Abstract
Introduction:
Reported incidence of acute cor pulmonale (ACP) in patients with acute respiratory distress syndrome (ARDS) varies from 10% to 84%, despite being subjected to lung protective ventilation according to the current guidelines. The objective of this review is to find pooled cumulative incidence of ACP in patients with ARDS undergoing lung protective ventilation.
Materials and Methods:
We searched MEDLINE, EMBASE, Cochrane Library, KoreaMed, LILACS, and WHO Clinical Trial Registry. Cross-sectional or cohort studies were included if they reported or provided data that could be used to calculate the incidence proportion of ACP. Inverse variance heterogeneity (IVhet) and random effect model were used for the main outcome and measures.
Results:
We included 16 studies encompassing 1661 patients. The cumulative incidence of ACP using IVhet analysis was 23% (95% confidence interval [CI] = 18%–28%) over 3 days of lung protective ventilation. Random effect analysis of 7 studies (1250 patients) revealed pooled odd ratio of mortality of 1.16 (95% CI = 0.80-1.67, P = 0.44) due to ACP.
Conclusion:
Patients with ARDS have a 23% risk of developing ACP with lung protective ventilation. Findings of this review indicate the need of updating existing guidelines for ventilating ARDS patients to incorporate right ventricle protective strategy.
Keywords: Acute cor pulmonale, acute respiratory distress syndrome, lung protective ventilation, mechanical ventilation, right ventricular dysfunction
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is often complicated by pulmonary vascular dysfunction leading to the variable degree of right ventricular (RV) dysfunction.[1] Mechanical ventilation which is central to the management of ARDS also interferes with cardiac function by decreasing RV preload and increasing after load.[2] Strategy to open lung for optimum oxygenation remained the focus of researchers for many years. However, its effect on pulmonary vasculature and RV was largely ignored. With the advent of point of care tool, numbers of research papers were published evaluating right heart function in ARDS patients. These studies reported very high incidence of acute cor pulmonale (ACP) due to the use of high tidal volume and high airway pressure.[3] This prompted the implementation of current guidelines of the National Heart, Lung, and Blood Institute, National Institute of Health (NIH) ARDS network mechanical ventilation protocol that recommends limiting plateau pressure (Pplat) below 30 cm H2O and positive end-expiratory pressure below 24 cm H2O.[4] The use of low volume ventilation in ARDS has reduced the incidence of ACP to some extent but could not eliminate it completely.[5,6] Many recent studies showed that a large number of patients still suffered from RV dysfunction and its associated mortality despite being subjected to lung protective ventilation according to the current guidelines.[5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22] However, most of these studies had small sample size and reported varied incidence of ACP ranging from 10% to 84% across the literature.[5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22] This necessitated us to undertake this systematic review of cross-sectional or cohort studies on cumulative incidence of ACP in patients with ARDS subjected to lung protective ventilation. This review was aimed for a critical appraisal of study quality and an attempt where possible to generate pooled cumulative incidence from meta-analysis.
MATERIALS AND METHODS
The method of review was decided prospectively before the commencement of the data search. The protocol was registered prospectively at the National Institute for Health Research international prospective register of systematic review (identifier CRD42017054688).[23] Amendments were made to the original protocol to calculate incidence proportion instead of prevalence. We undertook and reported our systematic review in line with the criteria outlined in the meta-analysis of observational studies in epidemiology guidelines.[24]
Data search
MEDLINE through PubMed, EMBASE through Ovid and Cochrane Library were searched from the year 2000 to 6th January 2017 using a search strategy combining keywords and related database-specific subject terms. We limited our search to the year 2000 when NIH ARDSNet Ventilator Protocol was published.[4] To retrieve further literature published in language other than English, we searched KoreaMed and LILCAS. We searched WHO Clinical Trial Registry (who.int/ictrp) to find out unpublished literature and ongoing studies. The details of search strategy are shown in the Appendix [e-Appendix 1]. New links displayed beside the online version of the published abstracts were followed and retrieved. Bibliography of retrieved articles was searched and checked for additional studies. For any confusion regarding data, the authors of the articles were contacted. We translated nonEnglish literature to English by Google Translate (https://translate.google.com/).
Selection of the studies
Any cross-sectional or cohort study that reported the incidence proportion of ACP or where incidence proportion data for ACP could be calculated was included in the meta-analysis. Studies were excluded from the analysis where the airway pressure was not limited to below 30 cm H2O during mechanical ventilation, and the study population included pediatric patients and pregnant women. The full inclusion and exclusion criteria are presented in e-Appendix 2.
Data extraction
A data extraction form was used to extract equivalent information from each study. Retrieved information were: year of publication, country of origin, study design, sample size, number of patients who suffered from ACP, the time of echocardiography examination, mortality and the definition of ARDS used. Incidence proportion with 95% confidence interval (CI) were extracted or calculated from the available data. Two authors (SD and SC) independently reviewed the articles; did quality assessment and ascertained the criteria for inclusion in the pooled data analysis. A third reviewer (PS) blinded to the primary reviewer's decision checked the article selection, data extraction, and risk of bias assessment. Any disagreement was resolved by consensus. Articles by the same author were carefully investigated to avoid duplication of studies included in the analysis.
Risk of bias assessment
Included studies were assessed for risk of bias using “JBL Critical Appraisal Checklist for Studies Reporting Prevalence Data”.[25] We assessed quality in two domains: study participation and outcome measurement. We omitted a domain about response rate, which was not relevant to our study.[25] The detailed method used for risk of bias assessment is presented in e-Appendix 3.
Analysis
Studies that met the inclusion criteria were included for meta-analysis. We conducted the meta-analysis to find out pooled incidence proportion using inverse variance heterogeneity (IVhet) under fixed-effect model assumption with a quasi-likelihood-based variance structure.[26] The proportions are transformed with double arcsine square root analysis to stabilize the variance. The incidence proportion was expressed over the period from the initiation of mechanical ventilation to the time of echocardiographic examination. Potential influence on pooled incidence proportion was investigated using subgroup analysis whenever possible. If the study permitted, odd ratio of mortality in patient with ACP was calculated using random effect model.
All calculation were done using MetaXL version 5.3 (www.epigear.com, Epigear International, Sunrise Beach, Queensland, Australia) add-in for Microsoft Excel and RevMen 5.3 (Copenhagen: The Nordic Cochrane, The Cochrane Collaboration, 2014).[27,28] We needed to use two different software as MetaXL alone cannot calculate odd ratio and RevMen cannot calculate pooled incidence proportion.
MetaXL computes the inconsistency index (I2), which has been proposed as a measure to quantify the amount of heterogeneity. Inconsistency (I2) describes the percentage of total variation across studies that are due to heterogeneity rather than due to chance. An I2 value above 75% indicates high heterogeneity.
Funnel plot and Doi plot were drawn to find out any publication bias. A quantitative measure of Doi plot asymmetry is called Luis Furuya-Kanamori (LFK) index. LFK index exceeding ±2 indicates major asymmetry.[27]
RESULTS
Our search returned a total of 9365 publications, and 44 articles were selected for full-text review. After removing correspondences, editorials, and reviews, 24 studies were read for inclusion. Finally, a total of 16 studies that fulfilled the inclusion criteria were included for quantitative analysis of pooled cumulative incidence. The review process and selection of included studies are presented in Figure 1. Email was sent to authors of two studies to know the timing of echocardiography examination. However, no reply was received.
Figure 1.
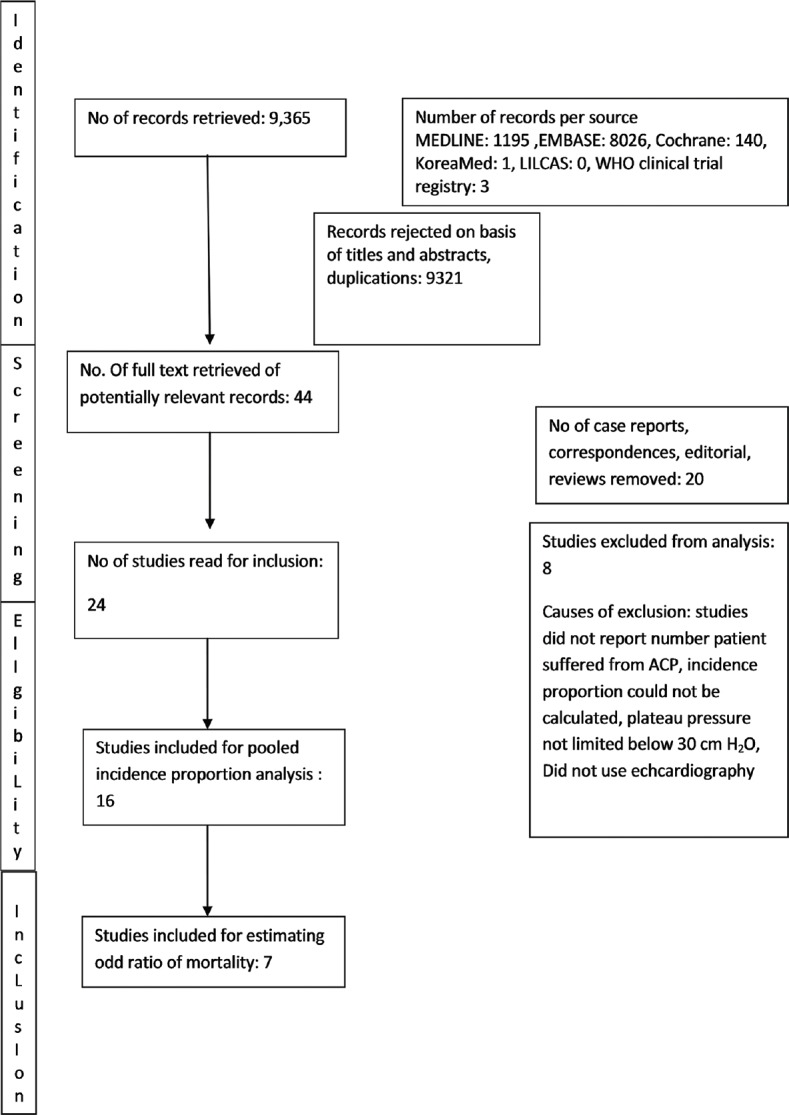
The review process and selection of included studies
The included studies had a total of 1661 patients who had ARDS and subjected to lung protective ventilation. Nine studies had prospective design, and the rest were retrospective. One study also included samples of three previously published studies.[19] The incidence proportion and mortality were calculated after excluding the samples of the previously published studies. One study was published in French language; the rest were in English.
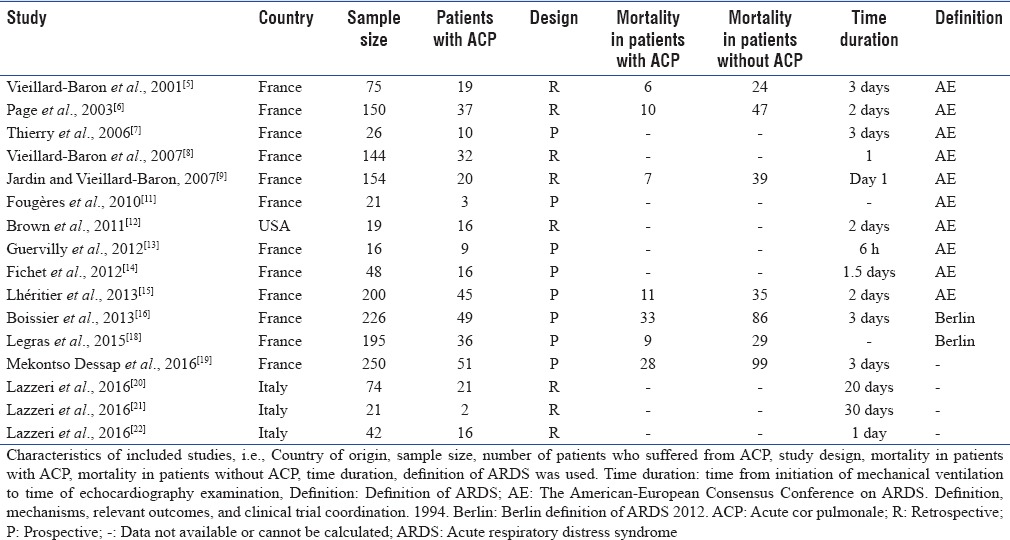
The characteristics of included studies are shown in Table 1. Seven studies with 1250 patients reported mortality in patients with ACP and in patients without it.[5,6,9,15,16,18,19]
Table 1.
Characteristics of included studies
A summary of the risk of bias of the included studies is provided in Table 2. Only 25% of the studies were considered to be at low risk of bias for study participation. This was because of poor method of sampling and inadequate number of sample size.
Table 2.
Risk of bias assessment of included studies
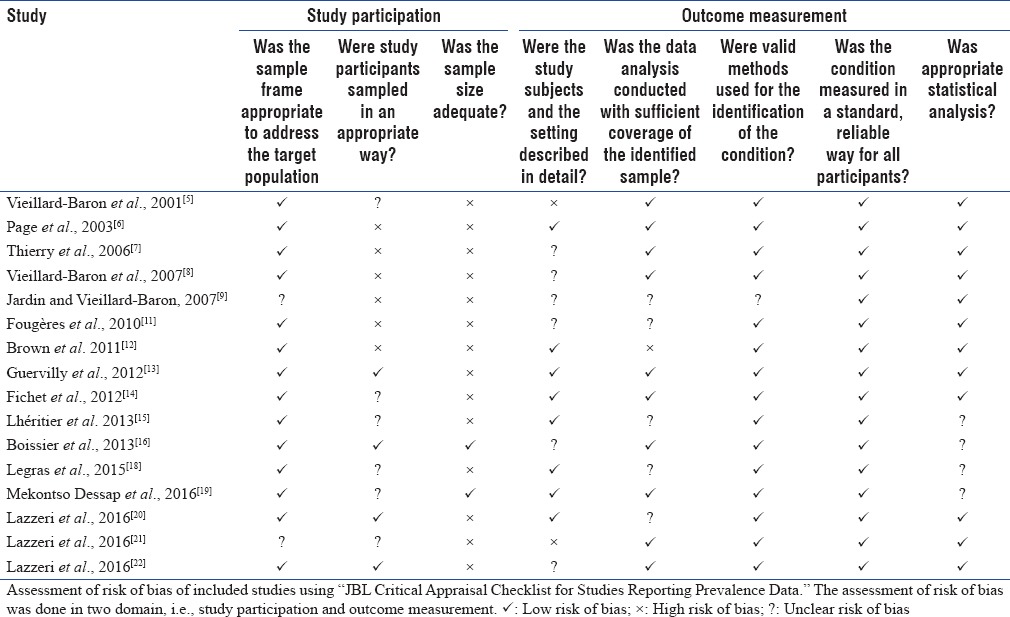
Seventy percent of the studies were at low risk of outcome measurement bias and used clearly defined diagnostic criteria, reliable-validated instruments, and a similar method and setting of outcome measurement for all participants.
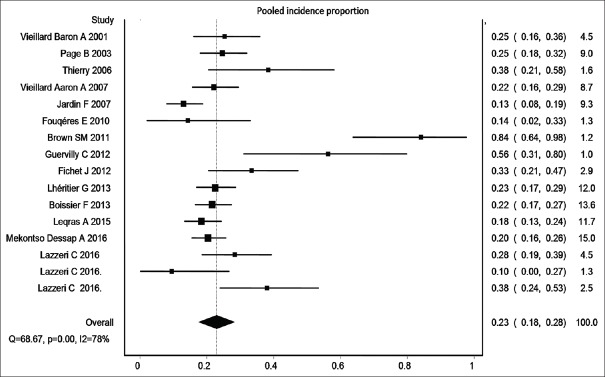
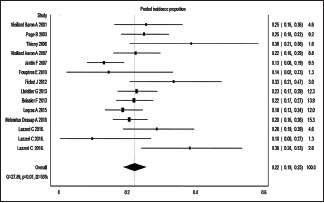
The incidence proportion ranged from 10% to 84%.[5,6,7,8,9,11,12,13,14,15,16,18,19,20,21,22] Three small studies reported incidence proportion more than 50%.[12,13] However, majority of the studies reported an incidence proportion between 10% and 30%.[5,6,7,8,9,11,12,13,14,15,16,18,19,20,21,22] Sixteen estimates were included in the meta-analysis. The overall IVhet pooled incidence proportion of ACP was 23% (95% CIs = 18%–28%) with a high level of heterogeneity (I2 = 78%) [Figure 2].
Figure 2.
Pooled incidence proportion of acute cor pulmonale in patients with acute respiratory distress syndrome undergoing lung protective ventilation
Funnel plot and Doi plot indicated the possibility of the presence of publication bias [e-Figures 1 and 2].
e-Figure 1.
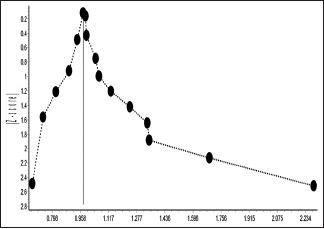
Major asymmetry in funnel plot
e-Figure 2.
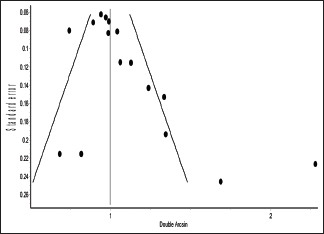
Significant asymmetry in Doi plot
Subgroup analysis was done excluding studies with <20 patients. Excluding two such studies that reported more than 50% incidence of ACP eliminated high level of heterogeneity [e-Figure 3].[12,13] This indicated that some unpublished small studies with low incidence of ACP might be the cause of major asymmetry of the funnel and Doi plot. However, cumulative meta-analysis with decreasing sample size did not show any major change of pooled incidence proportion due to inclusion of these two small studies [e-Figure 4]. Therefore, the presence of high heterogeneity and publication bias did not have any substantial effect on the result of the meta-analysis.
e-Figure 3.
Subgroup analysis after excluding two studies with sample size <20
e-Figure 4.
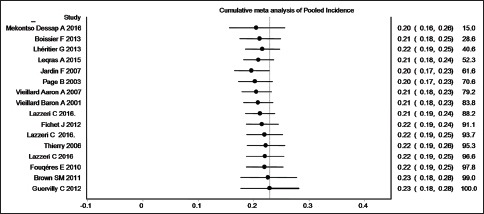
Cumulative meta-analysis of studies with decreasing sample size
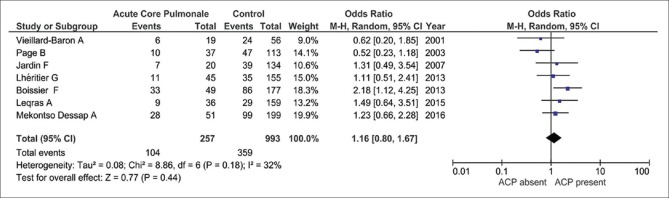
Total seven studies and 1250 patients were included in random effect analysis to calculate pooled odd ratio of mortality in patients with ACP.[5,6,9,15,16,18,19] Out of seven studies, five showed increased mortality in patients with ACP.[5,6,9,15,16,18,19] The pooled odd ratio of mortality in patients with ACP was 1.16 with 95% CI (0.80–1.67), which was not statistically significant (P = 0.44) [Figure 3].
Figure 3.
Odd ratio of mortality in patient with acute cor pulmonale using random effect model
DISCUSSION
The present systematic review and meta-analysis of 16 studies and 1661 ARDS patients subjected to lung protective ventilation revealed that pooled incidence proportion of ACP was 23% (95% CI = 18%–28%) during 3 days of lung protective ventilation. The odd ratio of mortality with ACP was 1.16 (95% CI = 0.80–1.67).
The deleterious effect of ARDS and mechanical ventilation on the right ventricle has been known for many years. ARDSNet protocol, which is in use for nearly one and half a decade, is primarily a lung protective ventilation strategy. Although lung protective ventilation improved mortality,[29] it still affects right ventricle adversely. These effects range from pulmonary artery hypertension to development of ACP.[16] This systematic review and meta-analysis give a quantitative estimation of the burden of ACP, which is the most serious form of RV dysfunction. The gold standard to diagnose pulmonary hypertension is right heart catheterization.[30] Two studies with 145 and 30 ARDS patients, respectively, who were managed with lung protective ventilation, used pulmonary artery catheter to evaluate RV function and pulmonary hypertension. Fourteen patients in each study suffered from pulmonary hypertension.[10,17]
Apart from RVED/LVED ratio, there are some other echocardiographic parameters that can also be used to measure RV function, for example, tricuspid annular plane systolic excursion (TAPSE), pulsed Doppler peak velocity at the tricuspid annulus, pulsed Doppler myocardial performance index, tissue Doppler myocardial performance index, fractional areas changes.[31] About 27%–48% patients with ARDS had TAPSE <16 mm while undergoing lung protective ventilation in five studies.[12,14,18,20,21]
It is not very clearly known about the factors that predispose to ACP. A very high incidence of ACP was reported in studies where airway pressure was not limited.[3,9] One study showed that high positive end-expiratory pressure and high partial pressure of carbon dioxide were associated with the development of ACP even when Pplat was maintained below 30 cm H2O.[32] Four factors, namely, pneumonia as cause of ARDS, driving pressure >18 cm H2O (difference between plateau and total positive end-expiratory pressure measured at zero flow)[33] partial pressure of arterial oxygen/fraction of inspired oxygen <150, partial pressure of carbon dioxide >48 mmHg was found to have contributed to the development of RV dysfunction in patients with ARDS.[19]
Various studies demonstrated the deleterious effect of RV dysfunction on hemodynamic. It decreases cardiac index, systolic, and mean arterial pressure. It increases heart rate, requirement of vasopressor, and incidence of shock.[15,16] ACP has been found to be associated with mortality in various studies.[9,10,15,16,18,19] However, the present study found a pooled odd ratio of mortality with ACP is 1.18, which is not statistically significant. This result may be due to the use of prone position ventilation in most of the studies that minimized the deleterious effect of ACP.[5,6,8,15,16,18,19] Present evidence makes it imperative to monitor RV morphology and its function while ventilating ARDS patients. The RV function and pulmonary circulation have been traditionally evaluated by pulmonary artery catheter. However, considering the invasiveness of the procedure and its associated complications, echocardiography can be used as an acceptable alternative.[34,35] To eliminate the discontinuous nature of hemodynamic monitoring by echocardiography, a miniaturized transesophageal echocardiography probe which can be kept inserted in the patients for several days has been developed, and it has shown to provide relevant hemodynamic information.[36]
In view of the potential risk associated with the current practice, it may be updated to include RV protective ventilation. This approach should include a stepwise ventilation strategy, keeping the pulmonary circulation and the right ventricle at the center of the decision-making process. It is based on the premise that “what is good for the right ventricle is also good for the lung and vice versa.”[37] This strategy may consist of limiting Pplat below 27 cm H2O, driving pressure below 17 cm H2O and PCO2 below 60 mmHg.[9,19,32,33] This strategy may also incorporate the early use of prone position ventilation or possibly extracorporeal membrane oxygenator if conventional ventilation does not meet the above criteria.[20,21,22,38] Current studies do not show evidence in support of high-frequency oscillation ventilation.[39]
We observed high heterogeneity in our study which is not due to chance (I2 = 78%). This heterogeneity may be due to different etiology of ARDS (studies where ARDS was caused by pneumonia had higher incidence of ACP), use of different definition of ARDS, different timing of echocardiography examination, use of adjunct therapy such as NO inhalation, publication bias, and inclusion of studies with small sample size.
This review has several strengths and limitations. This is the first review to give a quantitative summary of recent studies that examined the cumulative incidence of ACP in patients with ARDS subjected to lung protective ventilation. We conducted an extensive literature search that includes six databases. We did not limit ours search to any language. We tried to contact authors for clarification of data. Hence, we believe this review included most of the existing studies, and its result is a true reflection of cumulative incidence of ACP during mechanical ventilation of patients with ARDS. Its results, therefore, can be considered valuable addition to the present knowledge about mechanical ventilation of ARDS.
This review has some methodological limitations. Many studies included in the meta-analysis are retrospective and not of very good quality. The assessment of risk of bias showed that 75% studies had moderate to high risk of bias in patient participation domain. Many studies reported their results in prevalence data which was not accurately calculated. We calculated cumulative incidence from available data since time duration from the initiation of mechanical ventilation to the diagnosis of ACP for individual patient was not available to calculate incidence. In majority of studies, echocardiography was done within 3 days of mechanical ventilation, so we expressed cumulative incidence over 3 days of time.
The present systematic review and meta-analysis of 16 studies and 1661 patients revealed that patients with ARDS had a 23% (95% CI = 18%–28%) risk of developing ACP over <3 days of lung protective ventilation. This review suggests the need of updating existing guidelines for ventilating ARDS patients to incorporate right ventricle protective strategy.
AUTHORS CONTRIBUTION
SD, SC and PS are equally responsible for the integrity of this work from inception to manuscript preparation and contribution to study design, study selection, quality assessment, records review, data synthesis, data analysis and manuscript composition. AL contributed to the review process by searching literature, reviewing search records and manuscripts. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDICES: e-Appendix 1
Search strategy and the respective results and search date were shown.
MEDLINE Search (search date: 1.1.2017)
#1 Search “Ventricular Function, Right” [MH] (4832)
#2 Search “Ventricular Dysfunction, Right” [MH] (4494)
#3 Search “Heart Failure” [MH] (99601)
#4 Search “Pulmonary Heart Disease” [MH] (6132)
#5 Search “Pulmonary Artery Hypertension” [ALL] (1086)
#6 Search “Pulmonary Vascular Dysfunction” [ALL] (74)
#7 Search “Right ventricle dilation” [ALL] (15)
#8 Search “Acute Cor Pulmonale” [ALL] (275)
#9 Search “Right Heart Failure” [ALL] (2568)
#10 Search “Right Ventricle Dysfunction” [ALL] (81)
#11 Search “Acute Respiratory Failure” [ALL] (5461)
#12 Search “Mechanical Ventilation” [ALL] (32101)
#13 Search “ARDS” [ALL] (9249)
#14 Search “Acute Lung Injury” [ALL] (11882)
#15 Search “Lung Protective Ventilation” [ALL] (513)
#16 Search “Low stretch ventilation” [ALL] (12)
#17 Search “Acute Respiratory Distress Syndrome” [ALL] (9534)
#18 Search “Positive-Pressure Respiration” [MH] (22581)
#19 Search “Respiratory Distress Syndrome, Adult”[MH] (16343)
#20 Search “Respiration, Artificial”[MH] (66012)
#21 Search #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7OR #8 OR #9 0R #10 (114992)
#22 Search #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 (106129)
#23 Search # 21 AND #22 (1195) publication dated from 1.1.2000.
Chochrane Library Search (search date 29.12.2016)
#1 MeSH descriptor: [Ventricular Function, Right] explode all trees 210
#2 MeSH descriptor: [Ventricular Dysfunction, Right] explode all trees 167
#3 MeSH descriptor: [Heart Failure] explode all trees 6641
#4 MeSH descriptor: [Pulmonary Heart Disease] explode all trees 58
#5 “Pulmonary Artery Hypertension” 48
#6 “Pulmonary Vascular Dysfunction” 2
#7 “Right ventricle dilation” 2
#8 “Acute Cor Pulmonale” 5
#9 “Right Heart Failure” 75
#10 “Right Ventricle Dysfunction” 5
#11 MeSH descriptor: [Respiration, Artificial] explode all trees 5395
#12 MeSH descriptor: [Respiratory Distress Syndrome, Adult] explode all trees 684
#13 MeSH descriptor: [Positive-Pressure Respiration] explode all trees 2356
#14 “Acute Respiratory Distress Syndrome” 912
#15 “Low stretch ventilation” 1
#16 “Lung Protective Ventilation” 63
#17 “Acute Lung Injury” 716
#18 “ARDS” 892
#19 “Mechanical Ventilation” 4888
#20 “Acute Respiratory Failure” 629
#21 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 7094
#22 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 9809
#23 #21 and #22 140.
EMBASE search (search date 31.12.2016)
Right Ventricle Function.ti, ab. Or exp heart right ventricle function/(6380)
Exp heart right ventricle failure/or heart right ventricle/or Right Ventricle Dysfunction.ti, ab. or echocardiography/(206465)
Right ventricle dilation. ti, ab. (25)
Cor pulmonale/or Acute Cor Pulmonale.ti, ab. (6842)
Acute Respiratory Failure.ti, ab. or exp respiratory failure or exp acute respiratory failure/(6380)
ARDS.ti, ab. or exp adult respiratory distress syndrome/(32960)
Acute Lung Injury.ti, ab. or exp acute lung injury/(18596)
Exp artificial ventilation/or positive end expiratory pressure/or lung ventilation/(183939)
Low-stretch ventilation.ti, ab. or exp positive end expiratory pressure/(45852)
Positive Pressure Ventilation.ti, ab. (6490)
1 or 2 or 3 or 4 (215393)
5 or 6 or 7 or 8 or 9 or 10 (274973)
11 and 12 (8988) 9027
Limit 13 to yr = “200. Current” (7987) 8026
Korea med (search date: 31.12.2016)
[https://www.koreamed.org/SearchBasic.php]
“Acute cor pulmonale” AND [“Adult Respiratory Distress Syndrome” OR “Mechanical Ventilation”] 0
“Right Ventricle Dysfunction” AND [“Adult Respiratory Distress Syndrome” OR “Mechanical Ventilation”] 1
“Right Heart Failure” AND [“Adult Respiratory Distress Syndrome” OR “Mechanical Ventilation”] 0
Lilacs database (search date: 31.12.16)
[http://lilacs.bvsalud.org/en/]
“Acute cor pulmonale” AND [“Adult Respiratory Distress Syndrome” OR “Mechanical Ventilation”] 0
“Right Ventricle Dysfunction” AND [“Adult Respiratory Distress Syndrome” OR “Mechanical Ventilation”] 0
“Right Heart Failure” AND [“Adult Respiratory Distress Syndrome” OR “Mechanical Ventilation”] 0
WHO clinical trial registry (search date: 31.12.16)
[http://apps.who.int/trialsearch/Default.aspx]
(Acute cor pulmonale) AND (Adult Respiratory Distress Syndrome) 2
(Acute cor pulmonale) AND (Mechanical Ventilation) 0
(Right Ventricle Dysfunction) AND (Adult Respiratory Distress Syndrome) 0
(Right Ventricle Dysfunction) AND (Mechanical Ventilation) 0
(Right Heart Failure) AND (Adult Respiratory Distress Syndrome) 1
(Right Heart Failure) AND (Mechanical Ventilation) 0
e-Appendix 2
Inclusion and exclusion criteria

e-Appendix 3
“JBL critical appraisal checklist for studies reporting prevalence data” to asses of risk of bias of included studies. The checklist is adapted according to the present study. There two main domain: Study participation and outcome measurement.
Study participation
-
Was the sample frame appropriate to address the target population?
Low risk: Target population here adult population with ARDS defined by the American-European Consensus conference guidelines or Berlin definition. The cohort does not include only a specific subgroup of patients.
High risk: ARDS is not defined by American-European Consensus conference guidelines or Berlin definition. The cohort includes only a specific group of patients.
Unclear: No mention of any definition.
-
Were study participants sampled in appropriate way?
Low risk: Explicitly mention that random or consecutive sampling method has been used.
High risk: Convenience sampling method was used.
Unclear risk: There is no statement about the selection of patients.
-
Is the sample size adequate?
It will be assessed whether there is any mention of sample size calculation. We calculated minimum sample size of 228 is required to find out 20% of incidence proportion in population size of 3022, with 0.95 confidence level, and 0.05 desired precision on the basis of following published literature.
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016 23;315:788-800.
- Mekontso Dessap A, Repesse X, Boissier F, Legras A, Charron C, Begot E, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70.
Low risk: Sample of study is more than 228 or sample size was calculated.
High risk: sample of study is less than 228 or no mention of sample size.
Outcome measurement
-
Were the study subjects and setting described in detail?
Low risk: Meets the below-mentioned criteria
- Inclusion and exclusion criteria and patient's demographic characteristics are described.
- Ventilation strategy is clearly described with data about airway parameters.
- The echocardiography protocol is described.
High risk: If the above-mentioned details are not mentioned
-
Was data analysis conducted with sufficient coverage of identified sample?
Low risk: If etiology of ARDS is mentioned. The patients are not of one specific demographic characteristics or subgroup.
High risk: If etiology of ARDS is mentioned. Most of patient has one specific etiology.
Unclear risk: No statement regarding etiology.
-
Were valid methods used for identification of the condition?
Low risk: ARDS was defined either by Berlin or American. Acute Cor Pulmonale was defined as right ventricle end diastolic area/left ventricle end diastolic area >0.6
High risk: Other definition of ARDS is used. No mention of acute cor pulmonale definition.
Unclear risk: No mention of ARDS definition. No mention of any definition about acute cor pulmonale.
-
Was the condition measured in a standard reliable way for all participants?
Low risk: Criteria to define ARDS and ACP was applied to all patients.
High risk: Criteria to define ARDS and ACP was not applied to all patients.
Unclear: If there is no mention about on how many patients criteria to define ARDS and ACP was applied.
-
Was there appropriate statistical analysis
Low risk: Incidence or prevalence are calculated appropriately. Numerator and denominator can be identified clearly. Patient with acute cor pulmonale is numerator. Patients with ARDS and who were mechanically ventilated are denominators. Unit time is the time between initiation of mechanical ventilation and echocardiography examination in a predetermined manner. Incidence proportion can be calculated over unit time.
High risk: Incidence or prevalence are not calculated appropriately. Incidence proportion cannot be calculated over unit time.
Unclear risk: Incidence or prevalence are not calculated appropriately. However, incidence proportion can be calculated over unit time.
Low risk: If meets all of the criteria mentioned below.
Incidence or prevalence is reported or can be calculated with confidence interval or other measure of precision.
Numerator and denominator can be identified clearly, and patient with acute cor pulmonale are numerator. Patients with ARDS and who were mechanically ventilated are denominators.
High risk: If meets all of the criteria mentioned below:
-
Incidence or prevalence is not calculated appropriately
Ans: Cumulative incidence or incidence proportion was calculated as described in methodology ; analysis.
-
Numerator and denominator cannot be identified clearly
Ans: Numerator : number of patient who developed ACP. Denominator : number of patients with ARDS who are subjected to lung protective ventilation.
-
Incidence proportion can be calculated over unit time.
Ans: The incidence proportion was expressed over the time period from the initiation of mechanical ventilation to the time of echocardiographic examination
Unclear: Incidence or prevalence is provided but without any mention of any parameter of precision
Ans: The cumulative incidence of ACP using IVhet analysis, was 23% (95% CI = 18% – 28%) over three days of lung protective ventilation. Random effect analysis of 7 studies (1250 patients) revealed pooled odd ratio of mortality of 1.16 (95% CI = 0.80 to 1.67, P = 0.44).Underlined are parameter of precision.
REFERENCES
- 1.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296:476–80. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 2.Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: Rationale for protecting the right ventricle. Chest. 2015;147:259–65. doi: 10.1378/chest.14-0877. [DOI] [PubMed] [Google Scholar]
- 3.Jardin F, Gueret P, Dubourg O, Farcot JC, Margairaz A, Bourdarias JP. Two-dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med. 1985;13:952–6. doi: 10.1097/00003246-198511000-00035. [DOI] [PubMed] [Google Scholar]
- 4.ARDSnet NIH NHLBI ARDS Network Mechanical Ventilation Protocol Summary. [Last accessed on 2016 Dec 18]. Available from: http://www.ardsnet.org/files/ventilator_protocol_2008_07.pdf .
- 5.Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: Incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551–5. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Page B, Vieillard-Baron A, Beauchet A, Aegerter P, Prin S, Jardin F. Low stretch ventilation strategy in acute respiratory distress syndrome: Eight years of clinical experience in a single center. Crit Care Med. 2003;31:765–9. doi: 10.1097/01.CCM.0000055402.68581.DC. [DOI] [PubMed] [Google Scholar]
- 7.Thierry S, Lecuyer L, Brocas E, Van de Louw A, Hours S, Moreau MH, et al. Interest of the brain natriuretic peptide as a marker of acute cor pulmonale in acute respiratory distress syndrome. Ann Fr Anesth Reanim. 2006;25:815–9. doi: 10.1016/j.annfar.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007;132:1440–6. doi: 10.1378/chest.07-1013. [DOI] [PubMed] [Google Scholar]
- 9.Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33:444–7. doi: 10.1007/s00134-007-0552-z. [DOI] [PubMed] [Google Scholar]
- 10.Osman D, Monnet X, Castelain V, Anguel N, Warszawski J, Teboul JL, et al. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35:69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 11.Fougères E, Teboul JL, Richard C, Osman D, Chemla D, Monnet X. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: Importance of the volume status. Crit Care Med. 2010;38:802–7. doi: 10.1097/CCM.0b013e3181c587fd. [DOI] [PubMed] [Google Scholar]
- 12.Brown SM, Pittman J, Miller Iii RR, Horton KD, Markewitz B, Hirshberg E, et al. Right and left heart failure in severe H1N1 influenza A infection. Eur Respir J. 2011;37:112–8. doi: 10.1183/09031936.00008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, et al. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2012;40:1539–45. doi: 10.1097/CCM.0b013e3182451b4a. [DOI] [PubMed] [Google Scholar]
- 14.Fichet J, Moreau L, Genée O, Legras A, Mercier E, Garot D, et al. Feasibility of right ventricular longitudinal systolic function evaluation with transthoracic echocardiographic indices derived from tricuspid annular motion: A preliminary study in acute respiratory distress syndrome. Echocardiography. 2012;29:513–21. doi: 10.1111/j.1540-8175.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 15.Lhéritier G, Legras A, Caille A, Lherm T, Mathonnet A, Frat JP, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: A multicenter study. Intensive Care Med. 2013;39:1734–42. doi: 10.1007/s00134-013-3017-6. [DOI] [PubMed] [Google Scholar]
- 16.Boissier F, Katsahian S, Razazi K, Thille AW, Roche-Campo F, Leon R, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39:1725–33. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 17.Ñamendys-Silva SA, Santos-Martínez LE, Pulido T, Rivero-Sigarroa E, Baltazar-Torres JA, Domínguez-Cherit G, et al. Pulmonary hypertension due to acute respiratory distress syndrome. Braz J Med Biol Res. 2014;47:904–10. doi: 10.1590/1414-431X20143316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legras A, Caille A, Begot E, Lhéritier G, et al. Acute respiratory distress syndrome (ARDS)-associated acute cor pulmonale and patent foramen ovale: A multicenter noninvasive hemodynamic study. Crit Care. 2015;19:174. doi: 10.1186/s13054-015-0898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mekontso Dessap A, Repessé X, Boissier F, Legras A, Charron C, Bégot E, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–70. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 20.Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Terenzi P, Bernardo P, et al. Right ventricle dilation as a prognostic factor in refractory acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation. Minerva Anestesiol. 2016;82:1043–9. [PubMed] [Google Scholar]
- 21.Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Terenzi P, Bernardo P, et al. Pulmonary vascular dysfunction in refractory acute respiratory distress syndrome before veno-venous extracorporeal membrane oxygenation. Acta Anaesthesiol Scand. 2016;60:485–91. doi: 10.1111/aas.12643. [DOI] [PubMed] [Google Scholar]
- 22.Lazzeri C, Bonizzoli M, Cozzolino M, Verdi C, Cianchi G, Batacchi S, et al. Serial measurements of troponin and echocardiography in patients with moderate-to-severe acute respiratory distress syndrome. J Crit Care. 2016;33:132–6. doi: 10.1016/j.jcrc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, Choupoo NS, Saikia P, Lahakar A. Incidence Proportion of Acute Cor Pulmonale in Patients with Acute Respiratory Distress Syndrome Subjected to Lung Protective Ventilation: A Systematic Reviews and Meta Analysis. PROSPERO: International Prospective Register of Systematic Reviews. [Last accessed on 2017 May 26]. Available from: https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017054688 . [DOI] [PMC free article] [PubMed]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 26.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials. 2015;45(Pt A):130–8. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–8. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 28.Version 5.3. Copenhagen: The Nordic Cochrane, The Cochrane Collaboration; 2014. Review Manager (RevMan) [Computer Program] [Google Scholar]
- 29.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;2:CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–8. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 34.Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F. Echo-doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002;166:1310–9. doi: 10.1164/rccm.200202-146CC. [DOI] [PubMed] [Google Scholar]
- 35.Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Peris A, Gensini GF. The potential role and limitations of echocardiography in acute respiratory distress syndrome. Ther Adv Respir Dis. 2016;10:136–48. doi: 10.1177/1753465815621251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieillard-Baron A, Slama M, Mayo P, Charron C, Amiel JB, Esterez C, et al. A pilot study on safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med. 2013;39:629–35. doi: 10.1007/s00134-012-2797-4. [DOI] [PubMed] [Google Scholar]
- 37.Repessé X, Charron C, Vieillard-Baron A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol. 2012;78:941–8. [PubMed] [Google Scholar]
- 38.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]