Abstract
Treatment support is anticipated to improve the results of radiofrequency ablation (RFA) treatment in cases in which visualization of tumors using the conventional B-mode is unclear. In the present study, the effectiveness of treatment support for RFA reducing the local recurrence rate of hepatocellular carcinoma (HCC) that are located directly adjacent to the diaphragm, and which are difficult to visualize with B-mode ultrasound imaging, was investigated. A total of 103 HCC tumors measuring <5 cm, which were located abutting the diaphragm, and which were difficult to visualize using the B-mode, were treated using RFA. Thirty-three of those HCC tumors were treated using RFA without treatment support, whereas the remaining 70 HCC tumors were treated using RFA with treatment support, including artificial pleural effusion, contrast-enhanced ultrasonography (CEUS) with the contrasting agent, Sonazoid™, and fusion imaging, either alone or in combination to improve the visualization of the tumors. The rate of local recurrence, and factors affecting local recurrence, were analyzed. Local recurrences were confirmed in 17 of the 103 nodules (16.50%). The overall rate of local recurrence was 13.1% at 6 months, and 20.2% at 12 months. The rate of local recurrence using RFA with artificial pleural effusion was significantly lower compared with those cases of HCC tumors treated without artificial pleural effusion (P=0.008). Similarly, the rate of local recurrence for CEUS RFA with Sonazoid™ was significantly lower compared with those cases of HCC tumors treated without Sonazoid™ (P=0.00081). In a multivariate analysis, CEUS RFA with Sonazoid™ and artificial pleural effusion contributed to the decrease in the rate of local recurrence (hazard ratios, 0.075 and 0.143, respectively). Based on these results, it is possible to conclude that CEUS with Sonazoid™ as a treatment support was the most effective method for reducing the rate of local recurrences abutting the diaphragm that are difficult to visualize using B-mode ultrasonography.
Keywords: hepatocellular carcinoma, radiofrequency ablation, treatment support, Sonazoid™
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men, and the seventh most common cancer in women worldwide, and it is the most common cause of cancer-associated mortality (1). Radiofrequency ablation (RFA) was first described by Rossi et al (2) as a local thermal ablation therapy for HCC. It has been endorsed as a curative treatment modality by several clinical management guidelines for early-stage, unresectable HCC (3–9). However, to further improve the treatment outcomes of RFA for HCC, it is important to accurately identify the position of tumors on performing ultrasonography. Even in cases for which the visualization of tumors is difficult with the conventional B-mode ultrasound imaging, performing RFA using treatment support such as artificial pleural effusion, fusion imaging, and contrast-enhanced ultrasound with Sonazoid™ (Perflubutane; Daiichi Sankyo Co., Ltd., Tokyo, Japan), is expected to improve treatment outcomes in clinical settings (10).
The aim of the present study was to evaluate which treatment support most effectively contributed to reducing the rate of local recurrences of HCC directly abutting the diaphragm, which are difficult to visualize using B-mode ultrasound imaging, and the factors that affect local recurrences.
Patients and methods
A total of 103 HCC tumors, measuring <5 cm located immediately abutting the diaphragm and difficult to visualize using B-mode ultrasound imaging, were evaluated at the Saiseikai Niigata Daini Hospital, Niigata, Japan, between January 2009 and December 2015, including 33 HCC tumors treated using RFA without treatment support and 70 HCC tumors treated using RFA with artificial pleural effusion, contrast ultrasound, and fusion imaging, either individually or in combination for an improved visualization of the tumors.
HCC was diagnosed using dynamic contrast computed tomography (CT) or dynamic contrast magnetic resonance imaging (MRI). Exclusion criteria included: i) A tumor diameter of ≥5 cm; ii) infiltrating type with intentionally insufficient RFA; iii) a ≥4-month interval between RFA and the first follow-up CT or MRI; iv) no follow-up CT or MRI; and v) an observation period <6 months.
The ultrasound device used was equipped with a fusion imaging system (the Volume Navigation System; GE Healthcare, Milwaukee, WI, USA) and contrast-enhanced ultrasonography (CEUS) installed in LOGIQ E9 (GE Healthcare, Milwaukee, WI, USA). Treatment was performed in all patients through the percutaneous approach under ultrasound guidance, using the Angiodynamics (RITA Medical Systems, Inc., Mountain View, CA, USA) StarBurst XL (nine arrays; 5 cm) thermal ablation catheter, and the model 1500 (150–200 W power) generator, as previously described (11). This device has the capability to produce scalable, spherical ablations (of at least 5 cm). RFA was performed percutaneously in the present study by two hepatologists (T.I. and M.I.) with more than 10 years' of clinical experience in performing liver tumor ablation. No differences existed in the results for success rates of RFA between the two operators.
Fusion imaging
Fusion imaging was performed using volume data from the Volume Navigation System (V-Navi) installed in the ultrasound device. After the volume data of the CT or MRI images for reference had been imported in the DICOM (Digital Imaging and Communication in Medicine) format, the cross-section of ultrasound images approximately parallel to the reference was registered. Subsequently, one characteristic corresponding landmark between the ultrasound images and the reference was marked for each image as CT or MRI, and the position was aligned. In order to improve the registration accuracy, particularly in the vicinity of the tumor, the positional registration was repeatedly performed as close to the tumor as possible. After the positional registration, the multiplanar reconstruction images of CT or MRI were synchronously displayed side by side with the real-time ultrasound images.
CEUS using Sonazoid™
Sonazoid™ (Perflubutane; Daiichi Sankyo Co., Ltd.) was used as the CEUS agent (0.5 ml/body) in all of the examinations. The target lesions were scanned following injection in the arterial and Kupffer phases. The arterial phase of CEUS imaging was defined as that which occurred from 10–60 sec following the injection of Sonazoid™, and the Kupffer phase as that occurring 10 min following injection (10,12).
Method of inducing artificial pleural effusion
To infuse 5% glucose solution into the right pleural cavity without injuring the lung, a small skin incision was made on the chest wall under local anesthesia. The procedure was performed on patients under conscious sedation using a combination of 25 mg intramuscular hydroxyzine (Atarax P; Pfizer Japan Inc., Tokyo, Japan) and 15 mg pentazocine (Pentagin; Daiichi Sankyo Co., Ltd., Tokyo, Japan) administered 15 min prior to treatment. Following the injection of 10 ml 1% lidocaine (Xylocaine; AstraZeneca K.K., Osaka, Japan) into the peritoneum along the puncture line, percutaneous puncture was performed using a 21-gauge needle under sonographic guidance. Subsequently a drainage catheter kit (Hanaco Medical Co., Ltd., Saitama, Japan) was intrathoracically inserted through the chest wall. Once the needle entered the pleural cavity and no tissue resistance was encountered, the inner stylet extracts and guidewire (Teromo Clinical Supply Co., Ltd., Tokyo, Japan) were inserted into the pleural area. The outer catheter was inserted through the guidewire. Glucose solution (5%) was infused intrathoracically to separate the lung and liver; hence, it was possible to obtain an image of the hepatic dome (13,14).
During follow-up observation following treatment, local recurrence was evaluated by subsequent CT scans or MRI. All the patients were followed up every 3 months with measurement of the levels of serum α-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP), and enhanced CT or enhanced MRI. The detection of local tumor progression was defined as a recurrent tumor within, or adjacent to, the treated tumor.
The rate of local recurrence, and factors affecting local recurrence, were subsequently determined.
Ethics statement
Data are available from the Saiseikai Niigata Daini Hospital Data Access/Ethics Committee for researchers who meet the criteria for access to confidential data. The present study was approved by the Institutional Review Board of Saiseikai Niigata Daini Hospital (Niigata, Japan), and the study was conducted in accordance with the principles of the Declaration of Helsinki. All the patients provided their written informed consent.
Statistical analysis
The primary endpoint of the current clinical study was local recurrence. Local recurrence rates were calculated using the Kaplan-Meier method and log-rank tests, and the generalized Wilcoxon test was used for statistical analysis. Regarding the patient characteristics, statistical analyses were performed using the Fisher exact test and the Wilcoxon rank-sum test. A Cox proportional hazards model was used to identify independent factors of local recurrence. Statistical processing was performed using StatView version 5.0 software (SAS Institute, Cary, NC, USA). All reported P-values are two-sided, and P<0.05 was considered to indicate a statistically significant difference.
Results
The patients' baseline characteristics are summarized in Table I. The mean age was 71.56±10.33 years and the male-to-female ratio was 80:23. Among the 103 enrolled patients, 18 patients were seropositive for hepatitis B surface antigen, 54 patients were seropositive for hepatitis C virus antibody, and 31 patients were seronegative for both the hepatitis B surface antigen and the hepatitis C virus antibody. The mean AFP value was 122.53±396.92 ng/ml, the mean DCP value was 643.51±2,256.19 mAU/ml, the total bilirubin value was 0.67±0.34 mg/dl, the serum albumin value was 3.67±0.46 g/dl, and prothrombin activity was 96.32±14.73%. The mean observation period was 12.660±10.312 (range, 6–56) months. Artificial pleural effusion was used in 35 cases, CEUS with Sonazoid™ in 35 cases, and fusion imaging in 25 cases. The backgrounds of the patients with or without a supportive method were, for the most part, not significantly different (as shown in Tables II–IV for the patients with or without Sonazoid™, fusion analysis and artificial effusion as supportive methods, respectively), with the exception of the prothrombin activity data in the backgrounds of patients with or without fusion imaging, or artificial effusion, as supportive methods (P=0.02 and 0.032, respectively). Furthermore, there were no complications resulting from the supportive methods.
Table I.
Baseline characteristics of the study population.
| Demographic variable | Mean ± S.D. | Range |
|---|---|---|
| Age (years) | 71.56±10.33 | 31–86 |
| Sex (male:female ratio) | 80:23 | |
| Etiology (HBV/HCV/non-HBV, non-HCV) | 18/54/31 | |
| Size (mm) | 25.78±9.76 | 10–60 |
| AFP (ng/ml) | 122.53±396.92 | 1.5–2,578.7 |
| DCP (mAU/ml) | 643.51±2,256.19 | 10.0–14,874.0 |
| Serum albumin (g/dl) | 3.67±0.46 | 2.5–4.7 |
| Prothrombin activity (%) | 96.32±14.73 | 54.9–127.1 |
| Total bilirubin (mg/dl) | 0.67±0.34 | 0.21–2.1 |
| Artificial effusion? (yes/no) | 35:68 | |
| Sonazoid™? (yes/no) | 35:68 | |
| Fusion imaging? (yes/no) | 25:78 | |
| Child-Pugh Score (5/6/7/8) | 67/23/8/5 | |
| Class (A/B) | 90/13 | |
| With/without prior TACE | 76/27 |
HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; TACE, transarterial chemoembolization; S.D., standard deviation.
Table II.
Comparison of the backgrounds of patients with or without Sonazoid™ as a supportive method.
| Demographic variable | With Sonazoid™ (n=35) | Without Sonazoid™ (n=68) | P-value |
|---|---|---|---|
| Age (years) | 73.14±9.11 | 70.75±10.88 | 0.267 |
| Gender (male:female ratio) | 27:8 | 53:15 | 0.927 |
| Size (mm) | 24.14±8.86 | 26.64±9.88 | 0.213 |
| AFP (ng/ml) | 227.04±611.45 | 68.74±203.67 | 0.054 |
| DCP (mAU/ml) | 558.14±1,607.47 | 687.44±2536.24 | 0.785 |
| Serum albumin (g/dl) | 3.66±0.39 | 3.68±0.51 | 0.889 |
| Prothrombin activity (%) | 94.58±14.61 | 97.22±14.82 | 0.392 |
| Total bilirubin (mg/dl) | 0.68±0.35 | 0.67±0.33 | 0.797 |
| With/without prior TACE | 25:10 | 51:17 | 0.696 |
| Child-Pugh class (A/B) | 32/3 | 58/10 | 0.375 |
AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; TACE, transarterial chemoembolization.
Table IV.
Comparison of the backgrounds of patients with or without artificial effusion as a supportive method.
| Demographic variable | With artificial effusion (n=35) | Without artificial effusion (n=68) | P-value |
|---|---|---|---|
| Age (years) | 69.839.09 | 72.45±10.87 | 0.223 |
| Gender (male:female ratio) | 27:8 | 53:15 | 0.927 |
| Size (mm) | 26.23±9.19 | 25.47±8.93 | 0.695 |
| AFP (ng/ml) | 114.77±439.82 | 126.52±76.35 | 0.887 |
| DCP (mAU/ml) | 205.57±401.60 | 868.91±2,141.41 | 0.158 |
| Serum albumin (g/dl) | 3.66±0.56 | 3.67±0.41 | 0.959 |
| Prothrombin activity (%) | 91.98±18.02 | 98.56±12.27 | 0.031 |
| Total bilirubin (mg/dl) | 0.73±0.42 | 0.64±0.22 | 0.223 |
| With/without prior TACE | 23:12 | 53:15 | 0.181 |
| Child-Pugh class (A/B) | 29/6 | 61/7 | 0.322 |
AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; TACE, transarterial chemoembolization.
Of the total 103 nodules, local recurrences were confirmed in 17 nodules (16.50%). The overall rate of local recurrence was 13.1% at 6 months, and 20.2% at 12 months. Of the 33 nodules that were not provided with treatment support, local recurrences were confirmed in 12 nodules (36.3%), with a rate of local recurrence of 32.4% at 6 months, and 58.5% at 12 months. Only five of the 70 nodules given treatment support revealed local recurrences (7.1%). The rate of local recurrence was 6.2, 6.2, and 8.7% over 6, 12, and 24 months, respectively. Therefore, these results indicated that the treatment support significantly lowered the rate of local recurrences (P<0.05). The rate of local recurrence when fusion imaging was used was 8.7% for all the subjects at 6, 12, and 24 months; however, the rate of local recurrence for RFA without fusion imaging was 14.7, 25.1, and 28.4% over 6, 12, and 24 months, respectively.
A significant difference in the rate of local recurrence was identified with the use of artificial pleural effusion. The rate of local recurrence in RFA with artificial pleural effusion was 6.0% at 6 and 12 months. The rate of local recurrence in RFA without artificial pleural effusion was 18.0% over 6 months, and 30.4% over 12 months, showing a significant increase (P=0.008).
A significant difference in the rate of local recurrence of RFA was also identified, depending on whether or not CEUS with Sonazoid™ was performed. The rate of local recurrence in CEUS RFA with Sonazoid™ was 0.0, 0.0, and 5.9% at 6, 12, and 24 months, respectively. The rate of local recurrence in RFA CEUS without Sonazoid™ was 20.6, 31.0, and 31.0% over 6, 12, and 24 months, respectively (P=0.0052) (Fig. 1), indicating a significant increase.
Figure 1.
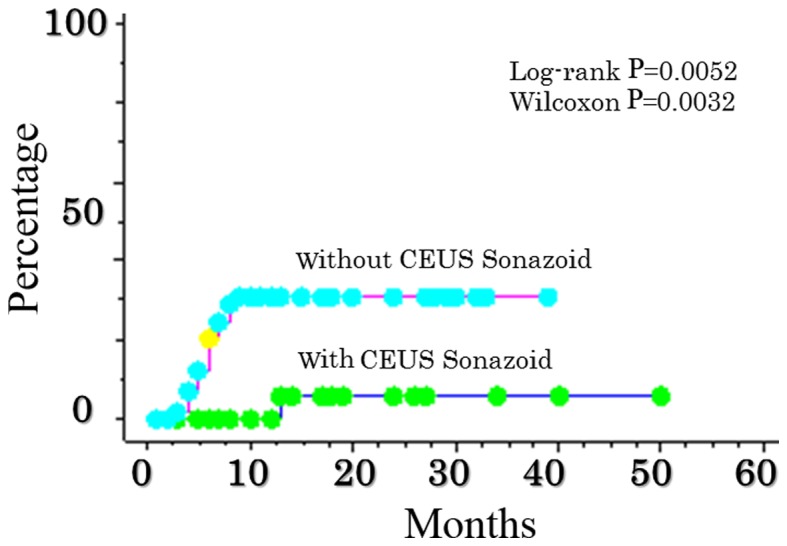
Comparison of local recurrence after RFA abutting the sub-diaphragm as determined from the use, or not, of CEUS ultrasonography with Sonazoid™. The rate of local recurrence in contrast ultrasonographic RFA with Sonazoid™ was 0.0, 0.0, and 5.9% at 6, 12, and 24 months, respectively. The rate of contrast ultrasonography without Sonazoid™ was 20.6, 31.0 and 31.0% over 6, 12 and 24 months, respectively (P=0.0052). CEUS, contrast-enhanced ultrasonography.
No significant differences in the rate of local recurrence according to the size of the tumors or measurements of hepatic function reserve, such as the Child-Pugh score, were identified. There was no local recurrence in 21 cases: Four of those cases were treated with artificial pleural effusion, fusion imaging, and Sonazoid™ CEUS; three cases were treated with fusion imaging and Sonazoid™ CEUS; seven cases were treated with artificial pleural effusion and Sonazoid™ CEUS; and seven cases were treated with artificial pleural effusion and fusion imaging.
In addition, in a multivariate analysis of these treatment factors, the use of CEUS with Sonazoid™ and artificial pleural effusion contributed to a significantly lower rate of local recurrences, with hazard ratios of 0.075 and 0.143, respectively (Table V).
Table V.
Prognostic factors related to local recurrence determined by multivariate analysis using the Cox proportional hazard model.
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Child-Pugh class | |||
| A | 0.948 | 0.210–4.281 | 0.944 |
| B | 1 | ||
| Size | |||
| ≥30 mm | 1.680 | 0.460–6.141 | 0.433 |
| <30 mm | 1 | ||
| Artificial effusion | |||
| With | 0.143 | 0.031–0.6.61 | 0.013 |
| Without | 1 | ||
| Fusion imaging | |||
| With | 0.223 | 0.047–1.052 | 0.058 |
| Without | 1 | ||
| Sonazoid™ | |||
| With | 0.075 | 0.010–0.571 | 0.012 |
| Without | 1 |
HR, hazard ratio, CI, confidence interval.
Discussion
HCC is the most frequent primary hepatic malignancy, and has been recognized as the predominant cause of mortality in patients with cirrhosis (1). Various types of nonsurgical treatments have been developed. RFA is able achieve complete tumor necrosis (2–9,15,16), and has become a principal treatment in numerous institutions for patients with small HCC tumors.
RFA was first used for the treatment of HCC in humans in 1993 (2) following extensive animal studies (17). RFA has since emerged as a popular local ablative therapy for unresectable HCC due to its efficacy and safety.
RFA is currently recognized as an effective local treatment (18) in patients with Barcelona Clinic Liver Cancer (BCLC) early stage HCC who are not eligible for surgical treatments. RFA has also been established as a safe, effective percutaneous technique for patients with HCC. RFA with a percutaneously inserted electrode is suitable as a method for a more complete ablation of tumors compared with other locoregional treatments, thereby leading to a reduction in the rate of local recurrence (19).
Local recurrence following the successful ablation of HCC using RFA is therefore an important issue. However, incomplete tumor ablation (20) and local tumor recurrence or progression remain problematic (21). In order to prevent local recurrence, Hirooka et al (22) reported that the safety margin for RFA should be defined as the blood drainage area, and ablation should aim at acquiring adequate safety margins. In the present study, CT during arterial portography (CTAP) and CT during arteriography (CTA) were performed on the patients with TACE. Furthermore, to assess the ablated area and complications, dynamic CT was performed within 3 days of the ablation. The goal of the treatment was to achieve complete ablation of the hypoattenuating areas visualized during the portal venous phases, and extending beyond the tumor itself. Additional ablation sessions were scheduled if the presence of residual lesions was confirmed. The diagnostic and treatment procedures were repeated until complete ablation was achieved during a single hospitalization.
Since it is usually performed under ultrasound guidance, cases in which the whole tumor could not be visualized due to being located immediately under the subdiaphragm, or in contact with adjacent organs, were considered difficult to treat using RFA. If complete necrosis of the tumor is attempted for these difficult cases using RFA, assisting techniques might be required in order to prevent local recurrence. When performing RFA with ultrasound guidance, it is important from the viewpoint of operative techniques, indications, and implementation criteria that the whole tumor is visualized. In recent years, RFA has become more precise and safer, with advances being made in ultrasound technologies, such as CEUS and fusion imaging. Treatment support can also be administered with artificial pleural effusion. It has already been reported that RFA with artificial pleural effusion is effective in treating difficult cases; however, there has been insufficient examination of RFA compared with other modalities.
In the present study, HCC tumors in which the whole tumor could not be visualized due to being located abutting the diaphragm were examined using RFA with CT/MRI fusion imaging, artificial pleural effusion, and CEUS with Sonazoid™. In January 2007, Sonazoid™ was approved as a new CEUS agent in Japan (10). With the development of Sonazoid™, a second-generation ultrasound contrast agent, low sound pressure irradiation is used to inhibit the collapse of bubbles, and the contrast effects may be observed continuously in real time. In the vascular phase, tumor hemodynamics may be visualized. In the post-vascular phase 10 min after administration, by taking advantage of its tendency to be taken up by Kupffer cells, tumors without Kupffer cells may also be clearly visualized.
Sonazoid™ CEUS is thought to offer visualization of tumors that is equivalent to that offered by CT angiography (23,24). Even nodules that were not able to be visualized using the B-mode could be clearly visualized using Sonazoid™ CEUS, and precise localized treatment was therefore possible. Even lesions that are clearly identified by contrast CT and MRI may not be clearly identified by B-mode ultrasound due to the background hepatic conditions or artifacts from previous cauterization. In such cases, observation with Sonazoid™ CEUS may be effective. In addition, compared with the historical controls that were in place prior to the availability of Sonazoid™, the number of RFA sessions per treatment is reported to have decreased (25). Therefore, the effectiveness of Sonazoid™ CEUS in RFA has been confirmed. When using Sonazoid™ with RFA, a puncture may be made while observing early staining on vascular imaging immediately following an injection of Sonazoid™. Alternatively, the puncture may be made for a Kupffer defect via Kupffer imaging 10–15 min after the injection. If it is already known that a Kupffer defect would not be clear, the puncture may be made using vascular imaging. However, as there is only a short period of time in which early staining is clear (a few sec), puncture with vascular imaging requires a high level of skill. In the present study, the effectiveness of RFA puncture with Sonazoid™ CEUS was examined using Kupffer imaging.
Furthermore, recently, another type of treatment support called ‘fusion imaging’ as V-Navi, a volume navigation system that allows for display and observation of real-time ultrasound imaging in combination with other modalities, has been developed (26). This system makes it possible to perform real-time observation of ultrasound images fused with three-dimensional ultrasound images that were previously collected. Although ultrasonography has numerous advantages over other modalities, it is considered inferior to CT in terms of its dependence on the examiner and objectivity. To compensate for these disadvantages, the V-Navi system was developed so that images obtained using other modalities could be added to the ultrasonography images, and fused for display. Although this may be extremely effective in certain cases, if the hepatic condition during treatment differs from that during CT imaging due to respiration, body position, and the condition of the surrounding organs, the CT and ultrasound images may not correctly overlap.
In the present study, images obtained following treatment deviated from those obtained prior to the operation in certain of the cases in which fusion imaging was used with artificial pleural effusion. The artificial pleural effusion technique, which is easier to perform than the artificial ascites technique, is used in numerous institutions to prevent lung obstruction and avoid complications (27,28).
Although no significant differences were observed in the suppression of local recurrence with fusion imaging only, the rate of local recurrence was sufficiently decreased in all the groups that received support treatment compared with the groups that did not. Therefore, a further examination of the indications pertaining to fusion imaging is required.
The limitations of the present study were its retrospective nature and the inclusion of only a small number of patients. Furthermore, CEUS with Sonazoid™ suffers from a weakness in being less suitable for imaging in deep positions. However, the present study identified that CEUS with Sonazoid™ was the most effective of the three types of treatment support in reducing the rate of local recurrences of lesions near the diaphragm that were difficult to visualize using the B-mode. In the future, further examinations with a larger number of sample sizes will be required to be performed. However, the results of the present study have suggested that the combined use of various types of treatment support for lesions adjacent to the diaphragm that are difficult to visualize using the B-mode is effective, as it makes achieving safe and favorable treatment outcomes possible.
Table III.
Comparison of the backgrounds of patients with or without fusion imaging as a supportive method.
| Demographic variable | With fusion (n=25) | Without fusion (n=78) | P-value |
|---|---|---|---|
| Age (years) | 74.00±8.26 | 70.78±10.89 | 0.177 |
| Gender (male:female ratio) | 23:2 | 57:21 | 0.089 |
| Size (mm) | 26.83±13.29 | 25.46±8.18 | 0.542 |
| AFP (ng/ml) | 19.31±37.03 | 155.62±451.34 | 0.135 |
| DCP (mAU/ml) | 578.64±1,727.24 | 664.29±2,410.69 | 0.869 |
| Serum albumin (g/dl) | 3.75±0.41 | 3.64±0.48 | 0.302 |
| Prothrombin activity (%) | 88.64±11.67 | 98.78±14.82 | 0.002 |
| Total bilirubin (mg/dl) | 0.68±0.26 | 0.67±0.35 | 0.882 |
| With/without prior TACE | 18:7 | 58:20 | 0.815 |
| Child-Pugh class (A/B) | 24/1 | 66/12 | 0.136 |
AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; TACE, transarterial chemoembolization.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J Interv Radiol. 1993;8:97–103. [Google Scholar]
- 3.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of liver diseases: Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. HCC Expert Panel of Japan Society of Hepatology: Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 5.Livraghi T. Treatment of hepatocellular carcinoma by interventional methods. Eur Radiol. 2001;11:2207–2219. doi: 10.1007/s003300100889. [DOI] [PubMed] [Google Scholar]
- 6.Rhim H, Lim HK, Choi D. Current status of radiofrequency ablation of hepatocellular carcinoma. World J Gastrointest Surg. 2010;2:128–136. doi: 10.4240/wjgs.v2.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22–29. doi: 10.1177/145749691110000105. [DOI] [PubMed] [Google Scholar]
- 8.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 9.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: Treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 10.Kindberg GM, Tolleshaug H, Roos N, Skotland T. Hepatic clearance of Sonazoid perfluorobutane microbubbles by Kupffer cells does not reduce the ability of liver to phagocytose or degrade albumin microspheres. Cell Tissue Res. 2003;312:49–54. doi: 10.1007/s00441-003-0698-0. [DOI] [PubMed] [Google Scholar]
- 11.Guglielmi A, Ruzzenente A, Battocchia A, Tonon A, Fracastoro G, Cordiano C. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480–484. [PubMed] [Google Scholar]
- 12.Watanabe R, Matsumura M, Chen CJ, Kaneda Y, Fujimaki M. Characterization of tumor imaging with microbubble-based ultrasound contrast agent, sonazoid, in rabbit liver. Biol Pharm Bull. 2005;28:972–977. doi: 10.1248/bpb.28.972. [DOI] [PubMed] [Google Scholar]
- 13.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Inoue T, Sakaguchi Y, Sakamoto H, Shiozaki H. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003;38:1066–1070. doi: 10.1007/s00535-003-1197-5. [DOI] [PubMed] [Google Scholar]
- 14.Minami Y, Kudo M, Kawasaki T, Chang H, Ogawa C, Shiozaki H. Percutaneous radiofrequency ablation guided by contrast enhanced harmonic sonography with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol. 2004;182:1224–1226. doi: 10.2214/ajr.182.5.1821224. [DOI] [PubMed] [Google Scholar]
- 15.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, Bartolozzi C. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Fornari F, Paties C, Buscarini L. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig livers. Tumori. 1990;76:54–57. doi: 10.1177/030089169007600114. [DOI] [PubMed] [Google Scholar]
- 18.European Association For The Study Of The Liver1, corp-author; European Organisation For Research And Treatment Of Cancer, corp-author. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, Omata M. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Pulvirenti A, Garbagnati F, Regalia E, Coppa J, Marchiano A, Romito R, Schiavo M, Fabbri A, Burgoa L, Mazzaferro V. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation; Transplant Proc; 2001; pp. 1516–1517. [DOI] [PubMed] [Google Scholar]
- 21.Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, Torre A Dela, Cho KC, Contractor D, Korogodsky M. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–764. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]
- 22.Hirooka M, Ochi H, Koizumi Y, Tokumoto Y, Hiraoka A, Kumagi T, Abe M, Tanaka H, Hiasa Y. Local recurrence of hepatocellular carcinoma in the tumor blood drainage area following radiofrequency ablation. Mol Clin Oncol. 2014;2:182–186. doi: 10.3892/mco.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M. Diagnostic imaging of hepatocellular carcinoma: Recent progress. Oncology. 2011;81(Suppl 1):S73–S85. doi: 10.1159/000333265. [DOI] [PubMed] [Google Scholar]
- 24.Mita K, Kim SR, Kudo M, Imoto S, Nakajima T, Ando K, Fukuda K, Matsuoka T, Maekawa Y, Hayashi Y. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol. 2010;16:4187–4192. doi: 10.3748/wjg.v16.i33.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, Kondo Y, Goto T, Ikeda H, Omata M, Koike K. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759–764. doi: 10.1111/j.1440-1746.2010.06559.x. [DOI] [PubMed] [Google Scholar]
- 26.Makino Y, Imai Y, Igura T, Kogita S, Sawai Y, Fukuda K, Hori M, Kudo M, Murakami T. Usefulness of the extracted-overlay function in CT/MR-ultrasonography fusion imaging for radiofrequency ablation of hepatocellular carcinoma. Dig Dis. 2013;31:485–489. doi: 10.1159/000355257. [DOI] [PubMed] [Google Scholar]
- 27.Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographicially guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583–588. doi: 10.2214/ajr.183.3.1830583. [DOI] [PubMed] [Google Scholar]
- 28.Fukuno H, Tamaki K, Urata M, Kohno N, Shimizu I, Nomura M, Ito S, Saito K. Influence of an artificial pleural effusion technique on cardio-pulmonary function and autonomic activity. J Med Invest. 2007;54:48–53. doi: 10.2152/jmi.54.48. [DOI] [PubMed] [Google Scholar]


