Abstract
Vibrio vulnificus is a gram-negative, highly invasive bacterium responsible for human opportunistic infections. We studied the antibacterial effects of toluidine blue O (TBO)-mediated photodynamic therapy (PDT) for V. vulnificus wound infections in mice. Fifty-three percent (10 of 19) of mice treated with 100 μg of TBO per ml and exposed to broad-spectrum red light (150 J/cm2 at 80 mW/cm2) survived, even though systemic septicemia had been established with a bacterial inoculum 100 times the 50% lethal dose. In vitro, the bacteria were killed after exposure to a lower light dose (100 J/cm2 at 80 mW/cm2) in the presence of low-dose TBO (0.1 μg/ml). PDT severely damaged the cell wall and reduced cell motility and virulence. Cell-killing effects were dependent on the TBO concentration and light doses and were mediated partly through the reactive oxygen species generated during the photodynamic reaction. Our study has demonstrated that PDT can cure mice with otherwise fatal V. vulnificus wound infections. These promising results suggest the potential of this regimen as a possible alternative to antibiotics in future clinical applications.
Photodynamic therapy (PDT) is an experimental treatment which shows great potential for the treatment of neoplastic and nonneoplastic diseases (11, 12). PDT involves a light-sensitive photosensitizer, light, and molecular oxygen (14). After excitation with visible light, highly cytotoxic singlet oxygen and other reactive oxygen species (ROS) are generated by either energy or electron transfer (14, 30, 32). The use of photosensitizers for microbial eradication can be traced back to before the age of chemotherapy (16, 38). The antimicrobial effects of PDT are increasingly recognized. The technique has been shown to have effects against a range of oral pathogens and also against drug-resistant bacteria. More importantly, there have been reports of the use of PDT to treat infections in selected animal models (13, 15, 17) and some clinical trials (16), with encouraging results. The activities of the virulence factors of gram-negative bacteria were also reduced with PDT (24). Among the many photosensitizers, toluidine blue O (TBO), a cationic phenothiazinium photosensitizer (16, 38), has been shown to be phototoxic to gram-negative bacilli with red-light irradiation (39). However, in vivo antimicrobial studies of TBO-PDT were mainly limited to oral infections (39).
Vibrio vulnificus is a gram-negative, motile, curved bacillus of the family Vibrionaceae (22). Most Vibrio species are free-living in marine or brackish water (6). Many cases of V. vulnificus infection have been reported from the coastal areas of the United States (4), Asia (6), and Europe (10). V. vulnificus causes primary sepsis, wound infection, and gastrointestinal illness in humans (6, 22). This organism is extremely virulent, and infections with this organism typically occur in patients with underlying liver disease 1 to 2 days after exposure. The mortality rate is up to 55% in septic patients and 25% in those with wound infections (6). Many factors have been implicated as possible virulence determinants for V. vulnificus, including the bacterial pili used for cell adherence (34), a polysaccharide capsule (40), extracellular toxins and enzymes (26, 29), resistance to phagocytosis (20, 42), resistance to the bactericidal effects of human sera (20, 40, 42), and the ability to acquire iron from transferrin (36).
In this study, we show the effect of TBO-PDT against V. vulnificus both in vitro and in vivo with a relatively low PDT dose. Interestingly, this treatment preserved the lives of 53% of mice challenged with a bacterial inoculum 100 times the 50% lethal dose (LD50). It works at least partly by reducing bacterial virulence factors. The present study has shown for the first time that PDT can cure mice with otherwise fatal V. vulnificus wound infections.
MATERIALS AND METHODS
Bacterial isolates.
V. vulnificus VV5823 (37), originally isolated from a septicemic patient at National Cheng Kung University Hospital, was selected for both the in vitro and the in vivo studies. The organisms were stored at −70°C in Protect bacterial preservers (Technical Service Consultants Limited, Heywood, England) before being cultured on Luria-Bertani (LB) agar (Difco Laboratories, Detroit, Mich.).
Animals.
Female BALB/c mice (age, 5 to 6 weeks) were used. The mice were anesthetized with pentobarbital sodium at about 30 mg/kg of body weight/h intraperitoneally for surgery and for subsequent PDT. Their backs were shaved. Surgical scissors and forceps were used to create one full-thickness excisional wound down to the panniculus carnosus. There was no visible bleeding within the wounds.
Photosensitizer and irradiation.
TBO solution was prepared by dissolving the powder (Sigma) in sterile 0.9% (wt/vol) NaCl. It was then filter sterilized by passage through 0.22-μm-pore-size membrane filters. The light source was a PDT-1200 lamp (Waldmann, Villingen-Schwenningen, Germany), and wavelengths from 560 to 780 nm were used (9). The light power at the irradiated surface was set at 80 mW/cm2 in all experiments. Plates or animals were placed at the center of the irradiated field. Hyperthermia induced by irradiation on the mouse skin or the plate surfaces was avoided by cooling the skin or surface with an electric fan during irradiation.
In vitro time-kill studies.
The bacteria were diluted in a six-well plate to concentrations of about 5.0 × 105 CFU/ml in 5 ml of fresh Mueller-Hinton broth. Fifty microliters of various concentrations of TBO was added to the wells to give final concentrations of 0.01, 0.1, 1.0, 10, and 100 μg/ml. After 2 min of incubation, TBO-containing wells were exposed to 100 J of red light/cm2 (20.8 min) at 80 mW/cm2 or were kept in the dark. TBO-negative controls were incubated with an equal volume of normal saline. Duplicate samples were removed for determination of the numbers of CFU at specified time intervals, as described previously (8, 37). All experiments were performed at least twice for confirmation of the results.
Photodynamic effect of TBO on V. vulnificus motility.
V. vulnificus cells in wells with different concentrations of TBO were treated with PDT as described above. Before and at different time points after PDT, 10 μl of the sample was put on a microscopic glass slide and examined under a light microscope coupled to a DP50-CU digital microscope camera (Olympus) connected to a personal computer. A video image was created by capturing digital pictures and was composed with software. To investigate whether TBO-PDT could damage the flagella of the V. vulnificus cells and thus affect their motility, flagella were stained by the protocol provided by the manufacturer of the stain (Fisher Scientific Co., Pittsburgh, Pa.) and examined under a light microscope.
Protease assays.
The proteolytic activities of samples were determined by an azocasein assay (24). Aliquots of protease-containing samples (500 μl) were transferred into Eppendorf tubes. The substrate azocasein (Sigma) was added to each tube as a 6% (wt/vol) solution in 0.5 M Tris buffer (Sigma) at pH 7.0 (250 μl); this was followed by aerobic incubation at 37°C for 4 h. Then, 750 μl of 20% (wt/vol) acetic acid was added to halt the reaction. The Eppendorf tubes were then centrifuged at 5,000 × g for 15 min. One milliliter of the supernatant was removed from each sample, and the absorbance at 440 nm was read. One unit of activity was defined as that which caused a change in the absorbance at 440 nm of 0.001 in 1 h. The control sample was treated as described above after the sample was boiled for 5 min to destroy the protease. The protease secretion ability of V. vulnificus after PDT treatment was determined by a modified well assay (5). Briefly, wells were cut into 2.5% skim milk agar plates with a 4-mm cork borer. Forty microliters of supernatant from liquid cultures with similar numbers of CFU of bacteria post-PDT was placed into each well. The plates were incubated at 37°C for 24 h. The diameters of the hydrolyzed skim milk halo produced around the wells were measured and compared.
Effect of ROS scavenger and stabilizer on photodynamic antibacterial effect.
ROS is one of the most important molecules involved in PDT-mediated cell killing (3, 11, 14, 30, 32, 38). The involvement of ROS in cytotoxic photosensitization was investigated by using one of two scavengers: proline (1) or l-tryptophan (3). Proline at 100 mg/ml (87 mM) or l-tryptophan at 5 mg/ml (24 mM) was added to the wells containing V. vulnificus and TBO immediately before irradiation. It is well known that proteins (31) and cations (27) in broth interfere with the effect of PDT. To exclude this interference, Mueller-Hinton broth was replaced by 100% deuterium oxide (D2O) (3) or normal saline for 2 h and during irradiation (with 100 J/cm2 and TBO at 0.05 μg/ml) to prolong the lifetime of singlet oxygen. Time-kill studies were then performed as described above.
TEM.
Transmission electron microscopy (TEM) studies were performed as described previously (34) to visualize the possible damage to bacterial pili and/or cell walls by TBO-PDT. Bacterial cells were negatively stained with 2% phosphotungstic acid (pH 7.2) on Parlodion-coated grids and were examined with a JEOL 100-B transmission electron microscope operated at 60 kV. At least 100 cells on each of three separate grids were examined.
Tissue culture adherence assay.
To investigate how TBO-PDT affects the adhesiveness of V. vulnificus to keratinocytes, quantitative adherence assays (34) were performed with human basal cell carcinoma (BCC) cells (41). BCC cell monolayers were grown in 24-well tissue culture dishes with RPMI and 10% fetal calf serum, in which each well was seeded with 2 × 105 cells and grown overnight at 37°C in 5% CO2. Serum in medium is an extremely complex mixture which contains food substances, metabolites, hormones, plasma proteins, and growth factors. Some proteins may enhance the attachment of cells to collagen and synthetic surfaces. To avoid the influences of these factors, serum-free medium was used in the adhesion assay. After being exposed to light or kept in the dark in the presence of TBO, a final V. vulnificus count of 4 × 107 CFU/ml was inoculated in triplicate, with 50 μl of the diluted bacteria inoculated onto cell monolayers in 1 ml of serum-free RPMI and then centrifuged at 700 × g for 10 min at 10°C. The tissue culture plates were incubated at 37°C in 5% CO2 for 1 h and then washed five times with phosphate-buffered saline (PBS) to remove nonadherent bacteria. After the final wash, the cells were covered with 1 ml of PBS and mechanically agitated by vigorous pipetting to suspend the epithelial cells and bacteria, followed by serial 10-fold dilution and quantitation by plating on LB agar. The assay results are presented as the percentage of cell-associated bacteria, defined as (numbers of CFU recovered/numbers of CFU inoculated) × 100.
Determination of inoculation time required for systemic infection.
V. vulnificus was inoculated into the wounds of five groups of mice and allowed to incubate for different time periods (5, 10, 15, 20, and 30 min). Each group contained six mice, and two independent experiments were done. After inoculation of 2.5 × 106 CFU of V. vulnificus per ml (100 times the LD50) (7), the bacteria were removed by vigorous flushing with PBS at the desired time points. The mice were killed at 18 h, after collection of a blood sample from the heart. Forty microliters of blood was inoculated in LB agar for determination of bacterial growth and proteolytic activity, as described above for the well assay.
In vivo studies.
A previously described (17) excisional wound model was used, with modification, for the in vivo studies. Initial studies were carried out to determine the degree of pathogenicity of the V. vulnificus strain used in this study. Mice with single wounds on the middle of the back with an area of 100 mm2 (8 × 12.5 mm) received inocula of mid-log-phase V. vulnificus (7) suspended to appropriate concentrations in 50 μl of PBS. All experiments were carried out in a room with subdued lighting or in the dark, except when illumination was taking place. After a 30-min interval to allow V. vulnificus to penetrate the wounds, 50 μl of TBO at various concentrations (0, 1, 10, or 100 μg/ml) was added to the wounds and was retained at the edges of the wounds for 2 min. Mice in the groups receiving PDT and light alone were then illuminated with 150 J/cm2 at 80 mW/cm2, delivered by the PDT-1200 lamp, while the others were kept in the dark. Mice were covered with a non-light-penetrating plastic sheet with a square opening (20 by 20 mm) aligned with the wounds during irradiation to avoid whole-body irradiation. After PDT, the mice were kept under subdued lighting for 72 h. This time point was selected because mice that survived for 72 h after bacterial inoculation could totally recover from the infections. Groups of four to eight mice each were used for each set of conditions. Each experiment was repeated three times. All of the mice treated with 0 or 1 μg of TBO per ml and either exposed to light or kept in the dark died within 24 to 36 h after treatment in the first experiment. These conditions were thus not repeated due to ethical considerations. The data from three independent experiments were pooled. The animal experiments were conducted in compliance with the relevant national guidelines for the care and treatment of animals of the Republic of China and were approved by the Chi Mei Medical Center.
Statistics.
One-way analysis of variance was performed to determine whether there were significant differences between the different test conditions. The Bonferroni correction was applied to allow for the effect of multiple tests. Regression analysis was performed to determine whether a dose-dependent relationship existed between the energy doses or TBO concentrations and a reduction in the bioactivities of the virulence factors.
RESULTS
Low-dose TBO with irradiation effectively kills V. vulnificus in vitro.
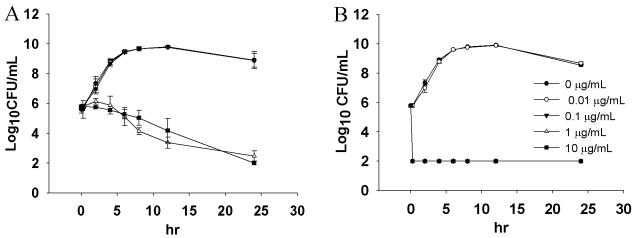
When approximately 5 × 105 CFU of V. vulnificus/ml was incubated with TBO in the dark, no influence on bacterial growth was noted at concentrations of 0.01 and 0.1 μg/ml (Fig. 1A). In our laboratory, 100 CFU/ml is the detectable limit of this assay (23), as no bacterial colonies were visible at this concentration. With higher concentrations of TBO (1 and 10 μg/ml), bacterial growth was inhibited (1 log10) during the initial 6 h, and thereafter, complete inhibition was found at 24 h. With TBO concentrations of 100 μg/ml, bacterial growth was completely inhibited after 2 h of incubation without light exposure (data not shown). This indicates that V. vulnificus is more sensitive to TBO than Porphyromonas gingivalis in the dark. The viability of P. gingivalis bacteria is not affected by TBO concentrations less than 1,000 μg/ml (35). No bacteria could be recovered after light exposure with TBO concentrations greater than or equal to 0.1 μg/ml (Fig. 1B). Bacterial growth was not affected at lower TBO concentrations. PDT provided nearly 6 log10 cell killing. The killing occurred almost immediately after illumination. This fast killing effect may be critical for the treatment of V. vulnificus infections, since the bacteria are highly invasive.
FIG. 1.
Inhibition of V. vulnificus growth (inoculum size, 5 × 105 CFU/ml) after the bacteria were incubated with different concentrations of TBO and kept in the dark (A) or exposed to 100 J of red light/cm2 at 80 mW/cm2 (B). Datum points are the means of triplicate determinations and two separate experiments, and bars indicate standard deviations. In panel B, the curves for 0.1, 1, and 10 μg/ml overlap completely.
TBO-PDT inhibits V. vulnificus motility.
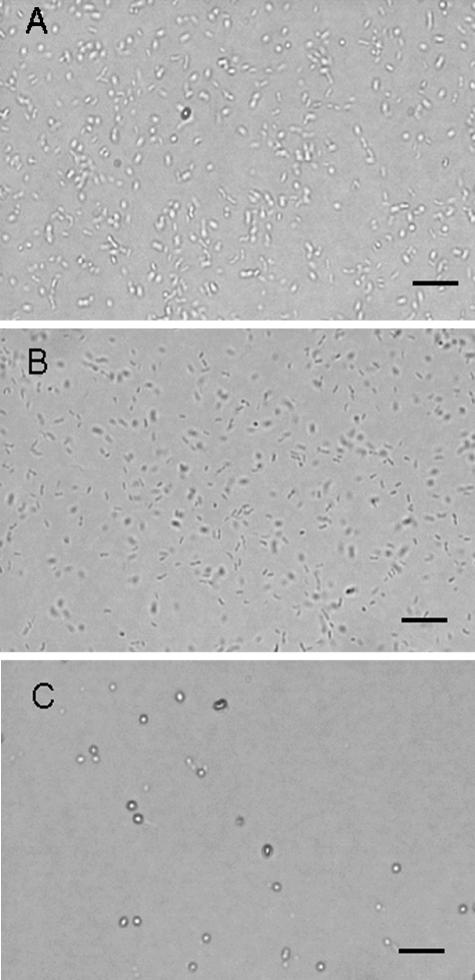
Representative data (108 CFU of V. vulnificus/ml exposed to light in the presence of 0.1 μg of TBO/ml) were selected to show the influence of TBO-PDT on V. vulnificus motility, as detected under a light microscope. Similar results were obtained for TBO at concentrations greater than 0.1 μg/ml. No influence was noted in the control samples (light alone) or the samples kept in the dark. Figure 2A shows that reflective V. vulnificus moved, rotated, and spun freely in all directions at a high speed (video images are not shown but are available on request). Thirty minutes after irradiation with 100 J of red light/cm2, however, motility was markedly reduced and could barely be seen on video captured at the same speed. The bacteria became less reflective upon examination under a microscopic (Fig. 2B). No movable bacteria could be detected at 1 h post-PDT. Almost all cells lost their rod shape and became round (Fig. 2C). Before PDT treatment, the long single flagellum of V. vulnificus could easily be seen after staining of the flagella (data not shown). By contrast, visible flagella were difficult to find shortly after PDT, even when the cell body remained intact (data not shown). This indicates that the flagellum structure of V. vulnificus is relatively susceptible to the stress of PDT and may play an important role in bacterial motility.
FIG. 2.
TBO-PDT inhibits V. vulnificus motility. V. vulnificus (108 CFU/ml) was incubated with TBO at 0.1 μg/ml before irradiation with red light. The V. vulnificus cells moved, rotated, and spun in all directions freely and at a high speed (A). Thirty minutes after irradiation, cell motility was markedly reduced and could barely be seen on the video at the same speed. The bacteria also became less reflective (B). No movable bacteria could be observed at 1 h post-PDT. Almost all cells lost their rod shape and became round (C). Bars, 25 μm.
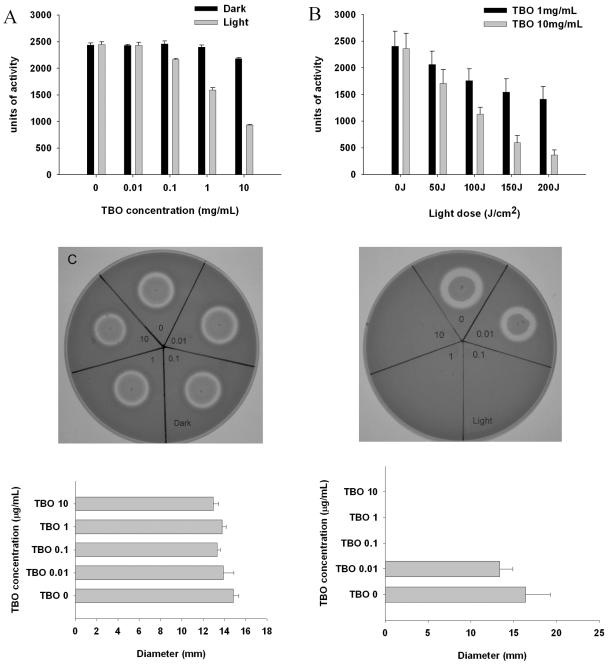
Low-dose TBO-PDT inhibits the proteolytic activity of V. vulnificus.
Figure 3A and B shows the proteolytic activity of V. vulnificus culture supernatants after treatment with various combinations of different concentrations of TBO and different doses of light. Neither TBO in the absence of light nor irradiation of the supernatants in the absence of TBO had any significant effect on proteolytic activity. With TBO concentrations of 1 and 10 μg/ml, proteolytic activities were reduced 34% (P < 0.001) and 57% (P < 0.001), respectively, compared to the proteolytic activities of V. vulnificus cells exposed to the same concentrations of TBO without light exposure (Fig. 3A). Irradiation of the supernatants in the presence of 1 or 10 μg of TBO/ml resulted in a substantial, light-dose-dependent decrease in their proteolytic activities (Fig. 3B). With the highest light dose used, the reduction in proteolytic activity was 84% (P < 0.001). The reduction of protease activity was dependent on the TBO concentration (P < 0.05) and the light dose (P = 0.002 for TBO at both 1 and 10 μg/ml). In the well assay, the protease secretion activity was totally inhibited by TBO concentrations greater than or equal to 0.1 μg/ml, which correlated with the results of the time-kill experiment (Fig. 3C, right panel). Interestingly, there was a trend for a lower TBO concentration of 0.01 μg/ml to provide an inhibitory effect when the cells were exposed to light, although this difference was not significant (P = 0.636). This PDT dose did not affect bacterial growth in vitro (Fig. 1B). Whether these results suggest that the intracellular protein synthesis machinery might be relatively sensitive to PDT will require further study.
FIG. 3.
Effect of photodynamic actions on V. vulnificus proteolytic activities as revealed by azocasein assay after exposure to different TBO concentrations and light doses. The protease-containing supernatants were kept in the dark or exposed to 100 J of red light/cm2 (A). Proteolytic activity was significantly reduced in the presence of TBO concentrations greater than 0.1 μg/ml with light exposure (P < 0.001; Bonferroni t method). The inhibition was TBO dose dependent (P < 0.05; linear regression). Irradiation of the supernatants in the presence of 1 or 10 μg of TBO per ml resulted in a substantial, light-dose-dependent decrease in proteolytic activity (B) (P = 0.002 for TBO at both 1 and 10 μg/ml; linear regression). Protease secretion and proteolytic activities were determined by the well assay (C). Samples were kept in the dark (left panel) or exposed to 100 J of red light/cm2 (right panel). The protease activity was totally inhibited by TBO at concentrations greater than or equal to 0.1 μg/ml when the samples were exposed to light, as shown by the absence of a clear halo in the agar. There was a trend for a very low TBO concentration (0.01 μg/ml) to result in protease inhibition when the sample was exposed to light, although this difference was not significant (P = 0.636). Datum points are the means of triplicate determinations and three separate experiments, and bars indicate standard deviations.
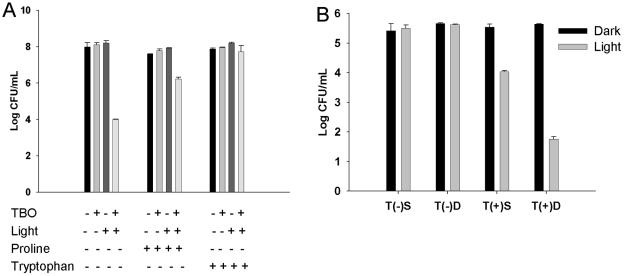
Scavengers of ROS reduce the TBO-PDT antibacterial effect, while stabilizer enhances the TBO-PDT antibacterial effect.
The antibacterial effect of TBO-PDT was markedly reduced in the presence of the ROS scavengers proline and l-tryptophan. Figure 4A shows that 2.2 and 3.7 log10 increases in the numbers of viable V. vulnificus cells were found with the addition of the two scavengers, respectively, compared to the numbers of viable cells obtained after treatment with TBO alone for bacteria treated with the same light dose (P < 0.001). The effect of replacing Mueller-Hinton broth with physiologic saline or D2O on the effectiveness of lethal photosensitization is shown in Fig. 4B. The presence of saline or D2O did not affect the growth of bacteria either kept in the dark or exposed to 100 J of red light/cm2 without TBO (the two sets of bars on the left in Fig. 4B). The antibacterial effect was enhanced in the presence of D2O, TBO, and light. The viable counts were reduced 3.9 log10 with D2O treatment and 1.5 log10 with saline treatment compared to the counts for the control kept in the dark (P < 0.001). These results suggest the involvement of ROS in cell killing and were in agreement with previous findings for TBO-PDT for P. gingivalis (3).
FIG. 4.
Free radicals and singlet oxygen scavengers reduced the antibacterial effect of TBO-PDT, while stabilizers enhanced the antibacterial effect of TBO-PDT. Increases in the numbers of viable V. vulnificus cells of 2.2 and 3.7 log10 were found with the addition of the scavengers proline and l-tryptophan, respectively, compared to the numbers obtained by treatment with TBO alone and the same light dose (P < 0.001; Bonferroni t method) (A). Effect of D2O on lethal photosensitization (B). The presence of saline (S) or D2O (D) did not affect bacterial growth when the samples were either kept in the dark or exposed to 100 J of red light/cm2 alone (left two sets of bars). The viable counts were reduced 3.9 log10 in the D2O-treated groups and 1.5 log10 in the saline-treated groups exposed to light compared to the counts for the control kept in the dark (P < 0.001; Bonferroni t method).
TBO-PDT disrupts the flagellum structure and causes cell wall pebbling.
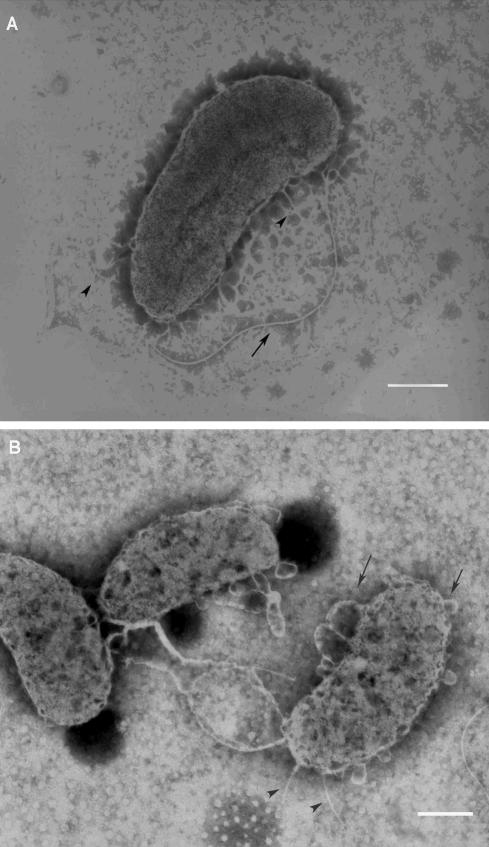
The ultrastructural damage after TBO-PDT was illustrated by TEM studies. Figure 5A shows the long single flagellum, fine hairlike pili, and intact cell wall of V. vulnificus before PDT treatment. Immediately after irradiation in the presence of TBO, the flagellum was fragmented and could hardly be seen by TEM. This finding is compatible with the observations made by light microscopy (data not shown). Many bubbles formed on the cell walls of the bacteria (Fig. 5B). These results suggest that the cell wall structure, and possibly the capsule as well, was severely damaged by PDT with the short 2-min incubation with TBO and subsequent 21 min of irradiation. Interestingly, some pili of the cells were still visible and remained intact (Fig. 5B), implying that the pilus proteins might be more resistant to PDT than the flagellum proteins.
FIG. 5.
TBO-PDT disrupts the flagellum and causes cell wall pebbling of V. vulnificus. TEM studies showed the typical long single flagellum (arrow), fine hairlike pili (arrowhead), and the intact cell wall of V. vulnificus before PDT treatment (A). Immediately after PDT treatment, the flagellum was fragmented and difficult to find. The cell wall structure was severely damaged, as revealed by the formation of many bubbles on the cell wall (arrow). The pili of the cells remained intact (arrowhead) (B). Bars, 0.5 μm.
Low-dose PDT reduces adherence of V. vulnificus to keratinocytes.
Table 1 shows that after exposure of cells to 100 J of red light/cm2 in the presence of 0.01 μg of TBO/ml, a dose with no effect on bacterial growth in vitro, the adhesiveness of the bacteria to BCC cells was significantly reduced (P < 0.001). These results imply that the protein machinery required for bacterial adhesion might be susceptible to photodynamic stress.
TABLE 1.
Adhesiveness of V. vulnificus cells to human epithelial cells after exposure to light or after being kept in the dark in the presence of TBO
| Treatmenta | % Adherence to BCC cells |
|---|---|
| D-0 | 1.64 ± 0.07 |
| L-0 | 1.64 ± 0.40 |
| D-0.01 | 1.65 ± 0.24 |
| L-0.01b | 0.29 ± 0.16 |
D, dark; L, light; 0 and 0.01, TBO at concentrations of 0 and 0.01 μg/ml, respectively.
P < 0.001.
Septicemia develops within 30 min of wound infection.
The systemic dissemination of V. vulnificus from the wound infections in the mice occurred rapidly. When the bacteria were allowed to incubate on the wounds for 5, 10, 15, 20, and 30 min, the rates of bacterial recovery from blood were 8, 25, 50, 50, and 83%, respectively. All mice died when V. vulnificus was incubated on the wounds for longer than 60 min.
TBO-PDT protects mice from V. vulnificus lethal wound infections.
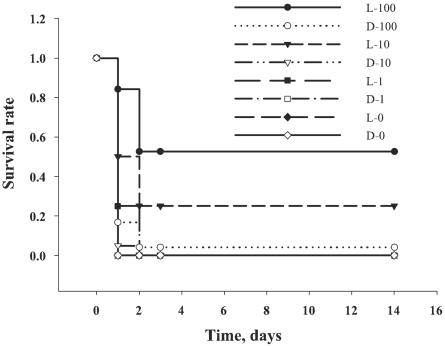
To provide a robust test of the ability of TBO-PDT to prevent death from a lethal wound infection, we used a bacterial challenge that was 100 times the LD50 (7) and a 30-min incubation to establish a systemic infection. Fifty microliters of bacteria plus 50 μl of TBO were sufficient to achieve an even spread over the wound and to be retained by the edges of the wound. Irradiation began after 2 min of incubation to allow the TBO to mix completely with the bacteria. The mice in the untreated control group, the group treated with light alone, and the group treated with TBO and kept in the dark usually died within 24 to 36 h of infection. Only 1 of 24 (4%) mice treated with TBO at 100 μg/ml and kept in the dark survived. In contrast, 10 of 19 (53%) mice exposed to TBO at 100 μg/ml and treated with light survived, as shown in Fig. 6. These mice showed symptoms of bacterial infection, such as weight loss, ruffled fur, and inactivity. However, they recovered quickly, and by 3 days after infection they were regaining weight and moving normally. An increase in the TBO concentration to 1,000 μg/ml did not significantly improve the rate of survival of PDT-treated animals compared to that of the controls (data not shown). V. vulnificus would probably be completely killed by this high concentration before the photodynamic action took place. Since the aim of TBO-PDT is the treatment of local infections, it was unexpected that about 50% of the mice remained alive, even though systemic infection had become established. One possible explanation is that PDT could effectively down-regulate bacterial virulence factors.
FIG. 6.
Kaplan-Meier survival plots for TBO-PDT-treated mice. Mice were kept in the dark (D) or were exposed to 150 J of red light/cm2 (L) with increasing TBO concentrations (0 to 100 μg/ml [as indicated by the numbers after L and D]). TBO at 100 μg/ml and light treatment (•), n = 19 animals; TBO at 100 μg/ml with the animals kept in the dark (○), n = 24 animals; TBO at 10 μg/ml and light treatment (▿), n = 20 animals; TBO at 10 μg/ml with the animals kept in the dark (□), n = 20 animals; TBO at 1 μg/ml and light treatment (▪), n = 4 animals; TBO at 1 μg/ml with the animals kept in the dark (□), n = 4 animals; TBO at 0 μg/ml and light treatment (♦), n = 4 animals; TBO at 0 μg/ml with the animals kept in the dark (⋄), n = 4 animals.
DISCUSSION
This study has demonstrated for the first time the efficacy of TBO-PDT in an animal model of potentially fatal wound infections caused by V. vulnificus. Fifty-three percent of the infected mice survived after irradiation with 150 J of broad-spectrum red light/cm2 in the presence of 100 μg of TBO per ml. The PDT-1200 lamp emits visible light at wavelengths from 560 to 780 nm (9) to cover the spectra of action of most available photosensitizers. The light intensity measured with the built-in light meter was the sum of the total spectrum of light emitted. Eighty milliwatts was set for irradiation in the present experiments. However, the maximal spectrum of action of TBO is at wavelengths of 620 to 660 nm. The light intensity of this spectrum of action was about one-fourth (20 mW/cm2) of the total intensity (80 mW/cm2). The light energy used for photodynamic action was thus only one-fourth of the total. On the other hand, a laser light source emits photons of a single wavelength. The readout of light intensity is exactly the same as the total intensity. The light energy accumulated from the PDT-1200 lamp at 630 nm was, therefore, far lower than that accumulated from a laser. Matevski et al. (28) found that a red-filtered xenon lamp was at least as effective as a laser for the photodynamic killing of P. gingivalis in vitro. Nevertheless, it is not known whether a single-wavelength laser or a broad-spectrum light source would have provided better PDT effects in our study because multiple factors, such as O2 saturation and serum in tissue, might affect the outcome of the therapy. Interestingly, although TBO-PDT was targeted to the treatment of a local infection, it might reduce the development or severity of the resulting septicemia by lowering the activities of virulence factors. A lower fluence rate provides a better photodynamic effect than a higher fluence rate, possibly due to differences in the available oxygen perfusion in vivo (11, 12, 14). However, a long irradiation time is not practical in most clinical settings. A fluence rate of 80 mW/cm2 was set throughout the experiments performed in this study. The results showed that light doses of 100 to 150 J/cm2 could provide effective photodynamic killing of V. vulnificus in the presence of low concentrations of TBO. Heat would not be generated with irradiation at a dose less than 150 mW/cm2 produced by a laser (11, 12). However, considerable heat will be generated at a low fluence rate with a broad-spectrum light source, such as the PDT-1200 lamp. Matevski et al. (28) found that the heat generated with a red-filtered xenon lamp might compromise future clinical applications for the treatment of periodontal infections. Continuous cooling of the irradiated surface is thus necessary to lessen the hyperthermic effects and discomfort generated during irradiation with a fluence rate greater than 40 mW/cm2 with the PDT-1200 lamp (T. W. Wong, unpublished data). Komerik et al. (25) used a 630-nm laser to treat P. gingivalis infections with TBO-PDT. In the presence of 100 μg of TBO per ml, only a 2-log10 reduction of bacteria could be achieved by irradiation with 48 J/cm2 at a fluence rate of 100 mW/cm2. Hamblin et al. (17) treated Pseudomonas aeruginosa-infected wounds with poly-l-lysine-chlorin e6 (Ce6) conjugate and a 665-nm diode laser in an open-wound animal model with a relatively high light dose (240 J/cm2) at 100 mW/cm2 and found that 90% of PDT-treated mice survived. Taken together, these results suggest that although V. vulnificus and P. aeruginosa may both cause potentially lethal infections, and the former is more susceptible to PDT.
The proteolytic activity of V. vulnificus was more vulnerable than that of P. aeruginosa to TBO-PDT at a low dose (a TBO concentration 10 times lower) (24). Inhibition of protein synthesis is one of the important factors in choosing an antibiotic which is effective against V. vulnificus infection (8) because cell wall-targeted antibiotics may cause the release of microbial toxins. Hamblin et al. (17) found that mice died from endotoxemia, despite successful killing of viable bacteria with poly-l-lysine-Ce6 conjugate PDT.
The rate of mortality from V. vulnificus septicemia in patients exceeds 50%, and the median time interval from hospitalization to death is approximately 2 days (6). Early intervention is thus critical. As shown in this study and in previous studies (25, 35), TBO binds to microorganisms almost immediately. Irradiation can be performed without any delay. Kato et al. (21) performed a phase II clinical study of PDT using mono-l-aspartyl chlorin e6 (Npe6) and a diode laser for the treatment of early superficial squamous cell carcinoma of the lung. Irradiation was performed 4 h after drug administration, and it was found that a complete response could be achieved in 82.9% of patients. Hongcharu et al. (18) used 3 h of occlusion with aminolevulinic acid (ALA) for the treatment of acne and showed a decrease in the amounts of follicular bacteria after a single treatment. Although there was no direct evidence of a time interval for Npe6 and ALA binding to bacteria, it is reasonable to predict that a longer incubation period may be needed for effective antibacterial photodynamic effects. The suitability of such treatment for V. vulnificus infection remains unclear. Conventional antibiotic therapy usually takes at least 2 h to provide the same cell killing effects (8, 37). Another advantage of TBO-PDT is the high safety margin of clinical use. TBO has been used (at about 10 mg/ml) for the surgical identification of tumor margins without causing human toxicity (38). Ingestion of 300 mg of TBO for the treatment of acute intoxication with aniline in a woman did not result in any adverse effects (19). TBO-PDT has also been shown to selectively kill microorganisms without damaging host tissues (25). Moreover, the cost of TBO is less than those of the other available photosensitizers, such as Photofrin, ALA, and Ce6.
PDT is used only for the treatment of the more accessible tumors, and the use of PDT to kill microorganisms may also be limited to localized infections due to the problems of systemic light delivery. Nevertheless, with the advent of optical fiber technology (2, 33), deep-seated infections, if not disseminated infections, should become amenable to treatment by the photodynamic approach. Gad et al. (13) reported encouraging results for the treatment of soft tissue infections in mice with poly-l-lysine-Ce6 conjugate PDT. The decreased mortality rate in the present study also suggests that PDT may prevent bacterial dissemination.
In summary, our study has demonstrated the success of treating highly invasive, potentially lethal V. vulnificus wound infections with TBO-PDT in an animal model. The advantages of TBO-PDT include (i) the short incubation time, (ii) the fast killing effect, (iii) the attenuation of bacterial virulence factors, (iv) the high therapeutic safety margin, and (v) the relatively low cost. Taken together, these characteristics suggest the potential for the development of clinical applications of this therapy. Future work should explore host factors in response to this treatment.
Acknowledgments
We thank Sek Wen Hui, Molecular and Cellular Biophysics Department, Roswell Park Cancer Institute, for excellent help with the TEM experiments; and we thank Ching-Hung Chen, Chi-Chung Chen, and Ching-Chien Lee for excellent technical assistance.
T.-W. Wong is supported by Physician Scientist Fellowship grant RE89P005 from the National Health Research Institute of Taiwan and National Science Council of Taiwan grant NSC932314B006027.
REFERENCES
- 1.Alia, P. Mohanty, and J. Matysik. 2001. Effect of proline on the production of singlet oxygen. Amino Acids 21:195-200. [DOI] [PubMed] [Google Scholar]
- 2.Arnfield, M., S. Gonzalez, P. Lea, J. Tulip, and M. McPhee. 1986. Cylindrical irradiator fiber tip for photodynamic therapy. Lasers Surg. Med. 6:150-154. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti, M., A. MacRobert, S. Meghji, B. Henderson, and M. Wilson. 1998. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem. Photobiol. 68:370-376. [PubMed] [Google Scholar]
- 4.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 5.Burger, M., R. G. Woods, C. McCarthy, and I. R. Beacham. 2000. Temperature regulation of protease in Pseudomonas fluorescens LS107d2 by an ECF sigma factor and a transmembrane activator. Microbiology 146:3149-3155. [DOI] [PubMed] [Google Scholar]
- 6.Chuang, Y. C., C. Y. Yuan, C. Y. Liu, C. K. Lan, and A. H. Huang. 1992. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin. Infect. Dis. 15:271-276. [DOI] [PubMed] [Google Scholar]
- 7.Chuang, Y. C., H. M. Sheu, W. C. Ko, T. M. Chang, M. C. Chang, and K. Y. Huang. 1997. Mouse skin damage caused by a recombinant extracellular metalloprotease from Vibrio vulnificus and by V. vulnificus infection. J. Formos. Med. Assoc. 96:677-684. [PubMed] [Google Scholar]
- 8.Chuang, Y. C., J. W. Liu, W. C. Ko, K. Y. Lin, J. J. Wu, and K. Y. Huang. 1997. In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob. Agents Chemother. 41:2214-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosbie, J., K. Winser, and P. Collins. 2002. Mapping the light field of the Waldmann PDT 1200 lamp: potential for wide-field low light irradiance aminolevulinic acid photodynamic therapy. Photochem. Photobiol. 76:204-207. [DOI] [PubMed] [Google Scholar]
- 10.Dalsgaard, A., N. Frimodt-Moller, B. Bruun, L. Hoi, and J. L. Larsen. 1996. Clinical manifestations and molecular epidemiology of Vibrio vulnificus infections in Denmark. Eur. J. Clin. Microbiol. Infect. Dis. 15:227-232. [DOI] [PubMed] [Google Scholar]
- 11.Dougherty, T. J., C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and Q. Peng. 1998. Photodynamic therapy. J. Natl. Cancer Inst. 90:889-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty, T. J. 2002. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 20:3-7. [DOI] [PubMed] [Google Scholar]
- 13.Gad, F., T. Zahra, K. P. Francis, T. Hasan, and M. R. Hamblin. 2004. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem. Photobiol. Sci. 3:451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomer, C. J., A. Ferrario, N. Hayashi, N. Rucker, B. C. Szirth, and A. L. Murphree. 1988. Molecular, cellular, and tissue responses following photodynamic therapy. Lasers Surg. Med. 8:450-463. [DOI] [PubMed] [Google Scholar]
- 15.Hamblin, M. R., D. A. O'Donnell, N. Murthy, C. H. Contag, and T. Hasan. 2002. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 75:51-57. [DOI] [PubMed] [Google Scholar]
- 16.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamblin, M. R., T. Zahra, C. H. Contag, A. T. McManus, and T. Hasan. 2003. Optical monitoring and treatment of potentially lethal wound infections in vivo. J. Infect. Dis. 187:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hongcharu, W., C. R. Taylor, Y. Chang, D. Aghassi, K. Suthamjariya, and R. R. Anderson. 2000. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J. Investig. Dermatol. 115:183-192. [DOI] [PubMed] [Google Scholar]
- 19.Iwersen-Bergmann, S., and A. Schmoldt. 2000. Acute intoxication with aniline: detection of acetaminophen as aniline metabolite. Int. J. Legal Med. 113:171-174. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, D. E., F. M. Calia, D. M. Musher, and A. Goree. 1984. Resistance of Vibrio vulnificus to serum bactericidal and opsonizing factors: relation to virulence in suckling mice and humans. J. Infect. Dis. 150:413-418. [DOI] [PubMed] [Google Scholar]
- 21.Kato, H., K. Furukawa, M. Sato, T. Okunaka, Y. Kusunoki, M. Kawahara, M. Fukuoka, T. Miyazawa, T. Yana, K. Matsui, T. Shiraishi, and H. Horinouchi. 2003. Phase II clinical study of photodynamic therapy using mono-l-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 42:103-111. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, M. T., F. W. Hickman-Brenner, and J. J. Farmer III. 1991. Vibrio, p. 384-395. In A. Balows, W. J. Hausler, Jr., K. L. Hermann, H. D. Isenberg, and H. J. Shadomy (ed.), Manual of clinical microbiology, 5th ed. American Society for Microbiology, Washington, D.C.
- 23.Ko, W. C., S. R. Chiang, H. C. Lee, H. J. Tang, Y. Y. Wang, and Y. C. Chuang. 2003. In vitro and in vivo activities of fluoroquinolones against Aeromonas hydrophila. Antimicrob. Agents Chemother. 47:2217-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komerik, N., M. Wilson, and S. Poole. 2000. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem. Photobiol. 72:676-680. [DOI] [PubMed] [Google Scholar]
- 25.Komerik, N., H. Nakanishi, A. J. MacRobert, B. Henderson, P. Speight, and M. Wilson. 2003. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob. Agents Chemother. 47:932-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreger, A., and D. Lockwood. 1981. Detection of extracellular toxin(s) produced by Vibrio vulnificus. Infect. Immun. 33:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambrechts, S. A., M. C. Aalders, D. H. Langeveld-Klerks, Y. Khayali, and J. W. Lagerberg. 2004. Effect of monovalent and divalent cations on the photoinactivation of bacteria with meso-substituted cationic porphyrins. Photochem. Photobiol. 79:297-302. [DOI] [PubMed] [Google Scholar]
- 28.Matevski, D., R. Weersink, H. C. Tenenbaum, B. Wilson, R. P. Ellen, and G. Lepine. 2003. Lethal photosensitization of periodontal pathogens by a red-filtered xenon lamp in vitro. J. Periodont. Res. 38:428-435. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi, N., C. Shimizu, S. Miyoshi, and S. Shinoda. 1987. Purification and characterization of Vibrio vulnificus protease. Microbiol. Immunol. 31:13-25. [DOI] [PubMed] [Google Scholar]
- 30.Moor, A. C. 2000. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B 57:1-13. [DOI] [PubMed] [Google Scholar]
- 31.Nitzan, Y., A. Balzam-Sudakevitz, and H. Ashkenazi. 1998. Eradication of Acinetobacter baumannii by photosensitized agents in vitro. J. Photochem. Photobiol. B 42:211-218. [DOI] [PubMed] [Google Scholar]
- 32.Oleinick, N. L., and H. H. Evans. 1998. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat. Res. 150:S146-S156. [PubMed] [Google Scholar]
- 33.Paiva, M. B., R. E. Saxton, G. A. Letts, P. S. Chung, J. Soudant, Q. Vanderwerf, and D. J. Castro. 1995. Interstitial laser photochemotherapy with new anthrapyrazole drugs for the treatment of xenograft tumors. J. Clin. Laser Med. Surg. 13:307-313. [DOI] [PubMed] [Google Scholar]
- 34.Paranjpye, R. N., J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibli, J. A., M. C. Martins, F. H. Nociti, Jr., V. G. Garcia, and E. Marcantonio, Jr. 2003. Treatment of ligature-induced peri-implantitis by lethal photosensitization and guided bone regeneration: a preliminary histologic study in dogs. J. Periodontol. 74:338-345. [DOI] [PubMed] [Google Scholar]
- 36.Simpson, L. M., and J. D. Oliver. 1987. Ability of Vibrio vulnificus to obtain iron from transferrin and other iron-binding compounds. Curr. Microbiol. 15:155-157. [Google Scholar]
- 37.Tang, H. J., M. C. Chang, W. C. Ko, K. Y. Huang, C. L. Lee, and Y. C. Chuang. 2002. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob. Agents Chemother. 46:3580-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy [PACT]. J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, M. 2004. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem. Photobiol. Sci. 3:412-418. [DOI] [PubMed] [Google Scholar]
- 40.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen, H. T., L. C. Chiang, K. H. Wen, C. C. Tsai, C. L. Yu, and H. S. Yu. 1996. The expression of cytokines by an established basal cell carcinoma cell line (BCC-1/KMC) compared with cultured normal keratinocytes. Arch. Dermatol. Res. 288:157-161. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida, S., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]