Abstract
Context:
Fine needle aspiration cytology (FNAC) of superficial or deep-seated lesion is an increasingly common practice, eliminating time consuming and costly diagnostic procedures and providing rapid and safe diagnosis.
Aims:
To assess utility of cell block preparation method in increasing sensitivity of cytodiagnosis in deep-seated image-guided FNACs.
Settings and Design:
This was a hospital-based observational study conducted in the Department of Pathology, over a period of one and a half years.
Materials and Methods:
A total of 46 cases of abdomino-pelvic and intrathoracic masses subjected to guided FNACs were included. Along with conventional smears, cell blocks were prepared by using AAF (95% Ethanol 34 ml + formalin 4 ml + Glacial acetic acid 2 ml) as fixative agent.
Statistical Analysis Used:
Done using MedCalc Version 12.7.5.0 to find out the sensitivity, specificity, and diagnostic accuracy of conventional smears and cell blocks.
Results:
The sensitivity, specificity, and diagnostic accuracy of cell blocks in our study was 71.11%, 100%, and 71.73%, respectively. The figures for FNA smears were 62.22%, 100%, and 63.04%, respectively.
Conclusions:
Cell block technique by AAF fixative is a simple, inexpensive procedure. Cell block method allows the recovery and processing of minute amounts of cellular material, facilitating better classification of tumor when reviewed along with cytological smears, the ability to obtain many sections for immunostains and other studies to be performed akin to paraffin sections produced in histopathology.
Key words: Cell block, conventional smears, image-guided FNACs
Introduction
Diagnostic cytology is the science of interpretation of cells that are either exfoliated from epithelial surfaces or removed from various tissues, and can be carried out by different methods, which includes collection and examination of exfoliated cells such as vaginal scrapes, sputum, urine, body fluids, etc., collection of cells by brushing, scraping, or abrasive techniques. George N Papanicolaou introduced cytology as a tool to detect cancer and pre-cancer in 1928.[1]
The accuracy of the cytologic examination from any body site depends greatly on the quality of collection, preparation, staining, and interpretation of the material. Inadequacy in any of these steps will adversely affect the quality of diagnostic cytology. Sometimes judgment is difficult; hence, residual material remaining after completion of cytologic preparations can be processed and paraffin embedded as cell blocks which enables the retrieval of minute amounts of cellular material.[2]
Even though the utility of cell blocks are greatly acknowledged, cell blocks are not routinely prepared along with deep-seated image-guided fine needle aspiration cytology (FNAC) smears. Hence, the present study has been undertaken to assess the utility of cell block preparation method in increasing the diagnostic accuracy especially in deep-seated image-guided FNAC.
Patients and Methods
This was an observational study conducted in the Department of Pathology, enrolling patients attending for image-guided FNAC in the Department of Radiodiagnosis. The study extended over a period of 18 months during which a total of 70 cases were studied. Only cases with FNAC sample followed by tissue biopsy were included. Patients with recurrent malignancies, past/current chemotherapeutic or prevention treatment were excluded.
Out of the 70 cases, 24 cases were excluded for not meeting the study criteria. Our final study group comprised 46 guided FNACs. Detailed clinical data which included the patient's history, physical examination findings, reports of relevant blood investigations, and radiological details including site, size, extent of mass, and its vascularity were recorded. The coagulation profile was routinely done in all patients and only those with normal coagulation profile were selected for the procedure. Patients were made aware of the procedure and informed consent was obtained from all patients.
For abdomino-pelvic lesions ultrasound (model VOLUSOM 730 pro) guided FNAC was the preferred method. For intrathoracic masses, computed tomography (CT)-guided (64 slice VCT GE light speed with 8 MHU) FNAC was done. Puncture site was marked using a radio-opaque marker. After introducing the needle another image was taken to ensure the position of the needle tip within the lesion. A third scan was done to look for any immediate complication (pneumothorax or hemorrhage) within 10 minutes of aspiration.
For FNAC, 20 and 22 gauge spinal needles with a central stylet were used depending on the depth of lesion. The skin entry site was sterilized and infiltrated with 2% lignocaine. The aspiration needle was then directed towards the lesion during suspended respiration. Presence of the needle tip within the lesion was ensured by the monitor display. After removing the stylet, a 20-ml syringe was attached to the needle hub. The plunger of the syringe was then pulled back and continuous suction was applied. When adequate material appeared in the hub, needle was withdrawn after releasing the suction pressure and smears were prepared.
The smears were made as quickly as possible to avoid coagulation of blood which hampers the smearing pattern by obscuring the parenchymal cells. Each aspirate was smeared on an average of four to five slides, few of which were immediately wet fixed in 95% isopropyl alcohol, stained by Papanicolaou method; a few were air dried stained by May–Grunwald Giemsa (MGG) method and were evaluated.
Excess material including the residual material in the needle hub was mixed with thrice the volume of AAF fixative (95% ethyl alcohol 34 ml + formalin 4 ml + Glacial acetic acid 2 ml) and was submitted for cell block preparation.
For preparing cell blocks, specimen with fixative was centrifuged for 10 minutes at 2000 rpm. The supernatant was discarded and cell button was resuspended in AAF fixative and centrifuged for 10 minutes at 3000 rpm. Tube was kept aside for 4–6 hours. Cell button was carefully removed after discarding the supernatant, wrapped in a filter paper and placed in a labeled tissue cassette. The specimen was then processed and embedded in the same way as that of routine biopsy specimens. Four tissue sections each measuring 3–4 μm in thickness, were cut from the cell blocks. Sections were stained with Hematoxylin and Eosin for morphological evaluation.
Histological correlation was obtained in patients who subsequently underwent other surgical procedures. The final diagnosis of each lesion was determined taking biopsy as the gold standard.
Immunohistochemical staining
In doubtful cases immunohistochemistry (IHC) was done on formalin-fixed paraffin embedded cell blocks using selected mouse monoclonal primary antibodies for confirmation. Details of primary antibodies used in this study are summarized in Table 1.
Table 1.
Panel of primary antibodies used in this study
Results
The present study was conducted over a period of one and a half years in a tertiary care center to assess the utility of cell block preparation method in increasing sensitivity of cytodiagnosis in deep-seated image-guided FNAC.
The applicability of cell blocks in guided-FNAC for the pre-operative diagnosis of abdomino-pelvic and intrathoracic lesions was assessed in 46 patients. Out of the total 46 patients, 30 (65%) were males and 16 (35%) were females. The maximum number of cases was seen between 61 and 70 years of age, showing a male predominance with a male to female ratio of 1.8:1. The mean age at presentation was 58.5 years ranging from 25–78 years. Of all the 46 cases, 25 (54%) cases were from abdomino-pelvic lesions and 21 (46%) were from intrathoracic lesions. Thirty-eight were CT-guided FNAC and 8 were USG guided.
Majority of the lesions in the abdomino-pelvic region were hepatic in origin (19.5%), followed by ovary (15.21%), retroperitoneum, pancreas, subhepatic (4.35% each), renal, right iliac fossa, right hypochondrium (2.17% each). Of the 21 intrathoracic lesions, 17 (36.95%) were from lung and 4 (8.70%) were from mediastinal masses. Site-specific histopathological diagnosis with FNAC and cell block results are summarized in Tables 2 and 3.
Table 2.
Comparison of diagnosis by cell block with FNA smears in abdomino-pelvic lesions (n=25)
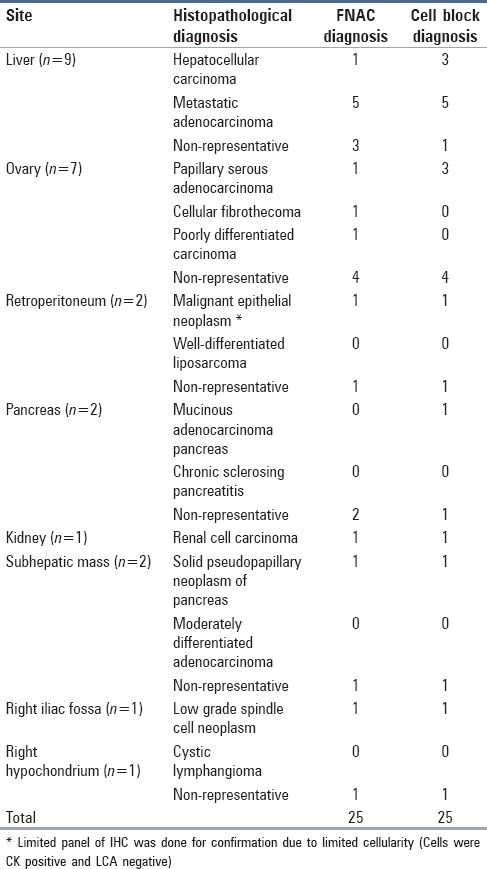
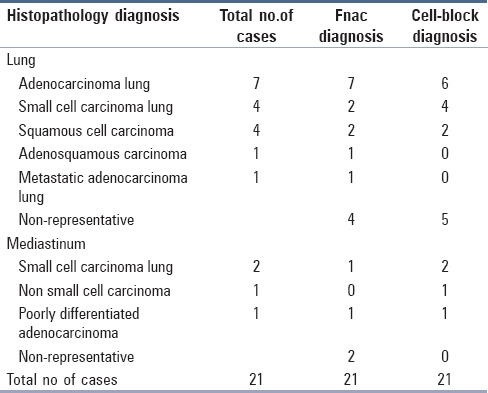
Table 3.
Comparison between fine needle aspiration cytology, cell block, and histopathology diagnosis in thoracic lesions
Statistical analysis
Statistical analysis was done using MedCalc Version 12.7.5.0 to find out sensitivity, specificity, and diagnostic accuracy of conventional cytological smears and cell blocks in image-guided FNAC. Results are summarized in Table 4.
Table 4.
Sensitivity and diagnostic accuracy of FNA smears and cell blocks

Discussion
The use of needle aspiration for purposes of diagnosis can be traced back to 1847 when Kun described a “new instrument for the diagnosis of tumors.” It was followed by occasional sporadic reports of this technique towards the end of the 19th century. In 1883, Leydon used needle aspiration to obtain cells to isolate pneumonic microorganisms, and three years later Menetrier used the technique to diagnose pulmonary carcinoma.[3]
FNAC of superficial lesion or deep anatomical site is an increasingly common procedure in the diagnosis of neoplastic lesions. The introduction of modern diagnostic imaging techniques has enabled the detection and location of lesions in sites, which are not easily accessible to surgical biopsies.
Even with many advantages sometimes FNA does not yield sufficient information for precise diagnosis and the risk of false negative or intermediate diagnoses always exists. An inconclusive diagnosis on FNAC may be due to poor spreading, air drying artifact, and presence of thick tissue fragments despite aspiration of adequate material.[4] Studies have shown that the cytological examination of specimens by means of smears, no matter how carefully prepared leaves behind a large residue that is not further investigated, but that might contain valuable diagnostic material.[5] The residual material can be evaluated in a simple and expedient fashion by treating it as cell block, embedded in paraffin and examined besides routine smears.[6]
The use of cell block for processing cytology fluids has been first reported in 1895 when Bahrenberg[7] allowed a large quantity of fluid to stand and clot spontaneously. After pouring off the supernatant fluid the clot was shrunken and hardened by successive addition of alcohol until a small stringy mass was obtained. This was finally embedded in celloidin and cut like tissue. He was able to find epithelial cells in two ascitic fluids with the aid of this technique. The autopsy in these cases revealed carcinoma involving peritoneum. The significance of Bahrenberg's method is obvious but it is regrettable that his work was buried in an obscure journal and was apparently unknown to any of the men who later attacked the subject.
Cell block preparations are used routinely for pleural, peritoneal fluids, bronchial washings, FNA, and other cytological specimens. Various methods for preparing paraffin- embedded cell blocks from FNAC have been reported, which include direct transfer of all centrifuged cellular material wrapped in lens paper[8,9,10] or embedding in plasma[11,12] or agar[13,14] and then processing as a routine histological specimen.
An alternative method of cell-block preparation is a modified technique using AAF fixative (95% ethyl alcohol 34 ml + formalin 4 ml + Glacial acetic acid 2 ml) combining the hemolytic property of glacial acetic acid with above fixatives. AAF fixative is considered to be the ideal fixative used for cell-block preparation of fluid specimens.[1]
In the present study, our main objective was to assess the role of cell block in guided- FNA of intrathoracic and abdomino-pelvic masses. A total of 46 cases were analyzed based on the cytological features. The final diagnosis was arrived at in corroboration with the clinical and the radiological features taking biopsy as gold standard.
Satisfactory aspirate for cytological diagnosis was obtained in 28/46 (60.87%) cases. The diagnostic sensitivity of smears alone was 62.22% comparable with Govind Krishna et al.[15] who reported a sensitivity of 71.1%. The diagnostic sensitivity of smears in other studies by Sidhalingreddy et al.[16] and Sobha Rani et al.[17] were 94.1% and 90%, respectively.
Satisfactory material on cell block was obtained in 32 cases (69.56%). This is comparable to the study done by Nathan et al.[18] in which cell block material was obtained in 300 cases (73.3%) out of 409 lesions. The results of the present study revealed that cell blocks were superior to conventional smears with a sensitivity of 71.11%. An overall improvement in the final diagnosis was noted when smears were complemented by cell block, with an increase in sensitivity of 84.44%. Even though diagnostic accuracy of cell block was superior to FNAC in the current study, there was no significant difference between conventional smears and cell block. (Chi square was 2.74 with P value 0.09).
The diagnostic yield of guided-FNAC in our study was low when comparing with others. As the technique is highly operator dependent the low cellular yield could be due to technical error in sampling, hemorrhagic or a paucicellular aspirate, failure to target lesion properly especially in small masses. As Dahnert et al.[19] have pointed out, once blood appears in the needle hub, it may flush the diagnostic elements into the syringe. Because the last material aspirated at the end of pass is the first expelled onto the slides, the smears may contain only blood, whereas the diagnostic material remains in the syringe and ultimately within the cell block.
Present study included 9 (19.57%) cases with clinical, biochemical and radiological evidence of liver lesion, distinction between Hepatocellular carcinoma (HCC) and metastatic tumors was possible with FNAC and cell blocks, and all cases were concordant with histopathology. To differentiate between primary and metastatic tumors on cytology can occasionally be difficult. The difficulty in cytological diagnosis arises at the end of malignant spectrum, i.e., distinguishing poorly differentiated HCC from metastatic malignancies.
Cytological diagnosis of HCC has often centered on the tumor cell itself without taking into consideration its stroma. The primary cytological features to differentiate hepatocellular carcinoma from metastatic tumors include polygonal cells with centrally placed nuclei, malignant cells separated by sinusoidal capillaries, and presence of bile. Other secondary criteria useful in differentiating HCC are intranuclear cytoplasmic inclusions and endothelial cells surrounding tumor cell clusters.[20]
In our study, the most important features encountered in favor of HCC were polygonal cells with centrally placed nuclei, intranuclear cytoplasmic inclusions, and endothelial cells surrounding tumor cell clusters.
The secondary deposits in the liver may reproduce the histology of primary lesions. The salient features separating HCC from metastatic adenocarcinoma are, tumor cells in HCC are polygonal or polyhedral whereas cells are usually cuboidal or columnar in adenocarcinoma; cells in HCC have abundant eosinophilic granular cytoplasm with one or two macronucleoli, where as adenocarcinoma cells may show mucin secretion and presence of more than two nucleoli is common. Trabecular arrangement is suggestive of HCC, whereas acinar or glandular arrangement is more favourable to adenocarcinoma. Inflammatory background is commonly seen in adenocarcinoma.[20]
The two important features in favor of metastatic adenocarcinoma observed in our study were cuboidal or columnar cells arranged in acinar or glandular pattern. Our study included 7 (15.22%) patients with ovarian masses, of which 6 were clinically diagnosed as carcinoma ovary and one as ovarian metastasis from carcinoma colon. Ovarian metastasis from colon carcinoma was ruled out by IHC and confirmed by biopsy. (Cells were positive for CK 7 [Figure 1] and were negative for CK20).
Figure 1.
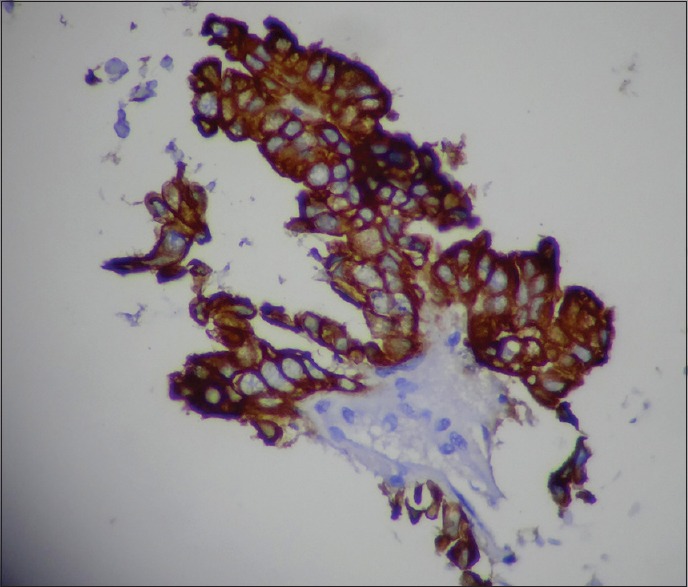
Papillary serous adenocarcinoma ovary. Cell block section showing intense cytoplasmic immunostaining for CK 7 (ICC : CK7 x400).
Punctation of the cystic ovarian tumors could be hazardous, which can cause leakage and a potential tumor cell implantation in the case of malignant tumors. The risk can be reduced significantly by doing a trans-vaginal or a trans-rectal aspiration. In the present study, though the approach was trans-abdominal in all cases, no such complications were encountered.
In the present study, both cytological smears and cell blocks were positive in three (42.86%) and showed false negative results in 4/7 (57.14%) cases. The evaluation of aspirated material by both FNAC and cell blocks showed non-diagnostic features in four (57.14%) cases. This could be due to aspiration of acellular fluid from cysts or due to presence of diagnostically insignificant macrophages, RBCs, and inflammatory cells. Many studies have highlighted this pitfall of cytological diagnosis.
The diagnosis of retroperitoneal lesions is one of the most difficult areas in surgical pathology. Present study included five cases from retroperitoneal space, with maximum number of cases from pancreas (40%). Cell block and FNA findings are illustrated in Table 1.
Study included 21 CT-guided FNACs from intrathoracic lesions, of which 17 (80.95%) were from lung and four (19.45%) from mediastinal masses.
Of the 21 cases, cytodiagnosis were made in 15 cases with a sensitivity of 71.43%. Cell block sections were positive for malignant cells in 16 cases with sensitivity of 76.19%. (Figure 2 demonstrating a case of Moderately differentiated squamous cell carcinoma lung).
Figure 2.
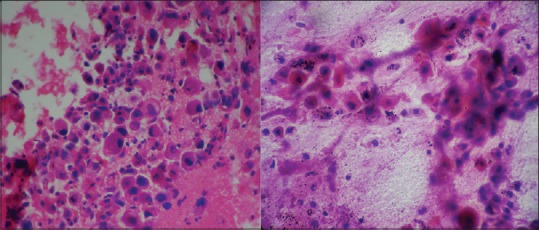
Moderately differentiated squamous cell carcinoma : FNAC (Pap stain x400) and cell block (H and E stain x400).
Study included four cases presented with mediastinal lymphadenopathy. All were neoplastic and had histological correlation with no false negative results in cell block study. On cell typing, small cell carcinoma (50%) was the most common neoplastic lesion encountered. In our study, the highest typing accuracy was seen in small cell carcinoma and may be attributed to abundant material and characteristic cytological appearance. IHC was done on one case, which showed strong diffuse positivity for chromogranin A and Synaptophysin.
Historically, all subtypes of non-small cell lung carcinomas (NSCLCs) were given similar chemotherapy, so further categorization of NSCLC into squamous-cell carcinoma (SCC), adenocarcinoma of the lung (LADC) was not important on fine-needle aspirates. But treatment in the present era for lung cancer is personalized and is based on the subtype of lung cancer (LADC vs SCC) and molecular status that is, epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements. Accurate subtyping of NSCLC into LADC and SCC and identification of the molecular status is mandatory for administrating the appropriate therapy. The opportunities for cytopathologists to influence therapy, and uncover strategies in the complex field of lung cancer are exciting and limitless especially in the presence of an adequate cytoblock.[21]
The results of present study revealed that the diagnostic accuracy of cell block technique was high when compared to conventional smears, as compared to other studies. Thus, our data supports the conclusion that though smears may contain adequate diagnostic material, the addition of cell block could further enhance the specificity of the diagnosis and thus both cell block and smears together can increase the diagnostic accuracy. However, if cell block analysis is not possible for all aspirations, the technique should be used selectively in cases that are difficult to diagnose in smears.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Manual for cytology, National cancer control programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. 2005 [Google Scholar]
- 2.Fischer AH, Zhao C, Li QK, Gustafson KS, Eltoum IE, Tambouret R, et al. The cytologic criteria of malignancy. J Cell Biochem. 2010;110:795–811. doi: 10.1002/jcb.22585. [DOI] [PubMed] [Google Scholar]
- 3.Ansari NA, Derias NW. Origins of Fine needle aspiration cytology. J Clin Pathol. 1997;50:541–3. doi: 10.1136/jcp.50.7.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KuIkarni MB, Prabhudesai NM, Desai SB, Borges AM. Scrape cell block technique for fine needle aspiration cytology smears. Cytopathology. 2000;11:179–84. doi: 10.1046/j.1365-2303.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 5.Thapar M, Mishra RK, Sharma A, Goyal V, Goyal V. Critical analysis of cell block versus smear examination in effusions. J Cytol. 2009;26:60–4. doi: 10.4103/0970-9371.55223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basnet S, Talwar OP. Role of cell block preparation in neoplastic lesions. J Pathol Nepal. 2012;2:272–6. [Google Scholar]
- 7.Zemansky AP., Jr Examination of tumour cells; Analysis of 113 cases checked against subsequent examination of tissue. Am J M.Sc. 1928;175:489–504. [Google Scholar]
- 8.Zito FA, Gadaleta CD, Salvatore CL, Filotico R, Labriolo A, Marzullo A. A modified cell block technique for fine needle aspiration cytology. Acta Cytol. 1995;39:93–9. [PubMed] [Google Scholar]
- 9.Brown KT, Fulbright RK, Avitabile AM, Bashist B. Cytologic analysis in FNA biopsy: Smears versus cell blocks. AJR Am J Roentgenol. 1993;161:629–31. doi: 10.2214/ajr.161.3.8352121. [DOI] [PubMed] [Google Scholar]
- 10.Wojcik EM, Selvaggi SM. Comparison of smears and cell blocks in the fine needle aspiration diagnosis. Acta Cytol. 1991;35:773–6. [PubMed] [Google Scholar]
- 11.Karnanuchow PN, Bouin RE. Cell block technique for fine needle aspiration biopsy. J Clin Pathol. 1992;35:688. doi: 10.1136/jcp.35.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burt AD, Smillie D, Cowan MD, Adams FG. Fine needle aspiration cytology: Experience with a cell block technique. J Clin Pathol. 1986;39:114–5. doi: 10.1136/jcp.39.1.114-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kung IT, Yuen RW. Fine needle aspiration of the thyroid. Distinction between colloid nodule and follicular neoplasm using cell blocks & 21 gauze needles. Acta Cytol. 1989;33:53–60. [PubMed] [Google Scholar]
- 14.Kung IT, Chan SK, Lo ES. Application of the immunoperoxidase technique to cell blocks preparations from the fine needle aspirates. Acta Cytol. 1990;34:297–303. [PubMed] [Google Scholar]
- 15.Krishna SR, Ananthakrishnan N, Narasimhan R, Veliath AJ. Accuracy of fine needle aspiration cytology of abdominal masses without radiological guidance. Indian J Pathol Micobiol. 1993;36:442–52. [PubMed] [Google Scholar]
- 16.Sidhalingreddy, Sainath K, Andola Fine needle aspiration cytology of intraabdominal lesions. J Clin Diagn Res. 2011;5:551–8. [Google Scholar]
- 17.Sobha Rani G, Faheem K, Sai Prasad, Sudhakar Reddy. Efficiency of ultrasound guided aspiration cytology in deep seated lesions- a diagnostic evaluation. Int J Med Health Sci. 2012;1:1–12. [Google Scholar]
- 18.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 19.Dahnert WF, Hoagland MH, Hamper UM, Erozan YS, Peirce JC. Fine needle aspiration biopsy of abdominal lesions: Diagnostic yield of different needle tip configurations. Radiology. 1992;185:263–8. doi: 10.1148/radiology.185.1.1523321. [DOI] [PubMed] [Google Scholar]
- 20.Rasania A, Pandey CL, Joshi N. Evaluation of FNAC in diagnosis of hepatic lesion. J Cytol. 2007;24:51–4. [Google Scholar]
- 21.Gupta N, Sekar A, Rajwanshi A. Role of FNAC, fluid specimens, and cell blocks for cytological diagnosis of lung cancer in the present era. J Cytol. 2015;32:217–22. doi: 10.4103/0970-9371.171219. [DOI] [PMC free article] [PubMed] [Google Scholar]


