Abstract
Bactericidal activity of human β-defensin 3 (hBD-3) against Streptococcus mutans and Actinobacillus actinomycetemcomitans was inhibited in a dose-dependent manner by the presence of saliva and/or serum. Increasing the concentration of hBD-3 partially overcame this inhibition. A fast bactericidal effect was observed against both bacterial strains, suggesting a potential therapeutic use for hBD-3 in the local treatment of oral infections.
Human defensins are small, cationic, and Cys-rich peptides with molecular masses ranging from 3 to 5 kDa (3). On the basis of sequence homology and the localization of six conserved cysteine residues, human defensins are classified into α and β families (3). Human defensins play an important role in innate immune responses because of their antimicrobial and immunomodulatory activities (3). Moreover, they are attractive candidates to consider as therapeutic agents because of their selectivity, speed of action, relative difficulty in selection of resistant mutants in vitro, and inherent immunological compatibility (4).
Human β-defensin 3 (hBD-3) was found to be expressed, at the mRNA level, in skin, placenta, and oral tissues (5). Interestingly, a lower hBD-3 mRNA expression level was observed in persons with periodontal disease than in those with healthy gingiva, suggesting a protective role for the peptide in the host immune response to infection by periodontal pathogens (2). hBD-3 has generated interest because it shows a broad spectrum of antimicrobial activity, also against many drug-resistant strains, in a salt-insensitive manner (5). Peptides that exert antimicrobial activity in artificial media or in buffers may be poorly active or even lack activity in the presence of complex biological fluids (10). Indeed, blood, serum, or saliva may inactivate antimicrobial peptides because of the presence of salts, inhibiting proteins, or peptidases with the ability to degrade the peptides over time (9, 11). Previous reports by us and others have demonstrated that hBD-3 has antimicrobial activity in sodium-phosphate buffer (SPB) or in conventional media against oral pathogens, including Streptococcus mutans, which is involved in cariogenesis, and Actinobacillus actinomycetemcomitans, the etiologic agent of juvenile periodontitis (6, 7). Nothing is known about the possible effects of saliva and serum on the bactericidal activity of hBD-3 against oral bacteria. Serum components can reach the mouth by the flow of a serum-like fluid through the junctional epithelium of the gingivae; their concentration is relatively low at healthy sites but increases during inflammation such as periodontitis. The aims of this study, therefore, were (i) to evaluate the bactericidal activity of hBD-3 against S. mutans and A. actinomycetemcomitans in a liquid assay containing saliva and/or serum and (ii) to compare, under the same experimental conditions, the kinetics of the bactericidal activity of hBD-3 with those of other antimicrobial agents used in the therapy of oral infections.
The bactericidal activity of hBD-3 against A. actinomycetemcomitans ATCC 43717 and S. mutans serotype c reference strain T282 (1) was evaluated by a liquid microdilution assay in 10 mM SPB (pH = 7.4) or in saliva and/or serum serially diluted in the same buffer. Whole saliva samples were obtained from six healthy donors, pooled, and centrifuged twice at 2,500 × g. The supernatant was sterilized by filtration and stored in aliquots at −20°C. Whole blood obtained from five healthy donors was allowed to spontaneously clot at room temperature. After centrifugation at 300 × g, sera were mixed and heat inactivated (56°C for 30 min.). A. actinomycetemcomitans and S. mutans were grown in tryptone soy broth (Oxoid, Basingstoke, United Kingdom) with 0.5% yeast extract (TSBYE) at 34 and 37°C, respectively. Synthetic hBD-3 (Sigma-Genosys) was diluted in 0.1% acetic acid; chlorhexidine digluconate, amoxicillin, and metronidazole (Sigma) were diluted in deionized water. Exponentially growing bacteria were resuspended in SPB to a density of 107 CFU/ml. Ten microliters of each bacterial suspension was exposed for various times to different concentrations of hBD-3 in 100 μl of SPB alone or in the presence of 80, 40, and 20% saliva or 10, 5, and 2.5% (vol/vol) serum. At the end of the incubation period, 10-fold dilutions of each sample were carried out in TSBYE and 0.2 ml of each dilution was plated onto TSYE agar. The number of CFU was determined after 3 days of incubation in a microaerophilic atmosphere at the temperatures indicated above. Each experiment was done three times. Bactericidal activity was defined as a reduction in viable bacteria of ≥3log10 CFU/ml at any of the incubation times tested.
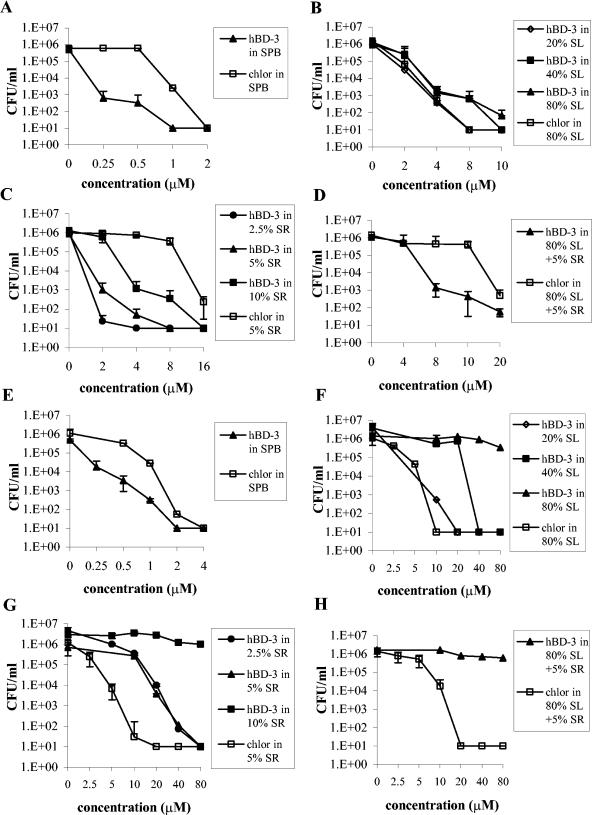
hBD-3 showed bactericidal activity against S. mutans in the presence of saliva (80, 40, and 20%), serum (10, 5, and 2.5%), or both (saliva, 80%; serum, 5%) although at concentrations higher than those used in SPB (Fig. 1A to D). In addition, the bactericidal activity of hBD-3 was compared with that of chlorhexidine, a commonly used oral antiseptic agent, in the presence of 80% saliva, 5% serum, or both. In the presence of 80% saliva, chlorhexidine exhibited bactericidal activity against S. mutans at a concentration two times lower than that of hBD-3 (Fig. 1B), while in 5% serum alone (Fig. 1C) or in combination with 80% saliva (Fig. 1D), hBD-3 showed a bactericidal effect at concentrations four and two times lower than those of chlorhexidine, respectively.
FIG. 1.
Bactericidal activities of hBD-3 and chlorhexidine against S. mutans (A to D) and A. actinomycetemcomitans (E to H) after 1.5 h of incubation in SPB (A, E); 80, 40, or 20% saliva (B, F); 10, 5, or 2.5% serum (C, G); or 80% saliva combined with 5% serum (D, H). Data are means ± standard deviations of three independent experiments. chlor, chlorhexidine; SL, saliva; SR, serum.
hBD-3 showed bactericidal activity against A. actinomycetemcomitans in 40 and 20% saliva and in 5 and 2.5% serum, although at concentrations higher than those used in SPB (Fig. 1E to H). The peptide activity was completely abolished in the presence of 80% saliva (alone or combined with 5% serum) and in 10% serum (Fig. 1F to H). Chlorhexidine exerted a bactericidal effect at a concentration four times lower than that of the peptide in 5% serum (Fig. 1G). The bactericidal activity of hBD-3 was inhibited equally by intact and heat-inactivated serum (data not shown).
The killing kinetics of the peptide were evaluated on S. mutans in 80% saliva and on A. actinomycetemcomitans in 5% serum. As a component of dental plaque, S. mutans interacts essentially with saliva during cariogenesis while A. actinomycetemcomitans preferentially localizes in periodontal pockets, which are rich in a serum-like fluid. The kinetics of hBD-3 were compared with those of equimolar concentrations of chlorhexidine and amoxicillin (MIC = 1.4 μM) against S. mutans (as an example of a bactericidal antibiotic against streptococci) and of metronidazole (MIC = 93 μM) against A. actinomycetemcomitans (an antibiotic used in the treatment of periodontal diseases). As shown in Fig. 2A, hBD-3 showed a very fast killing kinetic against S. mutans by causing a bactericidal effect within 1 and 5 min of incubation at concentrations of 16 and 8 μM, respectively. It is noteworthy that chlorhexidine was bactericidal against S. mutans within 3 min of incubation at a concentration of 16 μM, whereas at an equimolar concentration, amoxicillin was effective only after 20 h (Fig. 2A). hBD-3 was bactericidal against A. actinomycetemcomitans after 30 min when assayed at 80 μM in 5% serum, whereas chlorhexidine exhibited a bactericidal effect within 3 min and metronidazole was ineffective even after 20 h of incubation (Fig. 2B).
FIG. 2.
Time-kill curves of S. mutans in 80% saliva (A) and A. actinomycetemcomitans in 5% serum (B) by hBD-3, chlorhexidine (chlor), amoxicillin (amox), and metronidazole (metro). The concentrations of hBD-3 and the antimicrobial agents reported are micromolar. CTRL, bacteria incubated in the absence of hBD-3 or antimicrobial agents but containing the appropriate amount of the corresponding solvents (0.1% acetic acid for hBD-3 and distilled water for the antimicrobial agents). Data are means ± standard deviations of three independent experiments.
This study demonstrates that S. mutans is highly sensitive to the bactericidal activity of hBD-3 in the presence of both saliva and serum, whereas A. actinomycetemcomitans displayed a low level of susceptibility to hBD-3 in the presence of serum and/or saliva. Since hBD-3, unlike other defensins, is insensitive to the presence of physiologic concentrations of salts (5), it can be argued that the partial inhibition of hBD-3 activity in oral fluids may be due to the activity of proteases or, alternatively, to an interaction between serum albumin or other serum and salivary proteins, with the peptide making it less bioavailable. It is also possible that components of oral fluids interact with the bacterial surface, masking binding sites for the peptide. The latter hypothesis is supported by the observation that although the two bacterial species tested in this study showed comparable susceptibilities to hBD-3 in SPB, they exhibited a marked difference in sensitivity to the peptide in oral fluids. Interestingly, the quick bactericidal activity exhibited by hBD-3 against S. mutans in the presence of saliva and the killing effect against A. actinomycetemcomitans in the presence of serum were reached at concentrations that may potentially be obtained by local delivery in vivo (8). Further studies aimed at the identification of hBD-3 derivatives able to retain bactericidal activity but with low affinity for molecular components of biological fluids will help to overcome the partial inhibition of the peptide observed in saliva and serum, supporting the possibility of a therapeutic use for hBD-3 in the local treatment of oral infections.
Acknowledgments
This work was supported by grants from Progetti M.I.U.R. prot. 2002067349_01, Rome, Italy.
REFERENCES
- 1.Batoni, G., F. Marchetti, F. Ota, E. Ghelardi, S. Barnini, S. Inoue, C. Uchiyama, K. Hirota, Y. Minato, M. R. Giuca, M. Gabriele, M. Campa, and S. Senesi. 1993. First characterization in Italy of clinical isolates of mutans streptococci by using specific monoclonal antibodies. Eur. J. Epidemiol. 9:483-488. [DOI] [PubMed] [Google Scholar]
- 2.Bissell, J., S. Joly, G. K. Johnson, C. C. Organ, D. Dawson, P. B. McCray, and J. M. Guthmiller. 2004. Expression of β-defensins in gingival health and in periodontal disease. J. Oral Pathol. Med. 33:278-285. [DOI] [PubMed] [Google Scholar]
- 3.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 4.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 5.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human β-defensin 3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 6.Joly, S., C. Maze, P. B. McCray, Jr., and J. M. Guthmiller. 2004. Human β-defensin 2 and 3 demonstrate strain-selective activity against oral microorganisms. J. Clin. Microbiol. 42:1024-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisetta, G., G. Batoni, S. Esin, F. Luperini, M. Pardini, D. Bottai, W. Florio, M. R. Giuca, M. Gabriele, and M. Campa. 2003. Activity of human β-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob. Agents Chemother. 47:3349-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosca, D. A., M. A. Hurst, W. So, B. S. C. Viajar, C. A. Fuj II, and T. J. Falla. 2000. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panyutich, A. V., and T. Ganz. 1991. Activated alpha-2-macroglobulin is a principal defensin-binding protein. Am. J. Respir. Cell Mol. Biol. 5:101-106. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka, D., K. T. Miyasaki, and R. I. Lehrer. 2000. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: a cathelicidin found in human leukocytes and epithelium. Oral Microbiol. Immunol. 15:226-231. [DOI] [PubMed] [Google Scholar]
- 11.Wang, Y., B. Agerberth, A. Lothgren, A. Almstedt, and J. Johansson. 1998. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J. Biol. Chem. 273:33115-33118. [DOI] [PubMed] [Google Scholar]