Abstract
Introduction:
International guidelines are promoting early enteral nutrition (EN) as a means of feeding critically ill adult patients to improve clinical outcomes. The question of how much calorie intake is enough to improve the outcomes still remained inconclusive. Therefore, we carried out a meta-analysis to evaluate the effect of low calorie (LC) versus high calorie (HC) delivery on critically ill patients' outcomes.
Methods:
We included randomized clinical trials (RCTs) that compared LC EN with or without supplemental parenteral nutrition with HC delivery in this meta-analysis irrespective of the site of nutritional delivery in the gastrointestinal tract. We searched PubMed, EMBASE, and Cochrane central register of controlled trials electronic databases to identify RCTs that compared the effects of initially different calorie intake in critical illness. The primary outcome was overall mortality.
Results:
This meta-analysis included 17 RCTs with a total of 3,593 participants. The result of analysis showed that there was no significant difference between the LC group and HC group in overall mortality (risk ratio [RR], 0.98; 95% confidence interval [CI], 0.87–1.10; P = 0.74; I2 = 6%; P = 0.38), or new-onset pneumonia (RR, 0.92; 95% CI, 0.73–1.16, P = 0.46; I2 = 38%, P = 0. 11).
Conclusion:
The current meta-analysis showed that there was no significant difference in mortality of critically ill patients initially between the two groups.
Keywords: Critically ill patients, enteral nutrition, high calorie, low calorie, overall mortality, supplemental parenteral nutrition
INTRODUCTION
International guidelines recommended the initiation of early enteral nutrition (EN) to all Intensive Care Unit (ICU) patients who are not expected to receive a full oral diet within 2 or 3 days of ICU admission.[1,2,3] In support of these evidence-based guidelines, results from clinical trials and meta-analyses demonstrated that a statistically significant reduction in mortality,[4,5,6] ventilator-associated pneumonia,[4,7] duration of mechanical ventilations, and length of ICU[6,8,9] when initiated within the first 24 h of ICU admission. This reduction in clinical outcomes in turns associated with reduction in cost of care.[7] Interestingly enough, there is no any strong evidence so far suggesting the harmfulness of early initiation of EN in critically ill patients.[10]
Controversy exists in studies so far examining the effects of energy delivery and clinical outcomes in critically ill patients admitted to ICU. On the one hand, cumulative energy deficit has been associated with unwanted adverse outcomes such as prolonged ICU stay, and therefore, infectious complications.[11,12] Randomized clinical trials (RCTs) also demonstrated that enteral intake of full-calorie requirement was associated with a trend toward improvement of mortality.[13] Moreover, consensus regarding the early use supplemental parenteral nutrition (SPN) as a way to supply sufficient energy does not exist.[14] On the another hand, permissive underfeeding was associated with improved outcomes compared with full feeding[6,15,16,17,18] and even in some studies, the later was found to be associated with significantly higher mortality in critically ill patients with acute lung injury.[19]
Therefore, the question of how much calorie should be given to critically ill patients admitted to ICU remained to be a hot topic of debate. In response to this question, two recent meta-analyses were carried out to find the energy target for critically ill patients and reported that the initial calorie of 33%–66% range was associated with a trend toward reduction in mortality.[20,21] However, the interpretation of published meta-analyses of trials comparing nutritional support through full feeding compared to permissive underfeeding in critically ill patients is complicated by small sample sizes due to their strict inclusion criteria, variable quality and bias. Recently, published RCTs are also challenging the benefits of delivering of a full calories compared to hypocaloric EN.[22,23,24,25,26] All together, these data revealed that the optimal and safe caloric intake in critically ill patients still remains inconclusive. Taking into account, the variation in the results of the currently available studies, we believed that a comprehensive updated meta-analysis of more recent RCTs is mandated. Therefore, this meta-analysis of 17 RCTs aimed to compare initial LC versus HC delivery in critically ill patients.
METHODS
The meta-analysis reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.[27]
Search strategy
Two investigators (LCH and ZM) independently searched electronic databases in PubMed, EMBASE, and the Cochrane database from inception to June 2016 using the terms “permissive underfeeding,” or “hypocaloric feeding,” or “trophic feeding,” or “gradual enteral nutrition,” or “low calorie nutrition,” or “standard enteral nutrition,” or “intensive enteral nutrition,” or “eucaloric enteral nutrition,” or “normocaloric enteral nutrition,” or “concentrated enteral nutrition,” or “hypercaloric enteral nutrition,” or “full feeding,” “overfeeding,” or “gastrostomy tube,” “delay feeding,” or “early feeding,” or “postpyloric feeding” or “nasogastric tube,” or “J tube” or “G tube” combined with the terms “critically ill patients,” or “critical illness,” or “ICU,” or “intensive care,” in duplicate. A manual search for additional relevant studies using references from retrieved articles was also performed. Conference abstracts and unpublished studies were excluded from the study. We restricted the searches to human studies with language restriction to English placed on the searches.
Types of studies, participants, and interventions
The same authors (LCH and ZM) independently assessed the inclusion criteria and if there was disagreement, a third author consulted (MM). We included the studies if they met the following criteria: the study design was an RCT, the population comprised critically ill adult patients admitted to the ICU, had significantly different in calorie delivery by EN and/or supplemental parenteral nutrition between two or more arms (P < 0.05), nutritional intervention was >48 h and the mortality was reported as primary or secondary endpoint. Studies were excluded if either of the groups received no nutrition or identical calorie, nutritional intervention < 2 days and if mortality was not reported.
Outcome measures
The primary outcome was the overall mortality reported by each trial at the last follow-up duration. For instance, if a trial reported 28, 60, and 90 days of mortality, we considered 90-day mortality as overall mortality. When it was not reported, we excluded the trial(s) from the analysis. Other secondary outcomes considered in this meta-analysis were the incidence of new onset pneumonia and sepsis, the lengths of ICU and hospital stay, duration of mechanical ventilation, the incidence of hypoglycemia and average doses of insulin used per day, incidence of renal replacement therapy (RRT), and gastrointestinal intolerance. Subgroup analyses were conducted to find if mortality differed either by mode of delivery (EN with or without SPN), based on severity score (Acute Physiology and Chronic Health Evaluation II [APACHE II] >20 vs. APACHE II ≤20), the body mass index (BMI) (BMI ≥25 vs. <25), the amount of calorie intake by low calorie (LC) group of standard requirements (LC <33.3%, 33.33% < LC <66.6%, LC > 66%) based on the recent meta-analyses.[20,21]
Data abstraction and quality assessment
Two independent authors (LCH and ZM) extracted data from all eligible studies on to a standardized data abstraction sheet. We extracted information on study authors, year of publication, characteristics of the studies such as age (years), BMI in kg/m2, severity scores, and total sample size (intervention plus control), and daily calorie delivered (kcal/day), average protein intake (g/day), and primary and secondary outcomes. The same authors independently assessed the included trials for bias according to the Handbook for Systematic Reviews of Interventions.[28] Disagreement also resolved by consulting the third author. The following parameters were assessed: sequence generation, allocation concealment, masking (blinding) of participants, personnel and outcome assessors, incomplete outcome data, and selective outcome reporting. Other sources of bias were a risk of bias related to the specific study design used or trial stopped early due to some data-dependent process or an extreme baseline imbalance in patients selected according to this handbook.
Statistical analysis
We followed the Cochrane handbook of data analysis and reported dichotomous outcome measures to assess the summary effects of treatment by calculated risk ratio (RR) with 95% confidence interval (CI). The overall weighted mean difference (WMD) with 95% CI was estimated for continuous variable. A random-effects model was used in this meta-analysis because of anticipated heterogeneity. Statistical heterogeneity among trials was expressed as the P value (Cochran's Q statistic), where a P < 0.05 and I2 statistic >50% indicated significant heterogeneity. Sensitivity analysis was done by sequentially deleting a single study each time in an attempt to identify the potential influence of an individual study on mortality and stability of the result. The analyses were carried out using RevMan 5.3 software (The Nordic Cochrane Center, Denmark) to create a forest plot and a summary finding tables.
Definitions
“High calorie” and “low calorie” simply to represent the intervention arms of individual trials received higher calorie relative to the comparator group (received LC) for the respective trials. They were not showing comparison of levels of calorie delivered between trials. Gastrointestinal intolerance was also defined high residual gastric volume, regurgitation, vomiting, noninfectious diarrhea, constipation, or abdominal distension. All definitions were defined according to the original studies although there were differences in definitions.
RESULTS
Literature searches and selection
The details of our search strategy were depicted in Figure 1. Our initial research of electronic databases such as PubMed, EMBASE, and Cochrane yielded 6120 articles, from which 1206 records remained after removing 4914 duplications. A total of 1185 articles were not included; 520 were not adults, 371 articles were not RCTs, 85 articles were reviews or meta-analyses, 90 articles were about parenteral nutrition, no full text available for 6 articles, 32 articles were about timing, 7 articles were about immunonutrition, 40 articles were residual volume, and 34 articles were other procedures. Of the remaining 21 potentially relevant articles for qualitative analyses, 4 articles were excluded; 2 articles reported that group received no LC nutrition; and the remaining 2 articles reported that the two groups received similar calorie.
Figure 1.
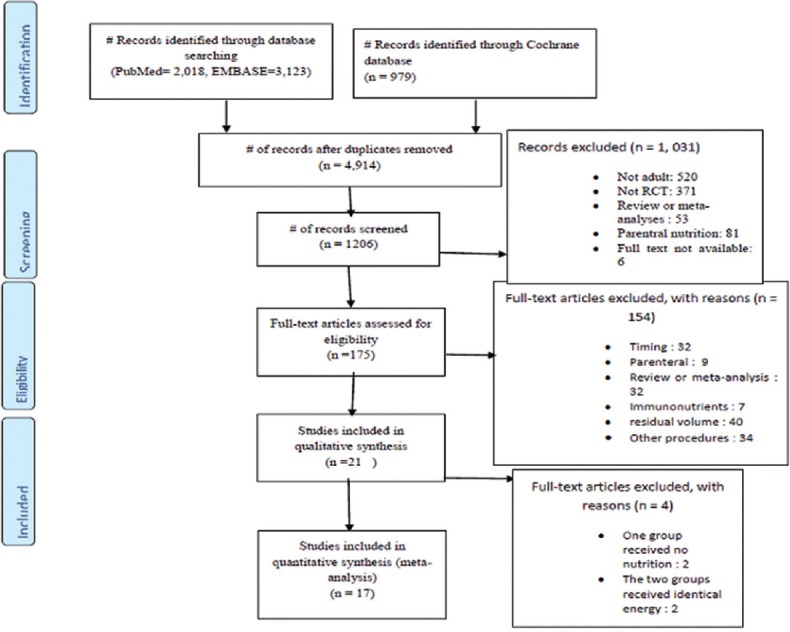
Study flow diagram according to Preferred Reporting Items for Systematic Reviews and Meta-analysis
Study characteristics
Finally, 17 RCTs published between 1992 and 2016 fulfilling the inclusion criteria were included in the final quantitative analyses.[6,13,15,18,19,22,23,24,25,26,29,30,31,32,33,34,35] The sample size of the included trials ranged from 19[35] to 1000[22] with a total number of 3593 patients, of which 1800 were assigned to the LC and 1793 to high calorie (HC) group. The mean age of the patients included in the study was >50 years with the exception of one which reported as <28 in the LC and <35 in the HC.[30] The mean BMI of patients was ≥25 kg/m2 in 11 studies,[6,13,18,19,22,23,24,25,26,29,34] 25< kg/m2 in two studies[31,32] and not available for four studies[15,30,33,35] The APACHE II scores in ten studies were >20[6,13,15,18,19,23,25,31,34,35] and ≤20 in five studies.[24,26,30,32,33] One study reported simplified acute physiology score (SAPS)[29] and one study used APACHE III scores[22] as shown in Table 1. The average daily calories delivered were different among all studies (P < 0.05). This ranges from 126 to 1480 kcal/day in LC group and 474–2086 kcal/day in HC delivery group. The mean daily percentage of calories in LC group in three studies was <33%,[15,22,23] 33%–66% in nine studies,[6,18,19,24,25,26,30,33,35] and >66% in five studies.[13,29,31,32,34] The mean calorie target in HC group was <70% in four studies,[15,30,33,35] >70% in seven studies,[6,18,19,22,23,24,25] and >90% in six studies.[13,26,29,31,32,34] Ten of the 17 RCTs reported amount gram of protein delivered per day and ranges from (mean ± standard deviation) 5.5 ± 5.3–86 ± 6 g/day in the LC group and 18.7 ± 15.4–83 ± 6 g/day for the HC delivery group[6,13,15,18,19,23,24,31,33,34] as indicated in Table 2.
Table 1.
The baseline characteristics of included patients in meta-analysis
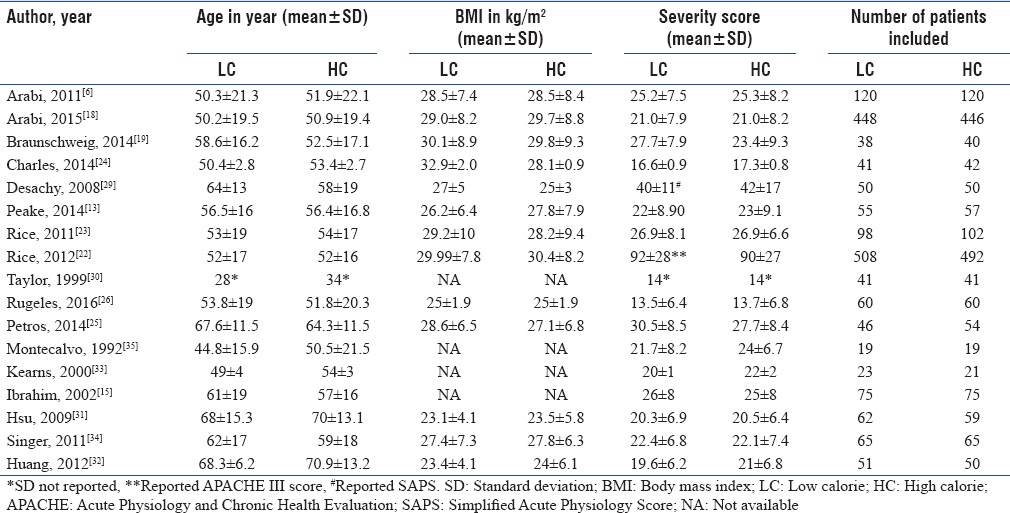
Table 2.
Amount of calorie delivered and overall mortality
Risk of bias in included studies
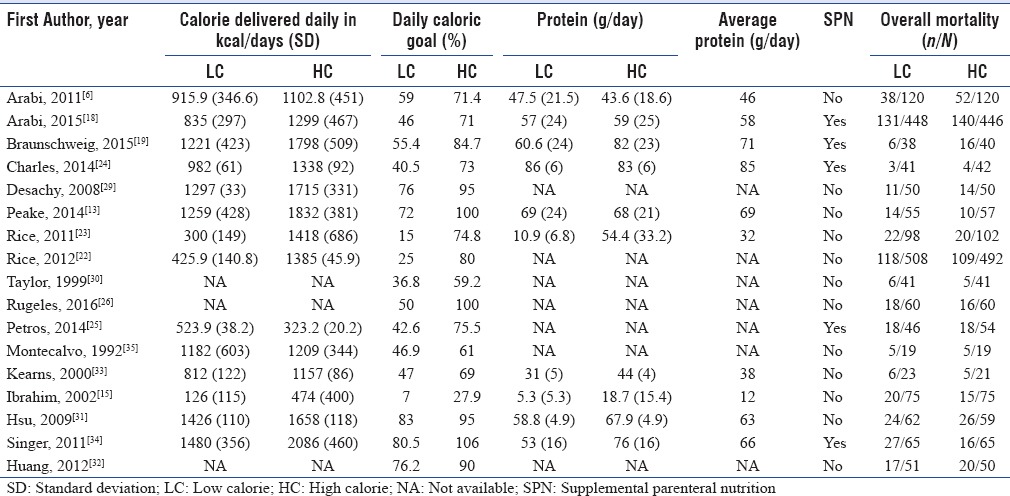
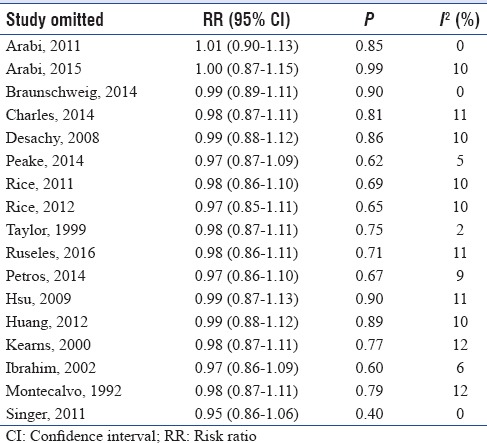
We used the Cochrane collaboration tool to assess the risk of bias of individual study. Only one study used a double-blind design because of the need for adjustment of the nutritional support according to feeding tolerance and gastric residual volume and at lower risk of bias across all domains.[13] Other five trials had also low risk of bias except for blinding of patients and personnel.[18,19,23,24,31] The rest trials had either high or unclear risk of bias in one or more domains in addition to bias in blinding domain as shown in Figures 2 and 3. Funnel plot was used to assess possible reporting or publication bias on mortality. Figure 4 shows the approximate symmetry of the funnel plot for mortality. We performed a sensitivity analysis by removing a single study at a time to evaluate the stability and robustness of the pooled mortality outcome. We obtained statistically similar results after omitting each of the studies as described in Table 3. This indicates that the good degree of stability in the findings of this meta-analysis about the primary outcome.
Figure 2.
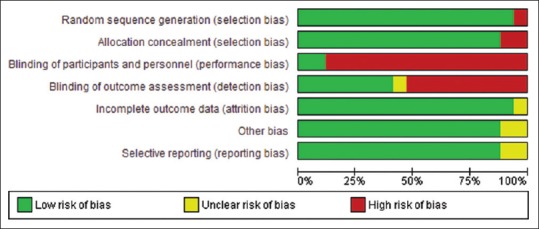
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies
Figure 3.
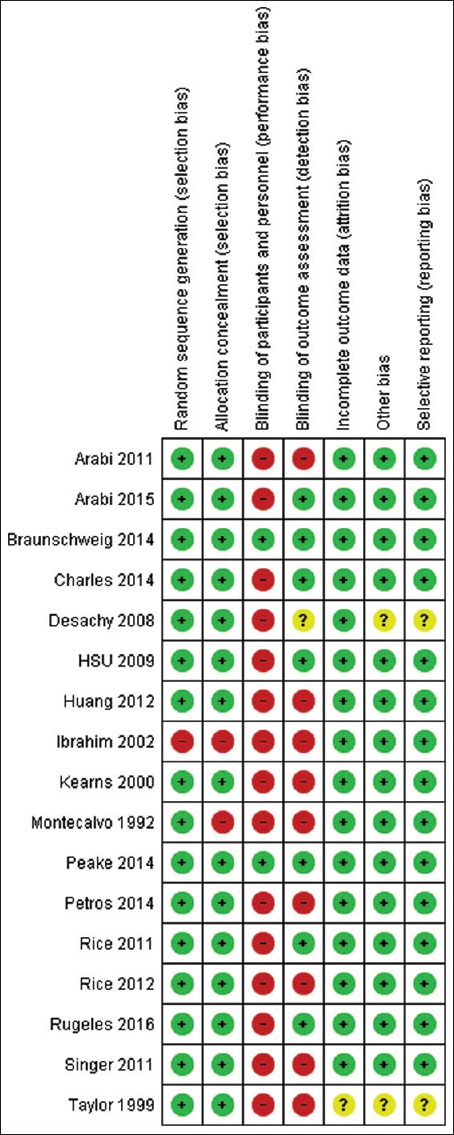
Risk of bias summary: Review authors' judgments about each risk of bias item for each included study
Figure 4.
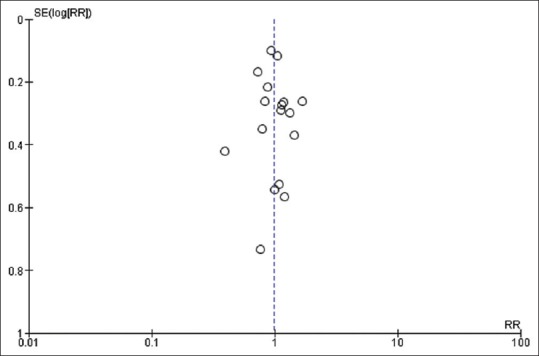
The impact of initial LC on mortality. LC: Low calorie; RR: Risk ratio; SE: Standard error
Table 3.
Sensitivity analysis for overall mortality by omitting each study in random-effects model
Clinical outcomes
Overall mortality
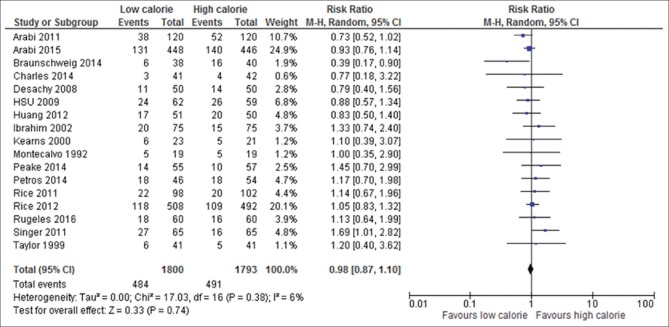
Mortality data were available in all 17 trials included in the meta-analysis; two trials reported 180-day mortality at the last follow-up,[6,18] one trail reported 90-day mortality,[13] one trail 60-day mortality,[22] one trial 28-day mortality,[26] one trail ICU mortality,[34] five trials hospital mortality,[15,23,25,29,32] and six trials reported unidentified mortality.[19,24,30,31,33,35] The aggregate result of this study showed that the overall mortality rate in LC delivery group was 484 (26.9%) of 1800 participants and 491 (27.4%) of 1793 in HC group which was not statistically significantly different (RR, 0.98; 95% CI, 0.87–1.10; P = 0.74; I2 = 6%; P = 0.38) as depicted in Figure 5. Close observation of these results shows that the absolute reduction of mortality by LC delivery is 1.6%. No significant difference effect was observed on mortality based on the subgroup analysis according to:
Figure 5.
Forest plot showing the impact of daily calories goals on overall mortality in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval
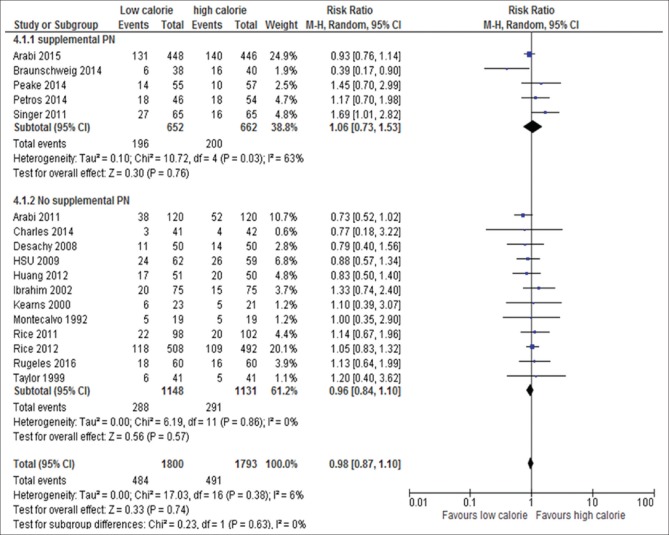
Mode of calorie delivery: EN with SPN (RR, 106; 95% CI, 0.73–1.53; P = 0.76; I2 = 63%, P = 0.03) versus without SPN (RR, 0.98; 95% CI, 0.84–1.10, P = 0.57; I2 = 0%, P = 0.86) [Figure 6]
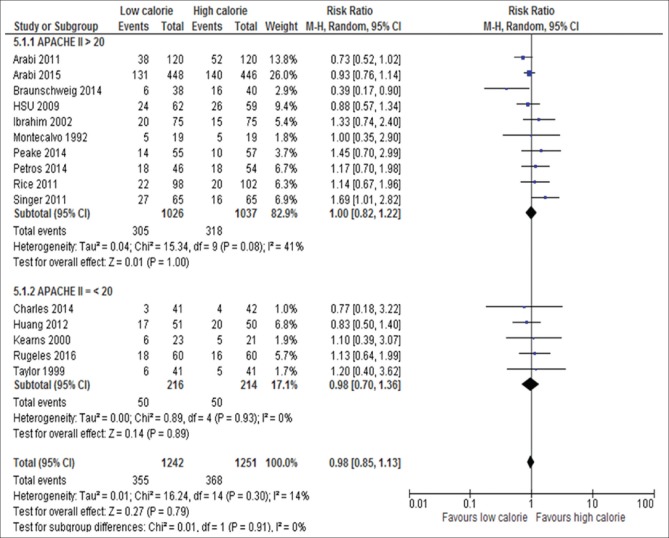
Severity score: APACHE II >20 (RR, 1.00; 95% CI, 0.82–1.22, P = 1.00; I2 = 41%, P = 0.08) versus APACHE II ≤20 (RR, 0.98; 95% CI, 0.70–1.1.36, P = 0.89; I2 = 0%, P = 0.93) [Figure 7]
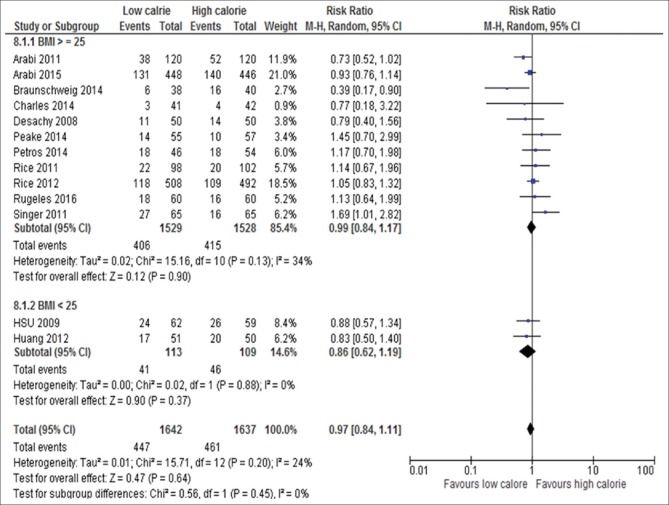
The BMI: BMI ≥25 kg/m2 (RR, 0.99; 95% CI, 084–1.16, P = 0.90; I2 = 34%, P = 0.13) versus <25 kg/m2 (RR, 0.86; 95% CI, 0.62–1.19, P = 0.37; I2 = 0%, P = 0.88) [Figure 8]
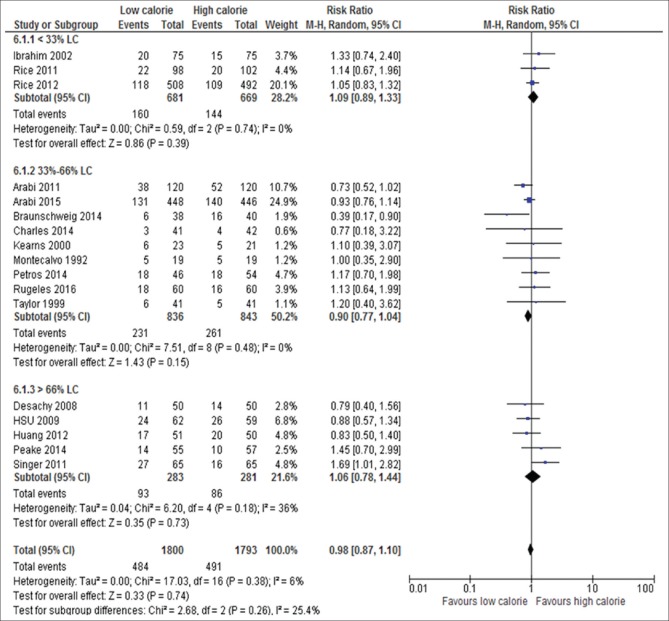
Amount of calorie delivered by LC of standard requirement: <30% LC (RR, 1.09; 95% CI, 0.89–1.33, P = 0.39; I2 = 0%, P = 0.74) versus 33%–66% LC (RR, 0.90; 95% CI, 0.77–1.04, P = 0.15; I2 = 0%, P = 0.48) versus >66% (RR, 1.06; 95% CI, 0.78–1.44, P = 0.73; I2 = 36%, P = 0.18) [Figure 9].
Figure 6.
Forest plot showing the impact of daily calories goals by EN with or without SPN on mortality in critically ill patients. M-H: Mantel-Haenszel; CI: Confidence interval; LC: Low calorie; EN: Enteral nutrition; PN: Parenteral nutrition
Figure 7.
Forest plot showing the impact of daily calories goals based on disease severity score on mortality in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval; APACHE II: Acute Physiology and Chronic Health Evaluation II
Figure 8.
Forest plot showing the impact of daily calories goals based on BMI on mortality in critically ill patients. M-H: Mantel-Haenszel; CI: Confidence interval; BMI: Body mass index
Figure 9.
Forest plot showing the impact of percentage of daily calories goals on mortality in critically ill patients. M-H: Mantel-Haenszel; CI: Confidence interval
Pneumonia
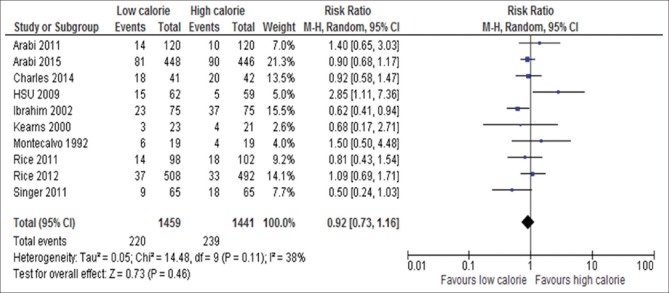
Ten of the 17 studies reported new-onset pneumonia which included 2950 patients.[6,15,18,22,23,24,31,33,34,35] The incidence of pneumonia was not significantly different between the groups (RR, 0.92; 95% CI, 0.73–1.116; P = 0.46; I2 = 38%; P = 0.11) as shown in Figure 10.
Figure 10.
Forest plot showing the impact of daily calorie goals on new onset pneumonia in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval
Blood stream infection or bacteremia or sepsis
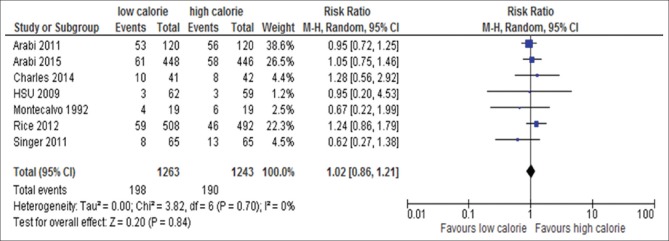
Seven out of the 17 trials reported either sepsis or bacteremia or bloodstream infection:[6,18,22,23,24,31,34,35] these studies included 2506 patients. Sepsis or bacteremia or bloodstream infection was not significantly different between the groups (RR, 1.02; 95% CI, 0.86–1.21; P = 0.84; I2 = 0%; P = 0.70) [Figure 11].
Figure 11.
Forest plot showing the impact of daily calorie goals on sepsis in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval
Lengths of Intensive Care Unit and hospital stay
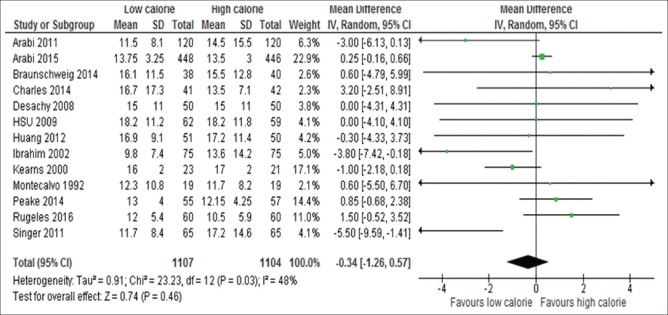
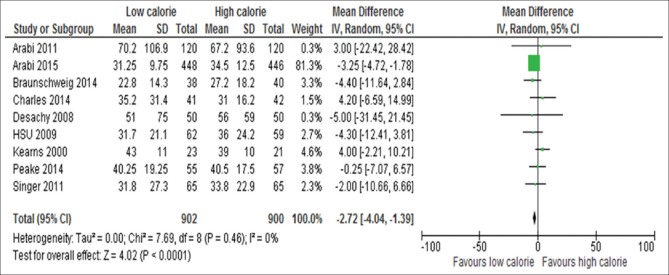
Information on the length of ICU stay was available for 13 of the 17 studies.[6,13,15,18,19,24,26,29,31,32,33,34,35] These 13 studies included 2211 patients and there was no significant difference between the two groups (WMD, −0.34; 95%, CI, −0–1.26 to 0.57, P = 0.46; I2 = 48%, P = 0.03) as shown in Figure 12. Information on the length of hospital stay available for nine studies[6,13,18,19,24,29,31,33,34] [Figure 13]. Patients initially receiving LC delivery had significantly lower length of hospital stays compared with those initially receiving HC delivery (WMD, −2.72; 95% CI, −4.04–1.39, P < 0.0001; I2 = 0%, P = 0.46).
Figure 12.
Forest plot showing the impact of daily calorie goals on lengths of ICU stay in critically ill adult patients. IV: Inverse variance; CI: Confidence interval; ICU: Intensive Care Unit; SD: Standard deviation
Figure 13.
Forest plot showing the impact of daily calorie goals on length of hospital stay in critically ill patients. IV: Inverse variance; CI: Confidence interval; SD: Standard deviation
Duration of mechanical ventilation
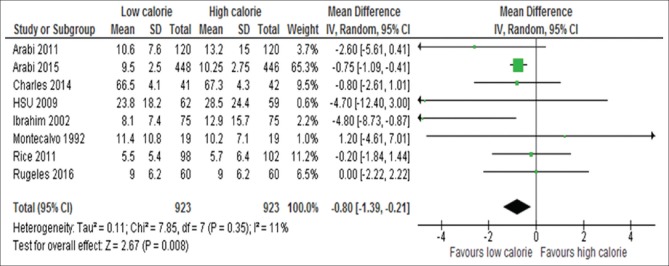
Data on duration of mechanical ventilation were available for eight studies:[6,15,18,23,24,26,31,35] these data included 1846 participants. The analysis of the data of these eight studies showed that patients assigned initially to LC delivery had significantly lower duration of ventilator dependency compared with those assigned to HC delivery (WMD, −0.80; 95% CI, −1.39 to − 0.21, P = 0.0001; I2 = 11%, P = 0.35) [Figure 14]. This indicates a 19.2 h reduction of ventilator dependency for patients received LC compared to those received HC artificial nutrition.
Figure 14.
Forest plot showing the impact of daily calorie goals on duration of mechanical ventilation in critically ill patients. IV: Inverse variance; CI: Confidence interval; SD: Standard deviation
Hypoglycemic
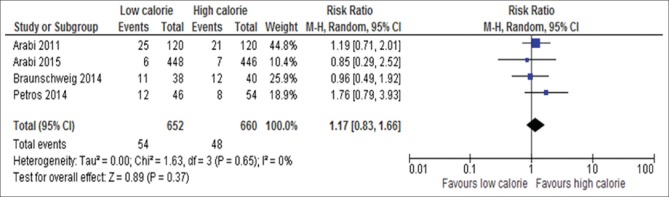
Four out of 17 trials reported data on incidence of hypoglycemia[6,18,19,25] as shown in Figure 15. The result of analysis of these data on 1312 participants showed that there was no significant difference between the two groups with regard to the occurrence of hypoglycemia (RR, 1.17; 95% CI, 0.83–1.66, P = 0.37; I2 = 0%, P = 0.65).
Figure 15.
Forest plot showing the impact of daily calorie goals on incident hypoglycemia in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval
Average dose insulin used per day
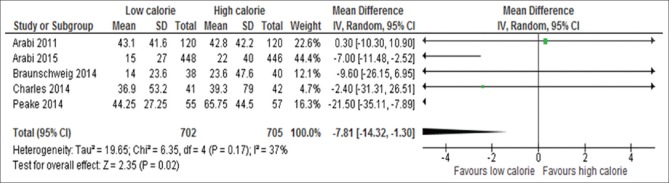
Five out of 17 trials reported the doses of insulin used per day[6,13,18,19,24] [Figure 16]. The five studies included 1407 participants. The result of analysis showed there was significant difference between patients assigned to LC compared with those assigned to HC with the former group received less daily dose of insulin than the latter group (WMD, −7.81; 95% CI, −14.32 to −1.30, P = 0.02; I2 = 37%, P = 0.17).
Figure 16.
Forest plot showing the impact of daily calorie goals on average of insulin used per day in critically ill adult patients. IV: Inverse variance; CI: Confidence interval; SD: Standard deviation
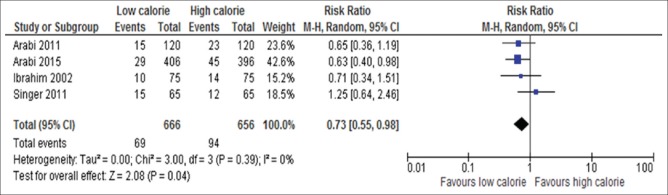
Incidence of renal replacement therapy
Four out of 17 trials include 1322 participants reported about incidence of RRT[6,15,18,34] [Figure 17]. Initially, delivery of LC was associated with less incidence of RRT compared with those assigned to initial HC nutrition (RR, 0.73; 95% CI, 0.55–0.98, P = 0.01; I2 = 0%, P = 0.92).
Figure 17.
Forest plot showing the impact of daily calorie goals on incident of RRT in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval; RRT: Renal replacement therapy
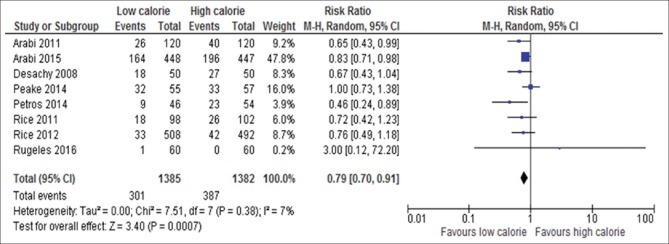
Gastrointestinal intolerance
Eight of the 17 studies reported gastrointestinal intolerance; these studies included 1347 participants[6,13,18,22,23,25,26,29] [Figure 18]. Patients received initial LC had significantly decreased risk of gastrointestinal intolerance compared with those initially received HC feeding (RR, 0.79; 95% CI, 0.70–0.91; P = 0.0007; I2 = 0%; P = 0.38).
Figure 18.
Forest plot showing the impact of daily calorie goals on gastrointestinal intolerance in critically ill adult patients. M-H: Mantel-Haenszel; CI: Confidence interval
DISCUSSION
In this meta-analysis of 17 RCTs enrolled around 3600 critically ill adult patients, compared to initial LC delivery of EN with or without SPN, HC delivery was not shown statistically significant reduction in mortality. The mortality rates in those initially underfeeding and full-feeding patients were 26.9% and 27.4%, respectively. Moreover, neither the subgroup analysis performed based on the presence or absence of SPN, baseline severity score, BMI nor stratified amount of calorie delivered by LC of standard requirement of nutritional interventions declared any evidence of survival benefits of initial HC delivery compared with under-feeding.
Two recently published meta-analyses have also reported the effect of calorie delivery on clinical outcomes of critically ill adult patients.[20,21] The subgroup analysis done by these authors based on tertiles of standard calorie requirement showed that mortality was significantly reduced in those fed on 33% to 66% calorie provision compared to those fed on HC. The current meta-analysis was unable to find the survival benefit of the middle tertile energy provision in the two meta-analyses mentioned above. The discrepancy could be more probably due to the difference in the sample size. Therefore, interpretation of the two meta-analyses should be with considerable causation since the result based on small sample size, low quality, and biased studies.
Why the aggregate result of 17 RCTs can't predict the treatment effect of different doses of artificial nutrition on mortality? Or why there is no statistically significant difference between high calories compared to low calorie delivery with regard to mortality in critically ill adult patients?
The answer is more likely due to the fact that artificial nutrition in critically ill patients is a medicine provided to malnourished patients, is unphysiologic, and may evoke complications and unwanted side effects that should be weighed against any expected effect as stated by Schetz et al.[36] Therefore, it seems that the so called “one-size-fits-all” seems inapplicable and individualization of nutritional therapy should be considered in daily clinical practice. Close observation of the trials included in this meta-analysis showed that almost all the patients included in each trial had normal nutritional status or slightly overweight (BMI >25 kg/m2) indicating that they might not derive benefits from overfeeding. This could partly explain lack of survival benefits of HC delivery compared to LC delivery in this meta-analysis. Similarly, a large multinational prospective observational study reported an inverse relationship between calorie input and mortality and risk of mortality was significantly for patients with BMI <25 or >35 kg/m2 compared with BMI of 25–35 kg/m2.[37]
Moreover, all of the participants in these studies received early EN that was approved to be beneficial in those patients who need nutrition (malnourished patients before ICU admission). What being tested in these studies was the amount of nutrition that the patients received and it is not expected that nutrition to be beneficial to all patients the same. A body of literatures reported that those patients at high risk nutritionally speaking are more likely derive an effect of increased delivery of protein and calorie on infection, resolution of organ failure or mortality.[36,38,39] Indeed, studies included in this meta-analysis also composed of patients at low risk of malnutrition before ICU admission. Therefore, it is perceivable that no matter how powerful the study is, if low risk patients are randomized to different doses of nutritional therapy, it is impossible to detect the treatment effect. We recommend better quality research concentrating on specific group of malnourished patients is therefore urgently needed.
We also found no significant between-group difference with respect to ICU-acquired infections (both new-onset pneumonia and sepsis), a finding that is consistent with the results of other studies.[6,22,23,24] The explanation for the absence of difference might be due to improvement in the current vascular access, and prevention of ventilatory associated pneumonia.
The length of hospital stays and duration of mechanical ventilation significantly shortened in LC delivery group, a result similar to a retrospective study which reported the reduced energy intake during 1st week in ICU was associated with a reduced length of hospital stays and mechanical ventilation.[40] However, the two recent meta-analyses failed to find the difference possibly due sample size.[20,21] Tian et al.[20] included in the analysis 4 out of 6 trials reported about length of hospital stay and 2 out of 6 studies reported on the duration of mechanical ventilations. Their explanation for the exclusion of those trails reported the endpoints of interest were the studies reported median instead of mean and they believed the comparison should not be done. However, we tried to overcome the problem by converting the median to mean by the formula reported in literature[41] to boost our sample size to detect the differences.
Regarding hypoglycemia and average insulin dose, LC was associated with lower blood glucose levels and reduced insulin requirements, findings that are consistent with those of other studies.[6,18,22] The current meta-analysis also showed that the incident of RRT in LC underfeeding group was significantly lower compared with the HC full-feeding group, a similar finding with another large RCT.[18] This notion supports the fact that higher calorie intake may be associated with kidney injury. It has been shown in animal model of acute renal injury that calorie restriction was renoprotective through several mechanisms including increasing insulin sensitivity.[42,43,44] Another study by secondary analysis of 1456 patients from RENA trial (after correction for multiple confounding variables and the application of different statistical modeling techniques) found that a lower mean delivery of caloric intake was not robustly independently associated with increased risk of death at 90 days, or with other major clinical outcomes.[45] We found also that there was significant gastrointestinal intolerance (regurgitation, vomiting, diarrhea, constipation, or abdominal distension) in HC feeding group compared with LC feeding group in contrast to the two recently done meta-analyses.[20,21] The difference seems due to the underpowered nature of those studies compared with the present study.
Strengths and limitations
Our meta-analysis has some strength that the previously done meta-analyses failed to find out due to the limited number of studies they included (small sample size). Including more studies using wide searching strategies and loss restrictive of inclusion criteria, we were able to reveal that there were significant differences between the permissive underfeeding and full-feeding with regards to length of hospital stays (measure of health care consumption), duration of mechanical ventilation and gastrointestinal intolerance. Moreover, some more endpoints which have good clinical implication for critically ill patients (incident of hypoglycemia, average daily dose of insulin used per day and RRT) were also included. Despite these differences, the collective results of our study and the two previous meta-analyses add to a growing body of literature that suggests over-feeding goals in critically ill patients do not improve clinical outcomes.
Despite our effort to reduce bias, the results of the study should be treated with caution because of some limitations. First, the disease severity reported by the studies differed; some reported APACHE II score, two studies reported APACHE III and one study SAPS. Second, the calculated calorie intake had significant variation which can potentially affect the aggregated results of our study. Third, in almost all of the selected studies there had been high exclusion criteria for high disease severity, important comorbidities and inclusion of patients with BMI of 20–35 kg/m2 thus limiting the generalizability of this finding. Forth, we are so conservative to give suggestion about the optimal dose of daily protein intake for a couple of reasons. It is difficult to consider the analysis of effect of protein intake on clinical outcomes due to the diverse protein dose among studies and the low dose of protein intake in all studies below the daily recommended dose by guidelines (1.2–1.5 g/kg) with the exception of one (Rugeles;[26] reported 1.7 g/kg/day). Lastly, there was apparent absence of a beneficial outcomes with more protein intake in secondary analyses of a randomized controlled trial (EPaNIC; n = 4640) performed in seven ICUs from three departments in two Belgian Hospitals.[46] Therefore, we feel that there is a need for good quality study about the optimal and safe range of daily protein intake in diverse groups of critically ill adult patients before any recommendation.
Finally, by any means, we are not announcing absolute clinical change by this work. However, we believe that this paper is a pivotal paper that may lead to considerable debate and discussion among practitioners and clinical scientists in the field and serve as an impetus for further research.
What do we learn from this meta-analysis?
This meta-analysis confirmed that HC intake is not associated with better outcome compared with LC intake in any endpoints analyzed so far in patients with low risk of malnutrition prior to ICU admission.
What is the role of nutrition in this group of patients then?
To achieve non-nutrition benefits early EN is of paramount importance than any other aspect of feeding in the critical care setting.[47,48,49] McClave et al. 2014[48] explained that early enteral nutrients stimulate the gastrointestinal response (maintaining blood flow and gut integrity, reducing gut-lung axis of inflammation, maintaining gut associated lymphoid tissue [GALT]), the endocrine response (improves insulin sensitivity, enhance fuel utilization) and the immune response (maintain GALT, decrease bacterial translocation, maintaining bacterial commensal). This nonnutritional benefits may be achieved by permissive underfeeding and is probably needed in every patients admitted to ICU, which has been suggested by this meta-analysis (i.e. no beneficial endpoint observed by overfeeding compared to underfeeding).
The bottom line is that patients at low risk of malnutrition before ICU admission should receive trophic feeding to achieve nonnutrition benefits of nutrients therapy. However, patients with reduced nutritional status (high-risk patients) before ICU admission should receive high dose closer to goal feeding to maintain lean body mass, correcting micronutrients deficits, antioxidants deficit, and maximizing protein synthesis as stated by McClave et al.[48]
CONCLUSION
The current meta-analysis showed that there was no significant difference between the LC and HC delivery groups in terms of the mortality in critically ill patients. However, initial LC delivery for critically ill patients resulted in shortening of length of hospital stay and duration of mechanical ventilation, low average daily dose of insulin use, low incidence of RRT, and gastrointestinal intolerance without significant effects on other secondary outcomes considered in the analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN Guidelines on enteral nutrition: Intensive care. Clin Nutr. 2006;25:210–23. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Crit Care Med. 2016;44:390–438. doi: 10.1097/CCM.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 3.Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: An update on current recommendations and implementation strategies. Nutr Clin Pract. 2014;29:29–43. doi: 10.1177/0884533613510948. [DOI] [PubMed] [Google Scholar]
- 4.Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Early enteral nutrition, provided within 24 h of injury or Intensive Care Unit admission, significantly reduces mortality in critically ill patients: A meta-analysis of randomised controlled trials. Intensive Care Med. 2009;35:2018–27. doi: 10.1007/s00134-009-1664-4. [DOI] [PubMed] [Google Scholar]
- 5.Khalid I, Doshi P, DiGiovine B. Early enteral nutrition and outcomes of critically ill patients treated with vasopressors and mechanical ventilation. Am J Crit Care. 2010;19:261–8. doi: 10.4037/ajcc2010197. [DOI] [PubMed] [Google Scholar]
- 6.Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: A randomized controlled trial. Am J Clin Nutr. 2011;93:569–77. doi: 10.3945/ajcn.110.005074. [DOI] [PubMed] [Google Scholar]
- 7.Doig GS, Chevrou-Séverac H, Simpson F. Early enteral nutrition in critical illness: A full economic analysis using US costs. Clinicoecon Outcomes Res. 2013;5:429–36. doi: 10.2147/CEOR.S50722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:117. doi: 10.1186/s13054-016-1298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapple LA, Chapman MJ, Lange K, Deane AM, Heyland DK. Nutrition support practices in critically ill head-injured patients: A global perspective. Crit Care. 2016;20:6. doi: 10.1186/s13054-015-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heighes PT, Doig GS, Sweetman EA, Simpson F. An overview of evidence from systematic reviews evaluating early enteral nutrition in critically ill patients: More convincing evidence is needed. Anaesth Intensive Care. 2010;38:167–74. doi: 10.1177/0310057X1003800126. [DOI] [PubMed] [Google Scholar]
- 11.Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux R N MC, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502–9. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: An observational study. Clin Nutr. 2006;25:37–44. doi: 10.1016/j.clnu.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Peake SL, Davies AR, Deane AM, Lange K, Moran JL, O'Connor SN, et al. Use of a concentrated enteral nutrition solution to increase calorie delivery to critically ill patients: A randomized, double-blind, clinical trial. Am J Clin Nutr. 2014;100:616–25. doi: 10.3945/ajcn.114.086322. [DOI] [PubMed] [Google Scholar]
- 14.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet. 2013;381:385–93. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V, et al. Early versus late enteral feeding of mechanically ventilated patients: Results of a clinical trial. JPEN J Parenter Enteral Nutr. 2002;26:174–81. doi: 10.1177/0148607102026003174. [DOI] [PubMed] [Google Scholar]
- 16.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: Consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124:297–305. doi: 10.1378/chest.124.1.297. [DOI] [PubMed] [Google Scholar]
- 18.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398–408. doi: 10.1056/NEJMoa1502826. [DOI] [PubMed] [Google Scholar]
- 19.Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, et al. Intensive nutrition in acute lung injury: A clinical trial (INTACT) JPEN J Parenter Enteral Nutr. 2015;39:13–20. doi: 10.1177/0148607114528541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian F, Wang X, Gao X, Wan X, Wu C, Zhang L, et al. Effect of initial calorie intake via enteral nutrition in critical illness: A meta-analysis of randomised controlled trials. Crit Care. 2015;19:180. doi: 10.1186/s13054-015-0902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi EY, Park DA, Park J. Calorie intake of enteral nutrition and clinical outcomes in acutely critically ill patients: A meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2015;39:291–300. doi: 10.1177/0148607114544322. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs. full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011;39:967–74. doi: 10.1097/CCM.0b013e31820a905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical Intensive Care Unit: A randomized controlled trial. Am J Clin Nutr. 2014;100:1337–43. doi: 10.3945/ajcn.114.088609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs. normocaloric nutrition in critically ill patients: A prospective randomized pilot trial. JPEN J Parenter Enteral Nutr. 2016;40:242–9. doi: 10.1177/0148607114528980. [DOI] [PubMed] [Google Scholar]
- 26.Rugeles S, Villarraga-Angulo LG, Ariza-Gutiérrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D. High-protein hypocaloric vs. normocaloric enteral nutrition in critically ill patients: A randomized clinical trial. J Crit Care. 2016;35:110–4. doi: 10.1016/j.jcrc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. The Preferred Reporting Items for Systematic Reviews and Meta-analyses. 2013. [Last accessed on: 2015 Nov 23]. Available from: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000097 .
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desachy A, Clavel M, Vuagnat A, Normand S, Gissot V, François B. Initial efficacy and tolerability of early enteral nutrition with immediate or gradual introduction in intubated patients. Intensive Care Med. 2008;34:1054–9. doi: 10.1007/s00134-007-0983-6. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525–31. doi: 10.1097/00003246-199911000-00033. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CW, Sun SF, Lin SL, Kang SP, Chu KA, Lin CH, et al. Duodenal versus gastric feeding in medical Intensive Care Unit patients: A prospective, randomized, clinical study. Crit Care Med. 2009;37:1866–72. doi: 10.1097/CCM.0b013e31819ffcda. [DOI] [PubMed] [Google Scholar]
- 32.Huang HH, Chang SJ, Hsu CW, Chang TM, Kang SP, Liu MY. Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–46. doi: 10.1016/j.jand.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM. The incidence of ventilator-associated pneumonia and success in nutrient delivery with gastric versus small intestinal feeding: A randomized clinical trial. Crit Care Med. 2000;28:1742–6. doi: 10.1097/00003246-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, et al. The tight calorie control study (TICACOS): A prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37:601–9. doi: 10.1007/s00134-011-2146-z. [DOI] [PubMed] [Google Scholar]
- 35.Montecalvo MA, Steger KA, Farber HW, Smith BF, Dennis RC, Fitzpatrick GF, et al. Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. The Critical Care Research Team. Crit Care Med. 1992;20:1377–87. doi: 10.1097/00003246-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Schetz M, Casaer MP, Van den Berghe G. Does artificial nutrition improve outcome of critical illness? Crit Care. 2013;17:302. doi: 10.1186/cc11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–37. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 38.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heyland DK, Wischmeyer PE. Does artificial nutrition improve outcome of critical illness? An alternative viewpoint! Crit Care. 2013;17:324. doi: 10.1186/cc12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichimaru S, Fujiwara H, Amagai T, Atsumi T. Low energy intake during the first week in an emergency Intensive Care Unit is associated with reduced duration of mechanical ventilation in critically ill, underweight patients: A single-center retrospective chart review. Nutr Clin Pract. 2014;29:368–79. doi: 10.1177/0884533614529162. [DOI] [PubMed] [Google Scholar]
- 41.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lempiäinen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1a-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf) 2013;208:410–21. doi: 10.1111/apha.12120. [DOI] [PubMed] [Google Scholar]
- 43.Ning YC, Cai GY, Zhuo L, Gao JJ, Dong D, Cui SY, et al. Beneficial effects of short-term calorie restriction against cisplatin-induced acute renal injury in aged rats. Nephron Exp Nephrol. 2013;124:19–27. doi: 10.1159/000357380. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, et al. Calorie intake and patient outcomes in severe acute kidney injury: Findings from the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy (RENAL) study trial. Crit Care. 2014;18:R45. doi: 10.1186/cc13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: A post hoc analysis. Am J Respir Crit Care Med. 2013;187:247–55. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 47.McClave SA, Codner P, Patel J, Hurt RT, Allen K, Martindale RG. Should we aim for full enteral feeding in the first week of critical illness? Nutr Clin Pract. 2016;31:425–31. doi: 10.1177/0884533616653809. [DOI] [PubMed] [Google Scholar]
- 48.McClave SA, Martindale RG, Rice TW, Heyland DK. Feeding the critically ill patient. Crit Care Med. 2014;42:2600–10. doi: 10.1097/CCM.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 49.McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305–15. doi: 10.1177/0884533609335176. [DOI] [PubMed] [Google Scholar]