Abstract
Localization of prostate cancer recurrence, particularly in the bones, is a major challenge with standard of care imaging in patients with biochemical recurrence following curatively intended treatment. Gallium-68-labeled prostate specific membrane antigen positron emission tomography/computed tomography (68Ga-PSMA PET/CT) is a novel and promising method for imaging in prostate cancer. The present study reports two cases of patients with prostate cancer with biochemical recurrence, with evidence of bone metastases on 68Ga-PSMA PET/CT images and low prostate specific antigen PSA levels (<2 ng/ml) and PSA doubling time >6 months. The bone metastases were verified by supplementary imaging with 18F-sodium fluoride PET/CT and magnetic resonance imaging as well as biochemical responses to androgen deprivation therapy. Therefore, 68Ga-PSMA PET/CT is promising for the restaging of patients with prostate cancer with biochemical recurrence, including patients with low PSA levels and low PSA kinetics.
Keywords: biochemical recurrence, 68Ga-prostate-specific membrane antigen, positron emission tomography, prostate-specific antigen, prostate-specific antigen kinetics
Introduction
Localization of prostate cancer recurrence is a major challenge in patients with biochemical recurrence following curatively intended treatment. The currently applied imaging modalities such as bone scintigraphy and abdominal pelvic computed tomography (CT) are too insensitive, particularly at low serum prostate-specific antigen (PSA) values (1). These two modalities only reliably show the site of relapse in patients with very high PSA levels (>10 ng/ml) (2). This is of particular importance as salvage radiotherapy in these patients is most effective at serum PSA values <0.5 ng/ml (3,4).
Established positron emission tomography (PET) tracers such as 11C- or 18F-choline or 11C-acetate are able to directly detect tumor tissue, which is also true at the bone level, where these tracers show tumor tissue, as opposed to reactive bone as with bone scans and 18F-sodium fluoride (NaF) PET/CT. This result may convey higher sensitivity for both osteoblastic and osteolytic bone lesions at low PSA levels (5). However, the majority of large series have included patients with a PSA level >2 ng/ml (6,7). Most studies that included patients with low PSA levels have shown infrequent bone metastases, and little information is provided concerning tumor characteristics in individual patients (8–11). Generally, choline PET is only indicated in biochemical failure if the PSA level is >2 ng/ml or the PSA level is rising rapidly (12).
Gallium-68-labelled prostate-specific membrane antigen (68Ga-PSMA) PET/CT has recently been introduced as a promising method for prostate cancer imaging, both for staging and restaging (13–17). In contrast to existing PET tracers, 68Ga-PSMA PET has revealed pathological sites of uptake, even with low PSA levels, in several retrospective series (13–17).
In 2015, the present authors initiated two prospective trials with 68Ga-PSMA PET/CT in prostate cancer, including a study of PSMA-11 (DKFZ-PSMA-11, also known as PSMA-HBED-CC) PET/CT in recurrent prostate cancer in comparison with magnetic resonance imaging (MRI) and NaF PET/CT (EudraCT no.; 2014-005073-37). The trial was approved by the Danish Health and Medicine Authority, The Danish Data Protection Agency, and the North Denmark Region Committee in Health Research Ethics. The patients received written and oral information and provided written informed consent, inclusive presentation of individual study results in a blinded fashion. The current study reports two cases of bone metastasis in patients with PSA levels <2 ng/ml and slow PSA kinetics.
Case reports
Case one
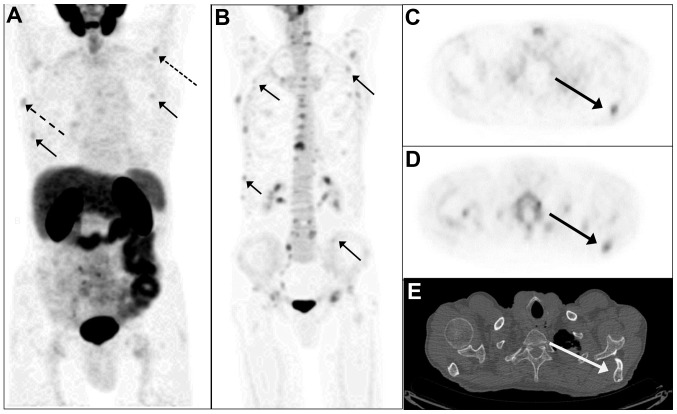
A 70-year old male was included in the present study in October 2015 due to biochemical recurrence of a prostate cancer. Five years previously, the patient underwent radical prostatectomy due to prostate cancer (T2c, Gleason 7 (3+4), with a PSA level of 10.9 ng/ml). The PSA values remained unmeasurable (<0.1 ng/ml) for 4 years but then increased to 0.4 ng/ml. The PSA doubling time (PSAdt) was 22.5 months. A 68Ga-PSMA-11 PET/CT scan with low-dose CT showed pathological PSMA uptake in several bone lesions, with corresponding morphological findings in the CT scan, including the right and left scapula, right 5th rib and left 3rd rib (Fig. 1). There was no pathological uptake in the lymph nodes, prostatic bed or soft tissues. In addition, the patient underwent an NaF PET/CT scan, which confirmed the skeletal findings on the 68Ga-PSMA PET/CT, but also revealed at least four additional bone metastases (ribs and the left iliac bone), but without corresponding changes on the CT. The NaF PET/CT scan identified numerous sites of enhanced uptake associated with benign, degenerative bone disorders. The MRI scan did not identify any enlarged lymph nodes in the pelvis. A slightly heterogeneous bone marrow signal was observed in the pelvis and lumbar spine on T1-weighted images but without the anatomical appearance of bone metastases, and a normal signal on the short TI-inversion recovery (STIR) sequences. In conclusion, the MRI was negative for bone metastases. Notably, the MRI covered the pelvis and spine only as recommended by the European Society of Urological Oncology guideline for nodes and bone (18). Thus, the MRI was not able to confirm the four skeletal lesions. The patient received leuprorelin acetate and six cycles of docetaxel. The PSA level dropped to an unmeasurable level (nadir PSA level <0.1 ng/ml).
Figure 1.
Prostate cancer imaging of biochemical recurrence. (A) MIP of the 68Ga-PSMA-11 PET image in the anterior view. The small, full arrows indicate pathologic PSMA uptake in the ribs (right 5th rib and left 3rd rib). The dotted arrow indicates a lesion in the left scapula, and the hatched arrow indicates uptake in the right scapula. (B) MIP of 18F-NaF-PET/CT confirmed the PSMA bone lesions shown in (A) and showed additional lesions in the ribs, the left scapula) and the right scapula (all full arrows). Axial 68Ga-PSMA PET image of the thorax shows pathologic PSMA-uptake in the left scapula (C) (indicated by the arrow), confirmed by 18F-NaF PET (D) and by mixed osteosclerotic and osteolytic lesions on low dose CT (E) (arrow). MIP, maximum-intensity projection; PSMA, prostate specific membrane antigen; PET, positron emission tomography.
Case two
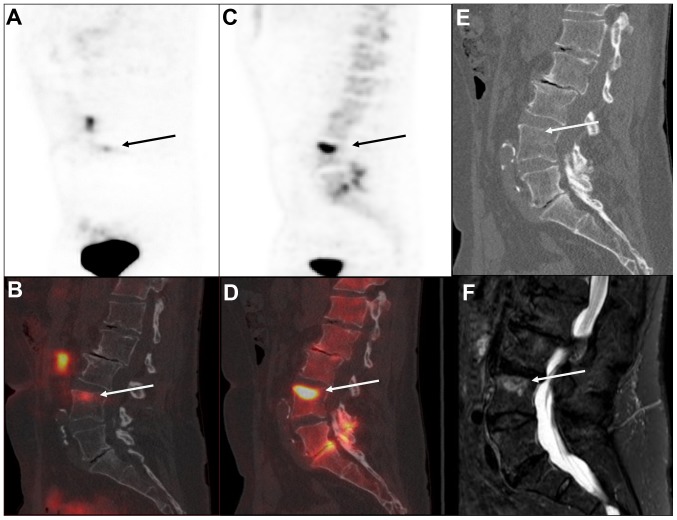
A 71-year old male was diagnosed with prostate cancer (pT2b, Gleason 7, with a PSA level of 16.1 ng/ml) in 2002. The patient received a radical prostatectomy with postoperative normalization of PSA levels (PSA level <0.1 ng/ml). Due to biochemical recurrence (a peak PSA level of 0.6 ng/ml) in 2006, the patient received salvage radiotherapy in 5 fields with a central dose of 48 Gray (34 fractions) supplemented with 12 months of treatment with flutamide. The PSA levels normalized within the first year following radiotherapy. In October 2014, the patient presented with an elevated PSA level (0.4 ng/ml) that further increased to 1.8 ng/ml at the time of evaluation in August 2016 (PSAdt 8.7 months). A 68Ga-PSMA-11 PET/CT scan with low-dose CT revealed PSMA uptake in four small lymph nodes on the left side of the pelvis and a skeletal lesion at the level of fourth lumbar vertebra (Fig. 2). Low-dose CT showed a discrete sclerotic lesion at L4. There was no pathological PSMA uptake in other sites. The NaF PET/CT confirmed the lesion at L4 as a bone metastasis. There were no additional malignant bone lesions identified. The MRI found that the four lymph nodes detected on 68Ga-PSMA PET/CT were of normal size. The skeletal lesion was suspicious for malignancy on the MRI scan due to a low signal in the T1 image and high signal on the STIR sequence. However, a slight depression of the upper discus at L4 caused the final MRI-based diagnosis to be equivocal for bone metastasis. The patient subsequently received treatment with bicalutamide, which caused the PSA level to decline to an unmeasurable level (<0.1 ng/ml).
Figure 2.
Imaging of bone metastasis in a patient with prostate cancer with biochemical recurrence. A single bone metastasis was identified in the upper part of the fourth lumbar vertebra indicated by the arrow in all the images. The lesion was identified by 68Ga-PSMA-11 PET/CT as shown in (A) the sagittal view of the 68Ga-PSMA-11 PET image with (B) the corresponding fused 68Ga-PSMA-11 PET/CT and (C) by 18F-NaF-PET with (D) corresponding fused images of NaF PET/CT. Morphological changes were recognized as (E) discrete sclerotic changes on the low-dose CT and (F) by magnetic resonance imaging exhibiting a high signal on the sagittal short TI-inversion recover image. PSMA, prostate specific membrane antigen; PET, positron emission tomography.
Discussion
Imaging in cases of biochemical recurrence of prostate cancer has been hampered by the lack of appropriately sensitive modalities, particularly for bone lesions. Bone scans and CT in general do not have adequate sensitivity, and choline PET is only indicated if the PSA level is >2 ng/ml or if the PSA has rapidly rising kinetics (e.g., PSAdt <6 months). The current study presents two cases of pathological 68Ga-PSMA uptake on PET/CT scans with PSA levels <2 ng/ml and slow PSA kinetics. These data, along with a number of cases identified in the published literature (Table I, data captured from a search of 1,858 references in an ongoing systematic review with a cutoff date of August 2016), indicated that 68Ga-PSMA PET/CT may be a valuable imaging technique to localize disease in very early biochemical recurrence.
Table I.
Published data with identification of bone metastases by 68Ga-PSMA PET/CT in patients with recurrent prostate cancer and a PSA level <2 ng/ml.
| Trigger PSA (ng/ml) | T stage at staging | PSA at staging (ng/ml) | Gleason at staging | Treatment | PSA nadir | Years since last Tx | PSA velocity (ng/ml/month) | PSAdt (months) | Number of bone metastases | Other sites | Concurrent imaging or biopsy of bone lesions | Post-imaging follow up | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | – | – | 9 | – | – | – | – | – | 1 | None | – | – | (18) |
| 0.1 | – | – | 9 | – | – | – | – | – | 3 | None | – | – | (19) |
| 0.1 | – | – | 7 | – | – | – | – | – | 3 | LN | – | – | (18) |
| 0.1 | pT3a | – | 7 | RP (Rx) | – | – | – | – | – | – | – | – | (13) |
| 0.19 | – | – | 7 | RP (Rx) | – | – | – | – | 1 | LN | – | – | (20) |
| 0.28 | – | – | – | ADT | – | – | – | – | – | Prostatic bed, LN | Positive bone on scintigraphy | (21) | |
| 0.4 | pT2c | 10.9 | 7 | RP (R0) | <0.1 | 5 | 0 | 22.5 | 4 | None | Confirmed by NaF PET/CT | Response to ADT and docetaxel with PSA decrease (nadir <0.1 ng/ml) | This report |
| 0.43 | – | – | 7 | – | – | – | – | – | 1 | None | Bone biopsy | – | (22) |
| 0.48 | – | – | 7 | – | – | – | – | – | 1 | None | – | – | (19) |
| 0.55 | pT3a | 8.3 | 7 | RP (Rx) | <0.007 | – | – | 15.4 | 1 | None | – | Last PSA 0.22 ng/ml | (23) |
| 0.56 | pT2c | 8.6 | 7 | RP (Rx) + RT (prostate) | <0.007 | – | – | 6.3 | 1 | None | – | Last PSA 0.12 ng/ml | (23) |
| 0.56 | pT2c | 10.8 | 7 | RP (Rx), RT (prostate), ADT | <0.007 | – | – | 1.0 | 2 | None | – | – | (23) |
| 0.7 | pT3a | 13.5 | 8 | RP (R1) | – | 12a | – | – | 2 | none | 2 suspicious lesions on MRI, 1 on CT | Response to RT with PSA decrease (nadir <0.1ng/ml) AND confirmation by post-treatment PSMA PET/CT | (24) |
| 0.8 | pT3b | 27.0 | 8 | RP (R1) | 4a | – | 1 | None | 1 suspicious lesion on MRI, 0 on CT | Response to ADT with PSA decrease (nadir <0.1 ng/ml) | (24) | ||
| 1.0 | pT3b | 9.1 | 7 | RP (R0) | – | 6a | – | – | 1 | LN | 1 suspicious lesion on MRI, 1 on CT | Response to RT and ADT with PSA decrease (nadir <0.1 ng/ml) AND confirmation by post-Tx PSMA PET/CT | (24) |
| 1.35 | – | – | – | RP (Rx) +ADT | – | – | – | – | Multiple | – | – | – | (25) |
| 1.5 | pT3b | 21.2 | – | RP (R1) | – | 7a | – | – | 1 | None | 1 suspicious lesion on MRI, 1 on CT | Response to ADT with PSA decrease (nadir <0.1 ng/ml) | (24) |
| 1.70 | – | – | 7 | – | – | – | – | – | 1 | None | – | – | (18) |
| 1.71 | – | – | 8 | RP (Rx) | – | – | – | – | 2 | LN | – | – | (20) |
| 1.8 | pT2b | 16.1 | 7 | RP (Rx) (2002), salvage RT (2006). | <0.1 | 8 | 0.02 | 8.7 | 1 | LN | Confirmation by NaF PET/CT. Equivocal on MRI | Response to ADT with PSA decrease (nadir <0.1 ng/ml) | This report |
| 1.86 | – | – | 8 | RP (Rx) + ADT | – | – | – | – | 3 | Prostate | – | – | (20) |
ADT, androgen-deprivation therapy; CT, computed tomography; LN, lymph node; MRI, magnetic resonance imaging; NaF, 18F-sodium fluoride; PSA, prostate-specific antigen; pT, pathological T stage; PET, positron emission tomography; PSAdt, PSA doubling time; RP (R1), radical prostatectomy with positive margins; RP (Rx), radical prostatectomy with unknown status for margins; RT, radiotherapy; Tx, treatment; ‘−’, not reported.
Time from year of diagnosis to year of publication of the paper.
68Ga-PSMA PET/CT identified pathological uptake in the bones in the first patient and in both the lymph nodes and the bones in the other patient. One of the current cases, as well as a number of cases presented in Table I, were identified to have a solitary site relapse in the bone. From a clinical point of view, the majority of published cases have a minimum amount of clinical and laboratory data to indicate if relapse was expected to occur at the prostate level, in lymph nodes or in the bones. The missing information includes data about resection margins, the nodal status at staging and/or surgery, and the levels and duration of post-treatment PSA levels (or time since curative treatment).
The presented data with 68Ga-PSMA PET/CT use in early recurrence are encouraging. Similar data with other PET tracers are scarce in the published literature. In several previous studies with choline PET/CT, no bone lesions were detected in early biochemical recurrence (10,11). Kjolhede et al (8) presented 5 patients with suspected bone lesions among 58 patients with biochemical recurrence and PSA levels of <2 ng/ml, but there were no data on PSA kinetics in patients with bone lesions. Castellucci et al (9) performed 11C-choline PET/CT in 605 patients with biochemical recurrence and PSA levels of 0.2–2.0 ng/ml and a median PSAdt of 6 months, and showed pathological bone uptake in 51/172 patients with choline-positive scans. The PSAdts of these 51 patients were not reported. It is appropriate to say that lesions detected by choline PET/CT must be cautiously interpreted due to low to moderate specificity; a study with verification by histology showed a low predictive value of 24% at the node level (19).
A number of trials are emerging that directly compare 68Ga-PSMA with other PET tracers and standard imaging in patients with biochemical recurrence. Although 68Ga-PSMA PET/CT appeared to detect more lesions than choline PET/CT, both on the whole patient level and at the level of individual lesions, the majority of studies include patients with high PSA levels, and there are limited data on skeletal involvement and characteristics of individual patients (20–22).
68Ga-PSMA PET/CT is developing rapidly (16,17). However, most studies are retrospective reports with inherent methodological deficiencies, including lack of compliance with the standards for reporting of diagnostic accuracy (STARD) criteria (23). One key feature of the STARD guidelines is the definition of the reference test. In the absence of a true reference, it remains unknown if PET uptake parallels tumor recurrence. This is well known with choline PET/CT (19), and it is shown with suspicious lesions without sclerosis on CT with NaF PET as in the current case no. 1. In the present cases, skeletal malignancy was confirmed in both the patients with positive findings with two functional methods (68Ga-PSMA and NaF PET) and at least one anatomical method (low-dose CT and/or MRI). In addition, both patients responded biochemically to anti-cancer treatment.
In conclusion, 68Ga-PSMA PET/CT has emerged as a very promising imaging technique for use in identifying tumor sites in patients with biochemical recurrence. In comparison with standard imaging modalities as well as existing PET tracers, 68Ga-PSMA PET/CT appeared to be sensitive at very low PSA levels (and in patients with slow PSA kinetics), which is optimal in terms of salvage radiotherapy.
Acknowledgements
The present study was supported by an unrestricted grant from the Obel Family Foundation.
Glossary
Abbreviations
- CT
computed tomography
- 68Ga
Gallium-68
- MRI
magnetic resonance imaging
- NaF
18F-sodium fluoride
- PET
positron emission tomography
- PSMA
prostate specific membrane antigen
- PSA
prostate specific antigen
- PSAdt
PSA doubling time
- STARD
the standards for reporting of diagnostic accuracy
References
- 1.Vargas HA, Martin-Malburet AG, Takeda T, Corradi RB, Eastham J, Wibmer A, Sala E, Zelefsky MJ, Weber WA, Hricak H. Localizing sites of disease in patients with rising serum prostate-specific antigen up to 1 ng/ml following prostatectomy: How much information can conventional imaging provide? Urol Oncol. 2016;34:482.e5–482.e10. doi: 10.1016/j.urolonc.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, Foley JP, Riffenburgh RH, Moul JW. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. doi: 10.1016/S0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, Anscher MS, Michalski JM, Sandler HM, Lin DW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, Buskirk SJ. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016;pii:JCO683425. doi: 10.1200/JCO.2016.68.3425. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 5.Ceci F, Castellucci P, Graziani T, Schiavina R, Chondrogiannis S, Bonfiglioli R, Costa S, Virgolini IJ, Rubello D, Fanti S, Colletti PM. 11C-choline PET/CT identifies osteoblastic and osteolytic lesions in patients with metastatic prostate cancer. Clin Nucl Med. 2015;40:e265–e270. doi: 10.1097/RLU.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 6.Fanti S, Minozzi S, Castellucci P, Balduzzi S, Herrmann K, Krause BJ, Oyen W, Chiti A. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016;43:55–69. doi: 10.1007/s00259-015-3202-7. [DOI] [PubMed] [Google Scholar]
- 7.Sobol I, Zaid HB, Haloi R, Mynderse LA, Froemming AT, Lowe VJ, Davis BJ, Kwon ED, Karnes RJ. Contemporary mapping of post-prostatectomy prostate cancer relapse with c-11-choline positron emission tomography and multiparametric magnetic resonance imaging. J Urol. 2017;197:129–134. doi: 10.1016/j.juro.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 8.Kjölhede H, Ahlgren G, Almquist H, Liedberg F, Lyttkens K, Ohlsson T, Bratt O. (18)F-choline PET/CT for early detection of metastases in biochemical recurrence following radical prostatectomy. World J Urol. 2015;33:1749–1752. doi: 10.1007/s00345-015-1547-y. [DOI] [PubMed] [Google Scholar]
- 9.Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, Pettinato C, Celli M, Lodi F, Fanti S. Early biochemical relapse after radical prostatectomy: Which prostate cancer patients may benefit from a restaging 11C-Choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55:1424–1429. doi: 10.2967/jnumed.114.138313. [DOI] [PubMed] [Google Scholar]
- 10.Giovacchini G, Picchio M, Briganti A, Cozzarini C, Scattoni V, Salonia A, Landoni C, Gianolli L, Di MN, Rigatti P, et al. [11C]choline positron emission tomography/computerized tomography to restage prostate cancer cases with biochemical failure after radical prostatectomy and no disease evidence on conventional imaging. J Urol. 2010;184:938–943. doi: 10.1016/j.juro.2010.04.084. [DOI] [PubMed] [Google Scholar]
- 11.Giovacchini G, Picchio M, Garcia-Parra R, Mapelli P, Briganti A, Montorsi F, Gianolli L, Messa C. [11C]choline positron emission tomography/computerized tomography for early detection of prostate cancer recurrence in patients with low increasing prostate specific antigen. J Urol. 2013;189:105–110. doi: 10.1016/j.juro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al. EAU guidelines on prostate cancer Part 1 Screening, diagnosis, and local treatment with curative intent update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, Eisenhut M, Boxler S, Hadaschik BA, Kratochwil C, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, Graner FP, Kübler H, Haberhorn U, Eisenhut M, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 15.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 16.Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R, Stief C, Thalmann GN, Picchio M. New clinical indications for (18)F/(11)C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: A systematic review of the literature. Eur Urol. 2016;70:161–175. doi: 10.1016/j.eururo.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, specificity and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Futterer JJ. European Society of Urogenital Radiology: ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passoni NM, Suardi N, Abdollah F, Picchio M, Giovacchini G, Messa C, Freschi M, Montorsi F, Briganti A. Utility of [11C]choline PET/CT in guiding lesion-targeted salvage therapies in patients with prostate cancer recurrence localized to a single lymph node at imaging: Results from a pathologically validated series. Urol Oncol. 2014;32:38.e9–e16. doi: 10.1016/j.urolonc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, Holland-Letz T, Hadaschik BA, Giesel FL, Debus J, Haberkorn U. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, Lapa C, Lassmann M, Riedmiller H, Czernin J, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-Choline-PET/CT. Clin Nucl Med. 2016;41:515–521. doi: 10.1097/RLU.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, Pfannenberg C, la Fougère C. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101e. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- 23.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC, STARD Group Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative. Fam Pract. 2004;21:4–10. doi: 10.1093/fampra/cmh103. [DOI] [PubMed] [Google Scholar]
- 24.Freitag MT, Radtke JP, Hadaschik BA, Kopp-Schneider A, Eder M, Kopka K, Haberkorn U, Roethke M, Schlemmer HP, Afshar-Oromieh A. Comparison of hybrid (68)Ga-PSMA PET/MRI and (68)Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:70–83. doi: 10.1007/s00259-015-3206-3. [DOI] [PubMed] [Google Scholar]
- 25.Demirkol MO, Acar O, Ucar B, Ramazanoglu SR, Saglican Y, Esen T. Prostate-specific membrane antigen-based imaging in prostate cancer: impact on clinical decision making process. Prostate. 2015;75:748–757. doi: 10.1002/pros.22956. [DOI] [PubMed] [Google Scholar]