) by analysis of variance. Significant differences in rates of mortality were determined by Fisher's exact test.
RESULTS
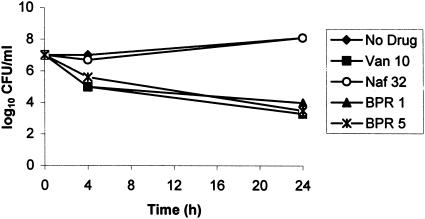
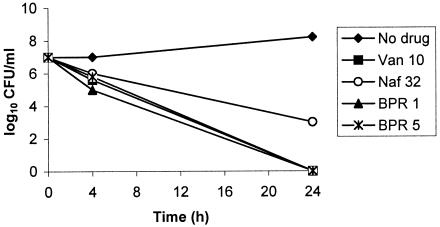
MICs of ceftobiprole were 2 μg/ml for MRSA strain 76 and 0.5 μg/ml for VISA strain HIP5836. MICs of vancomycin were 0.5 and 4 μg/ml for strains 76 and HIP5836, respectively. The MIC of 4 μg/ml for HIP5836 instead of 8 μg/ml, the breakpoint for VISA, probably reflects instability of the phenotype (2). In time-kill studies, ceftobiprole at a concentration of 1 or 5 μg/ml was bactericidal towards strain 76, producing an ∼3-log10-CFU/ml decrease in viable counts by 24 h (Fig. 2). Vancomycin at a concentration of 10 μg/ml was also bactericidal towards strain 76, whereas this strain was not inhibited by nafcillin at a concentration of 32 μg/ml. Ceftobiprole at a concentration of 1 or 5 μg/ml and vancomycin at 10 μg/ml were each bactericidal towards strain HIP5836, achieving ≥6-log10 decreases in viable counts by 24 h (Fig. 3). Nafcillin at 32 μg/ml, which is just above the MIC of 16 μg/ml for this strain, also was bactericidal towards strain HIP5836, with a decrease in viable counts of ∼3 log10 by 24 h.
FIG. 2.
Time-kill studies of MRSA strain 76 comparing ceftobiprole at 1 and 5 μg/ml (BPR 1 and BPR 5, respectively) with vancomycin at 10 μg/ml (Van 10) and nafcillin at 32 μg/ml (Naf 32).
FIG. 3.
Time-kill studies of VISA strain HIP5836 comparing ceftobiprole at 1 and 5 μg/ml (BPR 1 and BPR 5, respectively) with vancomycin at 10 μg/ml (Van 10) and nafcillin at 32 μg/ml (Naf 32).
Mean serum vancomycin concentrations (± standard deviations) achieved 1 h after dosing were 29 ± 9 μg/ml (nine rabbits), similar to those observed in humans. The half-life of vancomycin in this model is approximately 80 min (6). Mean serum ceftobiprole concentrations (± standard deviations) 1 h after dosing were 29 ± 7 μg/ml (16 rabbits), similar to those achieved in humans 1 h after an intravenous infusion of 500 to 750 mg (17). The half-life of ceftobiprole was estimated to be approximately 1 h, compared to approximately 3 h in humans (17).
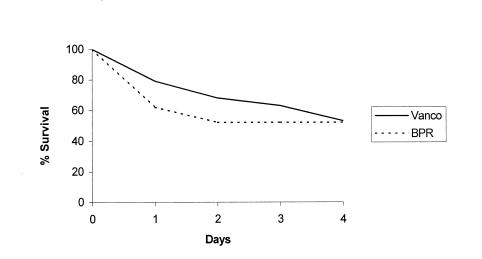
Ceftobiprole and vancomycin produced similar reductions in tissue burdens for infection caused by strain 76 (Table 1). Mortality for ceftobiprole- and vancomycin-treated rabbits was high; 10 of 21 rabbits (48%) and 9 of 19 rabbits (47%) died, respectively, with most deaths occurring during the first 2 days of treatment (Fig. 4). No resistance to ceftobiprole by strain 76 was detected in aortic valve vegetation, spleen, or kidney samples of untreated controls or ceftobiprole-treated rabbits.
TABLE 1.
Organism titers in vegetations, spleens, and kidneys of untreated and antibiotic-treated rabbits infected with MRSA strain 76
| Tissue | Mean organism titer (log10 CFU/g) ± SD (no. of sterile cultures)a
|
||
|---|---|---|---|
| No treatment (n = 8) | Ceftobiprole treatment (n = 13) | Vancomycin treatment (n = 13) | |
| Vegetation | 6.84 ± 0.39 (0) | 2.73 ± 1.58* (8) | 3.22 ± 2.39* (8) |
| Spleen | 6.02 ± 0.41 (0) | 2.36 ± 1.01* (7) | 2.07 ± 1.24* (10) |
| Kidney | 6.31 ± 0.69 (0) | 1.98 ± 0.57* (8) | 2.98 ± 1.80* (8) |
*, P was <0.001 versus the value for the control.
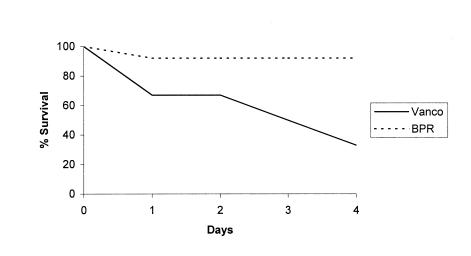
FIG. 4.
Survival curves for rabbits with endocarditis caused by MRSA strain 76, comparing treatment with ceftobiprole to treatment with vancomycin (Vanco).
Ceftobiprole was superior to vancomycin for endocarditis caused by VISA strain HIP5836 (Table 2). The burdens of organisms in vegetations, spleens, and kidneys were significantly lower in ceftobiprole-treated rabbits than in vancomycin-treated animals. Compared to what occurred in the untreated control group, vancomycin had no effect on the number of bacteria present in vegetations, whereas it did effect some reduction in bacterial counts in spleen and kidney. Mortality for the ceftobiprole-treated group (1 of 12 rabbits [8.3%]) was significantly lower (P = 0.022) than for the vancomycin-treated group (4 of 6 rabbits [67%]) (Fig. 5). No ceftobiprole-resistant populations of VISA strain HIP5836 were detected in tissue samples of controls or ceftobiprole-treated rabbits.
TABLE 2.
Organism titers in vegetations, spleens, and kidneys of untreated and antibiotic-treated rabbits infected with VISA strain HIP5836
| Tissue | Mean organism titer (log10 CFU/g) ± SD (no. of sterile cultures)a
|
||
|---|---|---|---|
| No treatment (n = 5) | Ceftobiprole treatment (n = 11) | Vancomycin treatment (n = 5) | |
| Vegetation | 6.73 ± 0.46 (0) | 2.25 ± 0.83* (7) | 6.84 ± 0.45† (0) |
| Spleen | 5.91 ± 0.52 (0) | 2.63 ± 1.24* (6) | 4.63 ± 0.73‡+ (0) |
| Kidney | 6.15 ± 0.34 (0) | 2.02 ± 0.58* (8) | 3.76 ± 1.23‡+ (0) |
*, P < 0.001 versus control value; +, P < 0.01 versus value with ceftobiprole; ‡, P < 0.05 versus control value; †, P < 0.001 versus value with ceftobiprole.
FIG. 5.
Survival curves for rabbits with endocarditis caused by VISA strain HIP5836, comparing treatment with ceftobiprole to treatment with vancomycin (Vanco).
DISCUSSION
Ceftobiprole was bactericidal in vitro, and administered as prodrug ceftobiprole medocaril, it was bactericidal in vivo towards both MRSA strain 76 and VISA strain HIP5836. The activity of ceftobiprole was superior to that of vancomycin for rabbits infected with the VISA strain and equivalent to that of vancomycin in rabbits infected with MRSA strain 76. No emergence of ceftobiprole-resistant populations was observed for either of these strains. These results are concordant with those obtained for a rat model of MRSA aortic valve endocarditis, in which ceftobiprole proved to be superior to vancomycin following 3 days of treatment for the two MRSA strains surveyed (8).
Mortality in rabbits infected with strain 76 was high, particularly during the first 48 h of therapy, irrespective of treatment with either ceftobiprole or vancomycin. This outcome is most likely a reflection of the overwhelming infection caused by strain 76 in this model and is not attributable to treatment failure, since surviving rabbits showed significant reductions in vegetation titers compared to those of untreated controls. Mortality was also high in vancomycin-treated rabbits infected with VISA strain HIP5836, which probably is attributable to treatment failure with vancomycin given that no decrease in bacterial counts in vegetations occurred during therapy. Only one ceftobiprole-treated rabbit died during the 4-day treatment period, whereas deaths occurred at a more or less steady rate among vancomycin-treated rabbits. These results suggest that ceftobiprole might be effective in patients in whom vancomycin fails, as it did in the patient from whom HIP5836 was isolated.
The feasibility of synthesizing beta-lactams that bind PBP 2a with high affinity is well established (4, 9, 12, 13, 16, 20). Solution of the PBP 2a crystal structure has shown that critical interactions with cephalosporin side chains occur in a pocket, closed in the free PBP 2a enzyme, that binds the 7-acyl amino side chain and in an extended groove that interacts with the 3′-cephem side chain through numerous noncovalent interactions (15). The cephalosporins that are active against MRSA have side chains that fill both side chain-binding sites. However, since this is also true of molecules that are less active against MRSA, the precise nature of the interactions that favor the formation of the reversible Michaelis complex and thus speed the acylation of the active-site serine needs further investigation. Predictably, the antimicrobial effect of those compounds that are effective inhibitors of both PBP 2a and the normal complement of sensitive penicillin binding proteins is inhibition of growth and eventual cell death at therapeutically relevant concentrations. Since resistance to compounds that bind with high affinity to PBP 2a does not occur as a single step (14), the emergence of resistance during therapy seems unlikely, which is in fact what was observed.
Ceftobiprole appears to have sufficient activity to be efficacious for human infections caused by MRSA (19), and the present in vivo data confirm this. Ceftobiprole has been safe and well tolerated in phase 1 and phase 2 clinical trials, with no severe or serious adverse effects reported (17-19). The activity of ceftobiprole is not expected to be affected by reduced susceptibility to vancomycin based on the known mechanisms of action of these compounds, in vitro data, and the results of endocarditis models of MRSA and VISA infection. The findings in this study, considered together with reports (8, 11; T. Bogdanovich, B. Bozdogan, and P. C. Appelbaum, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-928, 2004) on the refractoriness of staphylococci to develop endogenous resistance to ceftobiprole, suggest that ceftobiprole could be a valuable addition to the antibacterial armamentarium.
Acknowledgments
Li Basuino and Andres Madrigal provided technical support.
This work was supported by a research grant from Basilea Pharmaceutica AG, Basel, Switzerland.
REFERENCES
- 1.Azoulay-Dupuis, E., J. P. Bedos, J. Mohler, A. Schmitt-Hoffmann, M. Schleimer, and S. Shapiro. 2004. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob. Agents Chemother. 48:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle-Vavra, S., S. K. Berke, J. C. Lee, and R. S. Daum. 2000. Reversion of glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 44:272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozdogan, B., D. Esel, C. Whitener, F. A. Browne, and P. C. Appelbaum. 2003. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey Medical Center. J. Antimicrob. Chemother. 52:864-868. (First published 16 October 2003; http://jac.oupjournals.org/cgi/content/full/52/5/864.) [DOI] [PubMed] [Google Scholar]
- 4.Chambers, H. F. 1995. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for the penicillin-binding protein PBP 2a. Antimicrob. Agents Chemother. 39:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers, H. F., M. Kartalija, and M. Sande. 1995. Ampicillin, sulbactam, and rifampin combination treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis in rabbits. J. Infect. Dis. 171:897-902. [DOI] [PubMed] [Google Scholar]
- 6.Chambers, H. F., M. Sachdeva, and S. Kennedy. 1990. Binding affinity of penicillin binding protein 2a correlates with in vivo activity of beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 162:705-710. [DOI] [PubMed] [Google Scholar]
- 7.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung-Tomc, J. C., J. Clark, B. Minassian, M. Pucci, Y. H. Tsai, E. Gradelski, L. Lamb, I. Medina, E. Huczko, B. Kolek, S. Chaniewski, C. Ferraro, T. Washo, and D. P. Bonner. 2002. In vitro and in vivo activities of a novel cephalosporin, BMS-247243, against methicillin-resistant and -susceptible staphylococci. Antimicrob. Agents Chemother. 46:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heller, S., E. Marrer, M. G. P. Page, S. Shapiro, and L. Thenoz. 2004. Development of endogenous resistance by staphylococci to BAL9141 and comparators. Clin. Microbiol. Infect. 10(Suppl. 3):163.14759242 [Google Scholar]
- 12.Huang, V., W. J. Brown, and M. J. Rybak. 2004. In vitro activities of a novel cephalosporin, CB-181963 (CAB-175), against methicillin-susceptible or -resistant Staphylococcus aureus and glycopeptide-intermediate susceptible staphylococci. Antimicrob. Agents Chemother. 48:2719-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, A. P., M. Warner, M. Carter, and D. M. Livermore. 2002. In vitro activity of cephalosporin RWJ-54428 (MC-02479) against multidrug-resistant gram-positive cocci. Antimicrob. Agents Chemother. 46:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama, Y., H. Z. Zhang, and H. F. Chambers. 2004. PBP 2a mutations producing very-high-level resistance to beta-lactams. Antimicrob. Agents Chemother. 48:453-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim, D., and N. C. Strynadka. 2002. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 9:870-876. [DOI] [PubMed] [Google Scholar]
- 16.Ohtake, N., H. Imamura, H. Jona, H. Kiyonaga, A. Shimizu, M. Moriya, H. Sato, M. Nakano, R. Ushijima, and S. Nakagawa. 1998. Novel dithiocarbamate carbapenems with anti-MRSA activity. Bioorg. Med. Chem. 6:1089-1101. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt-Hoffmann, A., L. Nyman, B. Roos, M. Schleimer, J. Sauer, N. Nashed, T. Brown, A. Man, and E. Weidekamm. 2004. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2576-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt-Hoffmann, A., B. Roos, M. Schleimer, J. Sauer, A. Man, N. Nashed, T. Brown, A. Perez, E. Weidekamm, and P. Kovacs. 2004. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt-Hoffmann, A. H., M. Harsch, M. Heep, M. Schleimer, T. Brown, A. Man, and W. O'Riordan. 2004. BAL5788 in patients with complicated skin and skin structure infections caused by Gram-positive pathogens including methicillin-resistant Staphylococcus species. Interim pharmacokinetic results from 20 patients. Clin. Microbiol. Infect. 10(Suppl. 3):277. [Google Scholar]
- 20.Sumita, Y., H. Nouda, K. Kanazawa, and M. Fukasawa. 1995. Antimicrobial activity of SM-17466, a novel carbapenem antibiotic with potent activity against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 39:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenover, F. C., M. V. Lancaster, B. C. Hill, C. D. Steward, S. A. Stocker, G. A. Hancock, C. M. O'Hara, S. K. McAllister, N. C. Clark, and K. Hiramatsu. 1998. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J. Clin. Microbiol. 36:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]