Abstract
The link between the angiotensin-converting enzyme (ACE) insertion/deletion (I/D) gene polymorphism and the prevalence of type 2 diabetes mellitus (T2DM) developing in the Saudi Arabian population remains controversial. The aim of the present study was to evaluate the association between the ACE I/D gene polymorphism and the risk of developing T2DM and hypertension (HTN) in Saudi Arabian individuals. A total of 220 individuals consisting of 48 control subjects, 70 T2DM, 48 HTN, and 54 T2DM with HTN patients were recruited. Genotyping was performed by polymerase chain reaction initially and mistyping of the DD genotypes was conducted with an insertion-specific primer. The genotyping frequency for the II, ID and DD polymorphism of the ACE gene was 6.8, 42.6 and 48.6% in T2DM patients, 4.2, 50 and 45.8% in HTN patients, 5.6, 55.5 and 38.9% in T2DM patients with HTN and 58.3, 37.5 and 4.2% in control subjects, respectively. The frequency for the D allele was 70% in T2DM patients, 70.8% in HTN patients and 66.7% in T2DM patients with HTN as compared with 22.9% in the control subjects. The genotype and allele frequency of the ACE gene polymorphism varied significantly (P<0.05) in the patients when compared with the control subjects. The current study demonstrated that the ID/DD genotype and the D allele of the ACE gene I/D polymorphism were strongly associated with the risk of T2DM and HTN developing in a Saudi Arabian population.
Keywords: angiotensin converting enzyme, insertion/deletion gene polymorphism, type 2 diabetes mellitus, hypertension
Introduction
Diabetes mellitus (DM) and hypertension (HTN) are considered as major public health problems worldwide. The two rarely exist in isolation. The World Health Organization has ranked the Kingdom of Saudi Arabia (KSA) as having the seventh highest rate in the world, and the second highest rate of diabetes in the Middle East, with ~7 million individuals living with diabetes and >3 million who are pre-diabetics (1). In KSA, DM is a rapidly increasing medical health problem and is becoming a significant cause of morbidity and/or mortality (2).
In addition to traditional life-style risk factors, genetic background may also be pivotal in the pathogenesis of type 2 DM (T2DM) and HTN with the important and complex involvement of the renin angiotensin aldosterone system (RAAS) (3).
Polymorphisms of the angiotensin-converting enzyme (ACE) gene have been extensively investigated to ascertain the genetic susceptibility of HTN development (4). In addition, ACE significantly contributes to the pathogenesis of T2DM as RAAS blockade was demonstrated to improve insulin resistance (5). A meta-analysis reported the ACE insertion deletion (I/D) polymorphism as a candidate gene for the development of essential HTN in a Chinese population (6). Thus, the I/D polymorphism of the ACE gene may significantly influence the advancement of DM and/or HTN.
Different studies have described the link between the D allele of the ACE gene and the development of HTN (7) and T2DM in various populations (8). However, certain studies failed to show this association with the D allele (9). The contradictory results regarding the involvement of the ACE I/D polymorphism in HTN and T2DM are likely to be due to variance in ethnicity and/or gender (10,11). To properly manage this issue in a Saudi Arabian population, a multidisciplinary approach is required.
Yet, to date there is a paucity of information on the association between the ACE I/D polymorphism and the risk of T2DM in Saudi Arabian individuals. Therefore, the current study was designed to establish the association of ACE I/D polymorphism in Saudi Arabian subjects with T2DM and/or HTN.
Patients and methods
Ethical consideration and participants
Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine, Prince Sattam Bin Abdelaziz University (PSAU; Akharj, KSA). Participants included 220 subjects that were attending the outpatient medicine Clinic at PSAU Hospital over an 8-month period (January to August 2016). All participants provided written informed consent prior to beginning the study.
Inclusion criteria and study groups
Participants included subjects with T2DM and/or HTN. T2DM was defined by the American Diabetes Association (ADA) criteria as fasting blood glucose level >7.0 mmol/l (12). Subjects with persistent elevation of blood pressure ≥140/90 mmHg or who were on antihypertensive medications during the study period were considered hypertensive and, therefore, included in the study. Healthy individuals were randomly collected from volunteers and PSAU staff members without age or sex matching. Their inclusion criteria were defined as fasting blood glucose level <7.0 mmol/l, blood pressure <140/90 mmHg, negative family history of diabetes and HTN, and absence of any medication intake at the time of enrollment.
The study included four groups as follows: Control group (n=48); T2DM group (n=70); HTN group (n=48); and T2DM with HTN group (n=54). A questionnaire in Arabic and English language was competed by each participant to obtain information regarding socio-demographic status, history of diabetes, HTN and other co-morbidities. Weight and height of all participants were determined to compute their body mass index (BMI) using the formula, weight (kg)/(height; m)2 (13).
Biochemical analysis of blood samples
Blood samples were obtained from all subjects and plasma was removed by centrifugation at 1,000 × g (4°C) for 10 min and maintained at −30°C for subsequent analysis that included the triglycerides (TG), high density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) levels. The Friedewald formula (14) was used to calculate low density lipoprotein cholesterol (LDL-C). Lipid profile results were classified according to the Third Report of the National Cholesterol Education Program guidelines (15). The analysis was performed on a Hitachi-912 auto-analyzer (Hitachi, Ltd., Tokyo, Japan) using kits purchased from Roche Diagnostics GmbH (Mannheim, Germany).
Genotype determination
Saliva self-collection kits were used for genomic DNA extraction according to manufacturer's instructions (DNA Genotek, Inc., Kanata, ON, Canada). The samples were stored in a freezer at −80°C until further analysis. The extracted DNA was assessed for purity using a BioPhotometer (Eppendorf, Hamburg, Germany). The ACE gene I/D polymorphism was determined by fragment amplification using a polymerase chain reaction (PCR) technique followed by gel electrophoresis. The flanking primer pairs were as follows: Forward, 5′-GCCCTGCAGGTGTCTGCAGCATGT-3′ and reverse, 5′-GGATGGCTCTCCCCGCCTTGTCTC-3′ (synthesized by Research Biolabs Pte Ltd., Singapore) (16). Fragment amplification was performed with a 25-µl PCR master mix (New England BioLabs, Inc., Ipswich, MA, USA), which contained 20 pmol of each primer, 0.4 mmol/l deoxynucleotide triphosphate, 2 mmol/l magnesium chloride, 1X Taq buffer and 1 unit of NEB Taq DNA polymerase. The PCR was performed on a Thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with preliminary denaturation for 5 min at 94°C, followed by cycles of denaturation (94°C for 30 sec), annealing (58°C for 1 min) and extension (72°C for 2 min). These cycles were repeated 30 times followed by a final extension at 72°C for 5 min, the PCR amplicons were subsequently stored at 4°C. The desired DNA fragments were separated by agarose gel electrophoresis (Promega Corporation, Madison, WI, USA) performed in original electrophoresis tank (Elchrom Scientific AG, Cham, Switzerland). The PCR product (7 µl) and 5 µl of loading dye were mixed then loaded to 1% ethidium bromide gel. After 60 min at a 120-V current, the gel was removed and visualized using a ChemiDoc™ MP Gel imaging System (Bio-Rad Laboratories, Inc., USA) under UV light. The initial PCR results revealed three genotypes: A 490-bp band (II), a 190-bp band (DD) and the two bands (ID).
ID heterozygotes may be mistyped as DD homozygotes
Therefore, to augment the specificity of the DD homozygotes, another PCR amplification was performed with an insertion-specific primer pair used in each DD sample as follows: Forward, 5′-TGGGACCACAGCGCCCGCCACTAC-3′ and reverse, 5′-TCGCCAGCCCTCCCATGCCC-ATAA-3′. The PCR conditions were adjusted with an initial denaturation step at 94°C for 1 min, followed by 30 cycles of denaturation (94°C for 30 sec), annealing (67°C for 45 sec) and extension (72°C for 2 min). The second PCR product exhibited an I allele amplicon (335 bp) or no products in the DD allele homozygous samples. Genotyping was repeated twice on different occasions with similar results obtained.
Statistical analysis
Statistical analyses were conducted using SPSS (version 20.0; IBM Corp., Armonk, NY, USA). The descriptive data and laboratory results were tested for Gaussian distributions by Kolmogorov-Smirnov test and reported as means ± standard deviation. Differences in descriptive data were analyzed by one-way analysis of variance followed by Bonferroni post hoc multiple comparisons. Genotype and allelic frequencies in all groups were reported as numbers and percentage. The genotype data were examined for aberration from expected by Hardy-Weinberg equilibrium and the significant difference between groups was calculated using the χ2 test of independence and/or Fisher's exact test. Furthermore, the odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by multiple logistic regression analysis. P<0.05 was considered to indicate a statistically significant difference at 95% CI.
Results
Table I demonstrates that significant differences exist in BMI between the control group and the other investigated groups (P<0.05); however, when compared with each other, BMI was not significantly different between the T2DM, HTN and T2DM + HTN groups. The variables, systolic blood pressure (SBP) and diastolic BP (DBP) at the time of participation were significantly higher in the HTN groups compared with the control group (P<0.001), but was not significantly different between the patient groups (P>0.05). Regarding fasting blood glucose levels, significant differences were observed in all patient groups compared with the control group. HDL-C was significantly increased in the T2DM groups (P<0.05), although not in the HTN group. By contrast, no statistical differences in LDL-C, TG and TC levels were identified in any of the study groups.
Table I.
Demographic and biochemical results of the study groups.
| Parameter | Control group (n=48) | Diabetic group T2DM (n=70) | Hypertensive group HTN (n=48) | HTN + T2DM group (n=54) |
|---|---|---|---|---|
| Gender (M/F) | 28/20 | 28/42 | 22/26 | 24/30 |
| Age (years) | 32.2±10.9 | 42.9±12.5 | 39.8±13.9 | 49.8±10.4a,b |
| Body mass index (kg/m2) | 24.5±6.2 | 37.7±7.9a | 38.7±7.8a | 37.8±8.2a |
| Fasting blood glucose (mmol/l) | 4.7±0.8 | 9.7±1.2a | 7.5±0.6a | 10.7±3.4a,b |
| Systolic blood pressure (mmHg) | 126.1±8.7 | 140±20a | 158±18a | 154±17a |
| Diastolic blood pressure (mmHg) | 78.1±7.2 | 87±11a | 96±12a | 94±14a |
| Total cholesterol (mmol/l) | 5.21±1.33 | 6.12±1.55 | 5.76±1.62 | 5.99±1.42 |
| Low density lipoprotein-cholesterol (mmol/l) | 3.80±1.33 | 4.24±1.47 | 4.16±1.54 | 4.44±1.26 |
| High density lipoprotein-cholesterol (mmol/l) | 0.90±0.45 | 1.77±0.26a | 1.08±0.31 | 1.67±0.39a |
| Triglycerides (mmol/l) | 1.97±1.07 | 2.81±1.08 | 2.29±1.02 | 2.77±1.09 |
Values were presented as means ± standard deviation.
P<0.05 vs. Control group
P<0.05 vs. HTN group using one way ANOVA followed by post hoc Bonferroni test. T2DM, type 2 diabetes mellitus; HTN, hypertension.
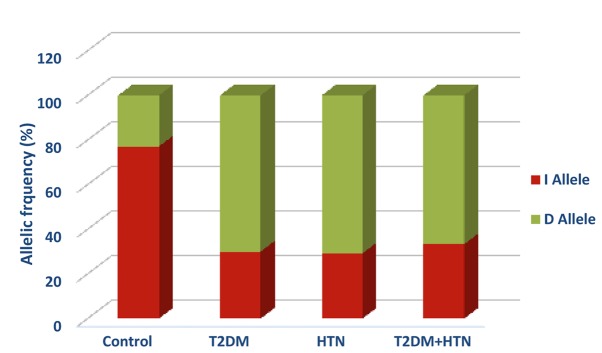
The ACE gene I/D polymorphism genotype frequencies followed the Hardy-Weinberg equilibrium in all of the studied groups (Table II). The control group exhibited higher frequencies of the II and ID genotypes than the DD genotype (58.3, 37.5 vs. 4.2% respectively). Compared with the control group, all diabetic-associated groups exhibited a lower frequency of II genotype than the ID and DD genotypes that was statistically significant (χ2=48.83; P<0.0001). The frequencies of the II genotype were 8.6, 4.2, and 5.6% in T2DM, HTN, and T2DM with HTN, respectively (Table II). In addition, the D allele was the dominant allele in diabetic, hypertensive, and hypertensive-diabetic patients with frequencies of 70, 70.8, and 66.7% respectively (Fig. 1). The D allele was significantly associated with T2DM diabetes (OR=7.85; 95% CI=3.90–15.78; P<0.0001), HTN (OR = 8.17; 95% CI = 3.73 – 17.88; p< 0.0001), and HTN-T2DM diabetes (OR=6.73; 95% CI=2.90–15.59; P<0.0001) compared with the control group (Table II). No significant differences were observed between D allele and other diabetes-associated co-morbidities.
Table II.
I/D polymorphism genotyping and allelic frequencies between the study groups.
| Parameter | Control group (n=48) | T2DM (n=70) | HTN (n=48) | HTN + T2DM group (n=54) |
|---|---|---|---|---|
| Genotype, n (%) | ||||
| II | 28 (58.3) | 6 (8.6) | 2 (4.2) | 3 (5.6) |
| ID | 18 (37.5) | 30 (42.8) | 24 (50.0) | 30 (55.5) |
| DD | 2 (4.2) | 34 (48.6) | 22 (45.8) | 21 (38.9) |
| χ2 test sig. (2-sided) | P<0.0001 between all groups | |||
| Allele, n (%) | ||||
| I | 74 (77.1) | 42 (30) | 28 (29.2) | 36 (33.3) |
| D | 22 (22.9) | 98 (70) | 68 (70.8) | 72 (66.7) |
| Total | 96 | 140 | 96 | 108 |
| Fisher's exact test sig. (2-sided) | P<0.0001a | P<0.0001a | P<0.0001a | |
| Odds ratio | 7.85 | 8.17 | 6.73 | |
| (95% confidence interval) | (3.90–15.78) | (3.73–17.88) | (2.90–15.59) |
P<0.05 vs. Control group using χ2 and/or Fisher's exact tests. T2DM, type 2 diabetes mellitus; HTN, hypertension.
Figure 1.
I/D polymorphism allelic frequencies between the studied groups. T2DM, type 2 diabetes mellitus; HTN, hypertension.
Discussion
It has previously been reported that an increased serum ACE level is genetically identified by a 287-bp fragment of the I/D polymorphism at chromosome 17 in the 16th intron of the ACE gene (17). The presence/insertion of this fragment is demonstrated as homozygosis II, ID for heterozygosis, while DD symbolizes the absence/deletion of a 287-bp Alu reduplicate series. DD genotypes, primarily the D allele, in contrast to the II genotypes and the I allele, are strongly associated with an increased risk of diabetes and diabetic complications (18), myocardial infarction (19) and HTN (20). By contrast, many studies denied the associated between the DD genotypes, and development of HTN (9) and diabetes (21). Furthermore, the I allele had a strong link with familial HTN in an Australian population (20).
Due to the inconsistent results of the above-mentioned studies, the ACE gene polymorphism was investigated in a Saudi Arabian T2DM patients with or without HTN in the present study. A strong link between the DD homozygous genotype and, primarily, the D allele of the ACE gene with T2DM and HTN was identified in Saudi Arabian subjects, when compared with the healthy control group (P<0.05; Table II). Previous studies in other populations showed high prevalence of the DD genotype (93.33%) and D allele (81.39%) of the ACE gene in diabetic and HTN patients as compared with control subjectss (7,21). In the present study the frequency of the D allele was found to be significantly different in hypertensive and T2DM patients with or without HTN compared with the control subjects (P<0.0001), a similar result was reported in a Taiwanese population (22).
In a Malaysian population, the angiotensinogen gene polymorphism was reported to be linked with HTN subjects, which clearly demonstrated an important role of the RAAS polymorphism in the development of HTN (23). However, another study denied the association of the renin gene and HTN, with or without T2DM (24).
Data from a study that involved Malaysian hypertensive subjects and angiotensinogen gene polymorphisms support the current results with regard to the diabetic and hypertensive groups' clinical characteristics and the ACE genotype distribution in terms of age, fasting blood glucose, SBP, DBP, and BMI, as the results are comparable (25). Additionally, the results of the present study are consistent with other studies regarding T2DM subjects in Taiwanese (26) and Iranian populations (27); however, there was negative association with gender, ethnicity and other confounding factors. Numerous factors may contribute to explaining these discrepancies that deny the link between the ACE I/D polymorphism, and T2DM and HTN, including ethnic differences or heterogeneity of the population, sampling bias, and potentially other ecological factors.
In addition, there were certain limitations of the present study; first, due to the controlled design, which was randomly performed, it did not allow for age- and sex-matched controls to be involved. Furthermore, the case subjects were relatively older than the control individuals. However, despite the somewhat small sample size of the current study compared with other epidemiological and association studies, the results confirm the assumption that the DD genotype, chiefly the D allele, is strongly associated with T2DM and/or HTN. In order to emphasize the association of the ACE I/D polymorphism with T2DM and HTN, further research with larger sample sizes is essential and further investigations are required to evaluate the possible correlation of other polymorphisms of RAAS genes with the risk of T2DM and HTN developing in a Saudi Arabian population.
In conclusion, the current study demonstrated strong evidence for the association of ACE gene I/D polymorphisms and the risk of T2DM and HTN in Saudi Arabian subjects. Furthermore, the D allele of the ACE I/D polymorphism was demonstrated as a valuable genetic marker for T2DM and HTN in Saudi Arabian subjects.
Acknowledgements
The authors would like to thank the Scientific Research Deanship of Prince Sattam Bin Abdulaziz University (Al-kharj, Saudi Arabia; grant no. 2015/03/4581), for providing financial support. The authors would also like to thank Dr. Taimour Langaee, Research Associate Professor, Department of Pharmacotherapy and Translational Research at the University of Florida (UF) College of Pharmacy and Director of the UF Center for Pharmacogenomics, for his kind invitation to Dr Rehab Ashour and supervision of the genotyping laboratory work.
References
- 1.Robert AA, Al Dawish MA, Braham R, Musallam MA, Al Hayek AA, Al Kahtany NH. Type 2 Diabetes Mellitus in Saudi Arabia: Major Challenges and Possible Solutions. Curr Diabetes Rev. 2017;13:59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 2.Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA, Al Sabaan FS. Diabetes Mellitus in Saudi Arabia: A Review of the Recent Literature. Curr Diabetes Rev. 2016;12:359–368. doi: 10.2174/1573399811666150724095130. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran V, Ismail P, Stanslas J, Shamsudin N, Moin S, Jas R Mohd. Association of insertion/deletion polymorphism of angiotensin-converting enzyme gene with essential hypertension and type 2 diabetes mellitus in Malaysian subjects. J Renin Angiotensin Aldosterone Syst. 2008;9:208–214. doi: 10.1177/1470320308097499. [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Cshang YP, Yan D, Weder A, Cooper R, Luke A, Kan D, Chakravarti A. Associations between hypertension and genes in the renin angiotensin system. Hypertension. 2003;41:1027–1034. doi: 10.1161/01.HYP.0000068681.69874.CB. [DOI] [PubMed] [Google Scholar]
- 5.Dahm KA Jandeleit, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin angiotensin system reduces the incidence of new onset diabetes. J Hypertens. 2005;23:463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]
- 6.Qu H, Lu Y, Lin S. Meta analysis on the association of ACE/ID polymorphism and essential hypertension in Chinese population. Zhonghua Yu Fang Yi Xue Za Zhi. 2001;35:408–411. (In Chinese) [PubMed] [Google Scholar]
- 7.Jeng JR, Harn HJ, Jeng CY, Yueh KC, Shieh SM. Angiotensin I converting enzyme gene polymorphism in Chinese patients with hypertension. Am J Hypertens. 1997;10:558–561. doi: 10.1016/S0895-7061(97)00036-8. [DOI] [PubMed] [Google Scholar]
- 8.Kennon B, Petrie JR, Small M, Connell JM. Angiotensin converting enzyme gene and diabetes mellitus. Diabet Med. 1999;16:448–558. doi: 10.1046/j.1464-5491.1999.00071.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiang FT, Lai ZP, Chern TH, Tseng CD, Hsu KL, Lo HM, Tseng YZ. Lack of association of the angiotensin converting enzyme polymorphism with essential hypertension in a Chinese population. Am J Hypertens. 1997;10:197–201. doi: 10.1016/S0895-7061(96)00345-7. [DOI] [PubMed] [Google Scholar]
- 10.Barley J, Blackwood A, Cartere ND, Crews DE, Cruickshank JK, Jeffery S, Ogunlesi AO, Sagnella GA. Angiotensin converting enzyme insertion/deletion polymorphism: association with ethnic origin. J Hypertens. 1994;12:955–957. doi: 10.1097/00004872-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 11.O'Donell CJ, Lindpaintner K, Larson MG, Rao VS, Ordovas JM, Schaefer EJ, Myers RH, Levy D. Evidence for association and genetic linkage of the angiotensin converting enzyme locus with hypertension and blood pressure in men but not women in the Framingham Heart Study. Circulation. 1998;97:1766–1772. doi: 10.1161/01.CIR.97.18.1766. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association, corp-author. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clinical Diabetes: A Publication of the American Diabetes Association. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO expert consultation. Appropriate body mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;157:163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay AJ, Morrissette H, Gagné JM, Bergeron J, Gagné C, Couture P. Validation of the Friedewald formula for the determination of low density lipoprotein cholesterol compared with β quantification in a large population. Clin Biochem. 2004;37:785–790. doi: 10.1016/j.clinbiochem.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Knoell DL, Johnston JS, Bao S, Kelley KA. A genotyping exercise for pharmacogenetics in pharmacy practice. Am J Pharm Educ. 2009;73:43. doi: 10.5688/aj730343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rigat B, Hubert C, Gelas F Alhenc, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marre M, Bernadet P, Gallois Y, Savagner F, Guyene TT, Hallab M, Cambien F, Passa P, Gelas F Alhenc. Relationship between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complication. Diabetes. 1994;43:384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 19.Samani N, Thompson JR, O'Toole L, Channer K, Woods KL. A meta analysis of the association of the deletion allele of the angiotensin converting enzyme gene with myocardial infarction. Circulation. 1996;94:708–712. doi: 10.1161/01.CIR.94.4.708. [DOI] [PubMed] [Google Scholar]
- 20.Zee RY, Lou YK, Griffiths LR, Morris BJ. Association of a polymorphism of the angiotensin I-converting enzyme gene with essential hypertension. Biochem Biophys Res Commun. 1992;184:9–15. doi: 10.1016/0006-291X(92)91150-O. [DOI] [PubMed] [Google Scholar]
- 21.Daimon M, Oizumi T, Saitoh T, kameda W, hirata A, Yamaguchi H, Ohnuma H, igarashi M, Tominaga M, Takeo KA. The D allele of the angiotensin converting enzyme insertion/deletion (I/D) polymorphism is a risk factor for type 2 diabetes in a population based Japanese sample. Endocr J. 2003;50:393–398. doi: 10.1507/endocrj.50.393. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh MC, Lin SR, Hsieh TJ, Hsu CH, Chen HC, Shin SJ, Tsai JH. Increased frequency of angiotensin converting enzyme DD genotype in patients with type 2 diabetes in Taiwan. Nephrol Dial Transplant. 2000;15:1008–1013. doi: 10.1093/ndt/15.7.1008. [DOI] [PubMed] [Google Scholar]
- 23.Say YH, Ling KH, Duraisamy G, Isaac S, Rosli R. Angiotensinogen M235T gene variants and its association with essential hypertension and plasma renin activity in Malaysian subjects: A case control study. BMC Cardiovasc Disord. 2005;5:7. doi: 10.1186/1471-2261-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasudevan R, Patimah I, Johnson S, Norashikin S. No association of BglI dimorphism of human renin gene in hypertensive subjects in Malaysia. Res J Biol Sci. 2008;3:1218–1822. [Google Scholar]
- 25.Ghazali DM, Rehman A, Rahman AR. Candidate gene polymorphisms and their association with hypertension in Malays. Clin Chim Acta. 2008;388:46–50. doi: 10.1016/j.cca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Tseng CH, Tseng CP, Chong CK, Sheu JJ, Cheng JC. Angiotensin converting enzyme gene polymorphism and stroke in type 2 diabetic patients in Taiwan. Eur J Clin Invest. 2007;37:483–491. doi: 10.1111/j.1365-2362.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 27.Nikzamir A, Nakhjavani M, Golmohamadi T, Dibai L. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with metabolic syndrome in Iranians with type 2 diabetes mellitus. Arch Iran Med. 2008;11:3–9. [PubMed] [Google Scholar]