Abstract
Zinc salt solutions administered as topical microbicides provided significant protection against herpes simplex virus type 2 infection in a mouse vaginal challenge model. However, at the therapeutic concentration, the salt solutions caused sloughing of sheets of vaginal epithelial cells. These observations limit the utility of zinc salts as microbicides and suggest that the application of zinc solutions to mucosal surfaces has the potential to cause damage that might increase susceptibility to secondary infections at a later time.
Zinc salts have been shown to have in vitro activity against a variety of pathogens, including the causative agents of a number of important sexually transmitted diseases (STD), such as human immunodeficiency virus, herpes simplex virus (HSV), and Chlamydia trachomatis (1, 9, 11, 12, 14, 17, 18). In addition to in vitro activity, mice inoculated intravaginally with HSV type 2 (HSV-2) and then treated either with 100 mM zinc-medicated collagen sponges or by an intravaginal 100 mM zinc salt douche had reduced incidences of genital herpes compared to controls (19, 20). Further, there are a number of reports of zinc salts being used topically for the treatment of recurrent herpes infections on the skin and mucosal surfaces (7, 10). Taken together, these observations suggested to us that zinc salts might have potential utility as topical microbicides. Topical microbicides are products that are designed to be applied directly to the vaginal or rectal epithelium prior to intercourse to prevent infection by STD pathogens (6, 22). They represent an attractive new approach to controlling the continuing STD epidemic for a variety of reasons. For example, microbicide use can be female initiated (potentially without partner notification). It is well recognized that women bear a disproportionate burden of STD infections but are frequently unable to negotiate the use of condoms for protection. Thus, a female-controlled protective measure would have obvious value. In addition, many of the products currently being developed as topical microbicides have shown activity against a number of different STD pathogens in preclinical testing. Thus, unlike vaccines, this approach offers the possibility that a single product could provide protection against multiple diseases. While a number of promising candidate products are being developed, none have, to date, undergone successful phase three clinical trials, and there is a real need for more research in this area to identify additional candidates.
In the studies reported here, we examined both the efficacy and safety of zinc salt solutions used as topical microbicides against HSV-2 genital infection in a mouse challenge model. We have used this model previously for the evaluation of other candidate topical microbicides, including a number that are currently undergoing clinical evaluation (2-4). Briefly, female Swiss Webster mice (weight, 18 to 21 g; Harlan, Houston, Tex.) were administered 0.1 ml of a suspension containing 3 mg of medroxyprogesterone acetate (Upjohn Pharmacia, Kalamazoo, Mich.) by subcutaneous injection in the shoulder region 7 days prior to virus challenge. On the day of challenge, animals were anesthetized with sodium pentobarbital, and the vaginal vault was swabbed with a moistened calcium alginate-tipped swab. An intravaginal instillation of 15 μl of the zinc salt or saline solution was administered to the anesthetized animals before the intravaginal challenge with 15 μl of inoculum containing 104 PFU of HSV-2 strain 186. We have previously established this inoculum as the minimum that routinely produces 90 to 100% infection in saline-treated control animals (2-4). The salts tested in these studies were zinc acetate, zinc chloride, zinc sulfate (all from Sigma Chemical Co., St. Louis, Mo.), and zinc gluconate (Alfa Aesar, Ward Hill, Mass.). All solutions were made in sterile deionized water. For zinc gluconate solutions, glycine was present at twice the molar amount of zinc as described previously for in vitro studies (1). Following HSV-2 challenge, vaginal swab samples were collected from all animals on day 2 postinoculation (p.i.) and stored frozen (−80°C) until they were assayed for the presence of virus by culture on Vero cell monolayers. Mice were evaluated up to day 21 p.i. for symptomatic infection (including hair loss and erythema around the perineum, chronic urinary incontinence, hind-limb paralysis, and mortality). Animals that did not develop symptoms were defined as infected if the virus was isolated from vaginal swab samples collected on day 2 p.i.
In initial protection studies, groups of mice were treated intravaginally with 100 mM solutions of zinc salts 20 s prior to virus inoculation. The concentration of zinc salt solution used was comparable to that used previously in treatment studies of mice (19, 20). At this concentration, only zinc sulfate provided significant protection against HSV-2 infection, with 40% of the animals being protected (P < 0.05 versus saline) (Table 1). To determine if protection could be improved, we next increased the concentration of the zinc salt solutions used in the animal studies to 200 mM. At this concentration, all three zinc salts tested, zinc acetate (P < 0.01), zinc sulfate (P < 0.01), and zinc gluconate (P < 0.001), provided significant protection compared to saline-treated control animals (Table 1). In contrast, no protection was seen for mice treated with saline solution in which the sodium chloride concentration was increased to 200 mM (Table 1).
TABLE 1.
Evaluation of zinc salts against HSV-2 in mice
| Study group and treatmenta | No. (%) of mice protected against:b
|
P valuec | |
|---|---|---|---|
| Disease | Infection | ||
| Study 1 | |||
| Zinc acetate (100 mM) | 4 (27) | 4 (27) | NS |
| Zinc chloride (100 mM) | 3 (20) | 2 (13) | NS |
| Zinc sulphate (100 mM) | 6 (40) | 6 (40) | <0.05 |
| Saline | 0 (0) | 0 (0) | |
| Study 2 | |||
| Zinc acetate (200 mM) | 9 (60) | 8 (53) | <0.01 |
| Zinc sulphate (200 mM) | 8 (53) | 7 (47) | <0.01 |
| Saline | 0 (0) | 0 (0) | |
| Study 3 | |||
| Zinc gluconate (100 mM) | 5 (33) | 2 (13) | NS |
| Zinc gluconate (200 mM) | 10 (67) | 9 (60) | <0.001 |
| Saline | 0 (0) | 0 (0) | |
| Study 4 | |||
| Sodium chloride (200 mM) | 0 (0) | 0 (0) | NS |
| Saline | 1 (7) | 1 (7) | |
Solutions (15 μl) were administered to animals 20 s before virus inoculation.
For each treatment in each study group, n = 15 mice. Animals were defined as infected if they developed symptoms or if virus was isolated from vaginal swabs collected on day 2 postinoculation.
P values were calculated versus saline by Fisher's exact test; all values are two tailed. NS, not significant.
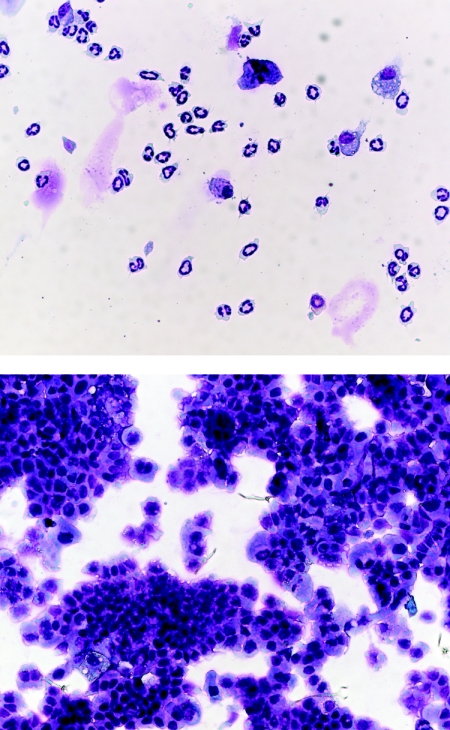
Recent clinical experiences with contraceptive gels containing the nonionic detergent nonoxynol-9 as a topical microbicide showed that frequent application produced a variety of adverse effects, including epithelial disruption and induction of inflammatory cells, and may increase the risk of human immunodeficiency virus infection (8, 16, 21). These findings highlighted the fact that topical microbicides would be applied frequently to mucosal surfaces by healthy women to prevent STD infections. Thus, to be accepted, they will need to not only to show efficacy but also to have outstanding safety profiles. The increased emphasis on early evaluation of microbicide safety that accompanied the nonoynol-9 findings is shown by recent reports describing a number of preclinical models for safety evaluation (5, 13, 15). In animals treated with zinc salts during the efficacy studies, there were no outward signs of adverse effects, such as irritation around the vaginal opening or altered behavior. However, to examine the safety of intravaginal treatment with the zinc salts in more detail, we next evaluated the entry of inflammatory cells into the vagina by using a modification of the vaginal lavage method we have described previously (15). Briefly, in two separate studies, groups of 10 animals were administered 200 mM solutions of zinc sulfate, zinc acetate, or saline as for efficacy studies. Six hours later, the vaginal vaults were washed three times with 50 μl of Hanks balanced salt solution plus 5% newborn calf serum to collect cells. Aliquots of cells from individual mice were spun onto glass slides by using a Cytospin 3 centrifuge (Shandon Life Sciences International, Ltd., Astmoor, Runcorn, England) and stained with a Hema 3 staining kit (Fisher Scientific, Inc., Pittsburgh, Pa.). Cell preparations were examined with a Zeiss Axiostar Plus microscope, and photomicrographs were obtained by using an AxioCam HRc digital camera and AxioVision 3.1 software (Carl Zeiss AG, Göttingen, Germany). Figure 1 shows that lavage samples from saline-treated mice contained only a modest number of cells that were predominantly neutrophils, characteristic of the normal vaginal leukocyte population (15). Samples from animals treated with zinc sulfate solution did not contain large inflammatory cell infiltrates; however, for all of the animals, these samples did contain sheets of vaginal epithelial cells, indicating that treatment resulted in substantial vaginal epithelial disruption (Fig. 1). Similar results were seen with vaginal lavage samples taken following treatment with zinc acetate solution (data not shown). It is possible that vaginal epithelial disruption could be significantly reduced by careful formulation of the zinc salts. However, our results suggest that the protection seen in the efficacy studies was due, at least in part, not to direct antiviral activity but to infected epithelial cells sloughing off before the virus could enter peripheral neurons. If this is the case, attempts to reduce toxicity by careful formulation are unlikely to be worthwhile.
FIG. 1.
Photomicrographs (×400) of cytospin preparations of vaginal lavage cells collected from mice 6 h after intravaginal administration of saline (upper panel) or a 200 mM zinc sulfate solution (lower panel). Samples from saline-treated animals show isolated cells, predominantly leukocytes. In contrast, samples from zinc sulfate-treated and zinc acetate-treated (data not shown) animals show rafts of sloughed vaginal epithelial cells.
In summary, when used as topical microbicides, zinc salt solutions may provide significant protection against HSV-2 infection in a mouse model, albeit at levels lower than those seen previously with some other topical microbicide candidates in this model (2-4). However, at a protective concentration, the salts produced vaginal epithelial disruption. While these studies utilize an animal model, and it is important to remember that there are morphological and physiological differences between the mouse and human vaginal epithelia, they raise serious safety concerns. Such adverse effects on vaginal mucosal integrity could actually enhance susceptibility to an STD pathogen if the user were exposed at a later time. Thus, zinc salt solutions would not appear to have utility as topical microbicides.
Acknowledgments
These studies were supported by National Institutes of Health grant PO1-AI-37940.
We thank Tedra Kelley for assistance with manuscript preparation.
REFERENCES
- 1.Arens, M., and S. Travis. 2000. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 38:1758-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourne, N., D. I. Bernstein, J. Ireland, A. J. Sonderfan, A. T. Profy, and L. R. Stanberry. 1999. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J. Infect. Dis. 180:203-205. [DOI] [PubMed] [Google Scholar]
- 3.Bourne, N., L. R. Stanberry, E. R. Kern, G. Holan, B. Matthews, and D. I. Bernstein. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 44:2471-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne, N., L. J. D. Zaneveld, J. A. Ward, J. P. Ireland, and L. R. Stanberry. 2003. Poly(sodium 4-styrene sulfonate): evaluation of a topical microbicide gel against herpes simplex type 2 and Chlamydia trachomatis infection in mice. Clin. Microbiol. Infect. 9:816-822. [DOI] [PubMed] [Google Scholar]
- 5.Catalone, B. J., T. M. Kish-Catalone, L. R. Bubgeon, E. B. Neely, M. Ferguson, F. C. Krebs, M. K. Howett, M. Labib, R. Rando, and B. Wigdahl. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Cruz, O. J., and F. M. Uckun. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10:315-336. [DOI] [PubMed] [Google Scholar]
- 7.Eby, G. A., and W. W. Halcomb. 1985. Use of topical zinc to prevent recurrent herpes simplex infections: review of the literature and suggested protocols. Med. Hypotheses 17:157-165. [DOI] [PubMed] [Google Scholar]
- 8.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 9.Geist, F. C., J. A. Bateman, and F. G. Hayden. 1987. In vitro activity of zinc salts against human rhinoviruses. Antimicrob. Agents Chemother. 31:622-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey, H. R., N. J. Godfrey, J. C. Godfrey, and D. Riley. 2001. A randomized clinical trial on the treatment of oral herpes with topical zinc oxide/glycine. Altern. Ther. Health Med. 7:49-56. [PubMed] [Google Scholar]
- 11.Greenberg, S. B., D. Harris, P. Giles, R. R. Martin, and R. J. Wallace, Jr. 1985. Inhibition of Chlamydia trachomatis growth in McCoy, HeLa, and human prostate cells by zinc. Antimicrob. Agents Chemother. 27:953-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haraguchi, Y., H. Sakurai, S. Hussain, B. M. Anner, and H. Hoshino. 1999. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 43:123-133. [DOI] [PubMed] [Google Scholar]
- 13.Keller, M. J., M. E. Klotman, and B. C. Herold. 2003. Rigorous pre-clinical evaluation of topical microbicides to prevent transmission of human immunodeficiency virus. J. Antimicrob. Chemother. 51:1099-1102. [DOI] [PubMed] [Google Scholar]
- 14.Merluzzi, V. J., D. Cipriano, D. McNeil, V. Fuchs, C. Supeau, A. S. Rosenthal, and J. W. Skiles. 1989. Evaluation of zinc complexes on the replication of rhinovirus 2 in vitro. Res. Commun. Chem. Pathol. Pharmacol. 66:425-440. [PubMed] [Google Scholar]
- 15.Milligan, G. N., K. L. Dudley, N. Bourne, A. L. Reece, and L. R. Stanberry. 2002. Entry of inflammatory cells into the mouse vagina following application of candidate microbicides: comparison of detergent-based and sulfated polymer-based agents. Sex. Transm. Dis. 29:597-605. [DOI] [PubMed] [Google Scholar]
- 16.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327-331. [DOI] [PubMed] [Google Scholar]
- 17.Suara, R. O., and J. E. Crowe, Jr. 2004. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 48:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugarman, B., and L. R. Epps. 1985. Zinc and Chlamydia trachomatis. Proc. Soc. Exp. Biol. Med. 179:382-387. [DOI] [PubMed] [Google Scholar]
- 19.Tennican, P., G. Carl., J. Frey, C. Theis, and M. Chvapil. 1980. Topical zinc in the treatment of mice infected intravaginally with herpes genitalis virus. Proc. Soc. Exp. Biol. Med. 164:593-597. [DOI] [PubMed] [Google Scholar]
- 20.Tennican, P. O., G. Z. Carl, and M. Chvapil. 1979. The diverse effects of topical and systemic administration of zinc on the virulence of herpes genitalis. Life Sci. 24:1877-1884. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2002. WHO/CONRAD technical consultation on nonoxynol-9, World Health Organization, Geneva, 9-10 October 2001: summary report. Reprod. Health Matters 10:175-181. [DOI] [PubMed] [Google Scholar]
- 22.Zeitlin, L., K. J. Whaley. 2002. Microbicides for preventing transmission of genital herpes. Herpes 9:4-9. [PubMed] [Google Scholar]