Abstract
Background:
We investigated the effect of cholinesterase inhibitors on all-cause discontinuation, efficacy and safety, and the effects of study design-, intervention-, and patient-related covariates on the risk-benefit of cholinesterase inhibitors for Alzheimer’s disease.
Methods:
A systematic review and meta-analysis of randomized placebo-controlled clinical trials comparing cholinesterase inhibitors and placebo was performed. The effect of covariates on study outcomes was analysed by means of meta-regression using a Bayesian framework.
Results:
Forty-three randomized placebo-controlled clinical trials involving 16106 patients were included. All-cause discontinuation was higher with cholinesterase inhibitors (OR = 1.66), as was discontinuation due to adverse events (OR=1.75). Cholinesterase inhibitors improved cognitive function (standardized mean difference = 0.38), global symptomatology (standardized mean difference=0.28) and functional capacity (standardized mean difference=0.16) but not neuropsychiatric symptoms. Rivastigmine was associated with a poorer outcome on all-cause discontinuation (Diff OR = 1.66) and donepezil with a higher efficacy on global change (Diff standardized mean difference = 0.41). The proportion of patients with serious adverse events decreased with age (Diff OR = -0.09). Mortality was lower with cholinesterase inhibitors than with placebo (OR = 0.65).
Conclusion:
While cholinesterase inhibitors show a poor risk-benefit relationship as indicated by mild symptom improvement and a higher than placebo all-cause discontinuation, a reduction of mortality was suggested. Intervention- and patient-related factors modify the effect of cholinesterase inhibitors in patients with Alzheimer’s disease.
Keywords: cholinesterase inhibitor, Alzheimer’s disease, discontinuation, efficacy, Bayesian meta-analysis
Significance Statement
In this article, we report the results of a systematic review and meta-analysis investigating the discontinuation, efficacy, and safety of cholinesterase inhibitors for Alzheimer’s disease. We included 43 randomized clinical trials involving 16106 patients. We used a Bayesian framework. While cholinesterase inhibitors showed a poor risk-benefit relationship, as indicated by small symptom improvement, and a higher all-cause discontinuation than placebo, a reduction in mortality was also found, which could indicate some disease progression-modifying effect these drugs. This finding could renew interest in clinical research on cholinesterase inhibitors. Nevertheless, the clinical relevance of reduction in mortality accompanied by only a small improvement in symptoms is uncertain. Finally, intervention- and patient-related factors, but not study design, were found to modify the effect of cholinesterase inhibitors in patients with Alzheimer’s disease. To the best of our knowledge, this is the largest meta-analysis in the field, the first to focus on clinically relevant outcomes, to find a reduction in mortality, and to identify patient-, intervention-, and study design-related covariates that modify the efficacy, safety, and discontinuation of cholinesterase inhibitors in patients with Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder that affects 60% to 70% of the 47.5 million people suffering from dementia worldwide (World Health Organization, 2015). AD causes progressive decline in cognition, behavior, and daily living activities, which can lead to complete dependency on caregivers before finally to resulting in death. From the initial diagnosis and the beginning cholinesterase inhibitor (ChEIs) therapy, men live 5.1 years and women 6.1 years, on average (Wattmo et al., 2014). The most common cause of death is pneumonia, followed by cardiovascular diseases (Brunnström et al., 2009; Foley et al., 2015).
ChEIs increase acetylcholine in the synaptic gap of the hippocampus and cortex neurons with the aim to improve cognitive function (Francis et al., 1999). Furthermore, since cholinergic transmission was found to be involved in mood regulation, ChEIs may improve psychiatric symptoms in patients with AD (Jeon et al., 2015). Donepezil, galantamine, and rivastigmine are Food and Drug Administration- and European Medicine Agency-approved ChEIs for AD and have become widely used. American and European guidelines recommend ChEIs as a first-line pharmacological treatment for mild to moderate AD, jointly with nonpharmacological treatment for cognitive disorders (Regional Health Council, 2011; Rabins et al., 2010). Nevertheless, the risk-benefit of ChEIs is still under discussion. Evidence of improvement on relevant clinically meaningful outcomes, for example, need for caregiver, institutional care, hospital admissions, disease progression through relevant health states, quality of life, and mortality are lacking (National Institute for Health and Care Excellence, 2011). The efficacy of these interventions has been assessed, essentially, on AD symptoms using rating scales. Outcomes of this type have several limitations, as they are subjective and therefore more likely to be biased due to blinding failure, particularly if the interventions studied have behavioral or physical effects that may unmask blinding. Furthermore, these outcomes may show a high risk of attrition bias due to systematic differences between the interventions studied in withdrawals from the study. In addition to this, ChEIs have been associated with a number of side effects such as nausea, vomiting, diarrhoea, abdominal pain, anorexia, headache, insomnia, muscle cramps, bradycardia, and syncope (Birks, 2006; California Workgroup on Guidelines for Alzheimer’s Disease Management, 2008). Since the efficacy of ChEIs is arguable and tolerability may be low, the risk-benefit relationship of these interventions is unclear. In this context, all-cause discontinuation is a pragmatic outcome that may help in weighing the efficacy of ChEIs for AD against their safety. Any intervention leading to a meaningful improvement in symptoms, with acceptable side effects, would be expected to yield a lower discontinuation rate than placebo, whereas when the efficacy of the drug does not compensate for its side effects, the discontinuation rate would be higher. Furthermore, discontinuation is not affected by attrition bias, because there are no missing data for this outcome. Discontinuation has been used in other areas such as schizophrenia (Stroup et al., 2003), depression (Cipriani et al., 2016), and attention deficit hyperactivity disorder (Cunill et al., 2015).
The aim of this study was to investigate the effect of ChEIs on all-cause discontinuation, efficacy, and safety in patients with AD. Furthermore, the between-study variability on efficacy and safety was large, with some randomized placebo-controlled clinical trials (RPCCTs) showing substantial symptom improvement compared with placebo, while others found no evidence of efficacy on relevant clinical outcomes (Corey-Bloom et al., 1998; Rogers et al., 1998; Wilcock et al., 2000; AD2000 Collaborative Group, 2004). With the aim of determining the reasons behind such variability, we grouped the factors explaining between-study variability into 3 categories: (1) factors related to the design of the study, such as the existence of a lead-in phase (Cunill et al., 2016) or the number of study sites (Undurraga et al., 2012), (2) intervention-related factors such as dose (Castells et al., 2011) and treatment duration (Pérez-Mañá et al., 2013), and (3) patient-related factors such as age (Stone et al., 2009) and the severity of the disease (Schwartz et al., 2014). To achieve these goals, a systematic review with meta-analysis and meta-regression was carried out. This method has the advantage that it allows for the investigation of covariates that vary between studies but not within study such as study-design related covariates.
Methods
Design and Search Strategy
A systematic review and meta-analysis was conducted. We included double-blind RPCCTs with a parallel design that compared authorized doses of donepezil, galantamine, or rivastigmine by Food and Drug Administration or European Medicine Agency with placebo in patients with AD. The length of intervention was 12 weeks minimum. We excluded studies that were available only as abstracts. The study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO): CRD42014015156.
The following electronic databases were searched: Medline, Cochrane Central Register of Controlled Trials, PsycINFO, Web of Knowledge, www.clinicaltrials.gov, www.clinicaltrialregister.eu, www.controlled-trials.com, and pharmaceutical databases (see supplementary Table 1 for the search strategies). The search was limited to clinical trials up to April 30, 2016. Systematic reviews (Lanctôt et al., 2003; Birks, 2012; Di Santo et al., 2013) and list references were revised to identify potential RPCCTs.
Data Extraction and Quality Assessment
Data extraction from the articles selected was performed independently by two reviewers (L.B., X.C.). We contacted authors and pharmaceutical companies to obtain unpublished data. The risk of bias was evaluated using the scale developed by the Cochrane Collaboration (Higgins et al., 2011a). This instrument ascertains the risk of bias on the basis of the description and suitability of the following: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other biases. A judgement relating to the risk of bias is given for each domain in terms of low, high, or unclear risk.
Outcomes and Covariates
The primary outcomes were all-cause discontinuation defined as the proportion of randomized patients who did not complete the study for any reason; discontinuation due to adverse events (AEs) and efficacy on cognitive function, assessed using the Alzheimer’s Disease Assessment Scale Cognitive subscale (Rosen et al., 1984) or the Mini-Mental State Examination (Folstein et al., 1975).
The secondary outcomes were (1) discontinuation due to lack of efficacy (LoE); (2) efficacy on global change from the baseline using the Clinician Interview-Based Impression on Change-Plus Caregiver Input (Schneider et al., 1997) or the Clinical Global Impression (Guy, 1976); (3) efficacy on neuropsychiatric symptoms using the Neuropsychiatric Inventory (Cummings et al., 1994) or the Behavioral Pathology in Alzheimer’s Disease Rating Scale (Reisberg et al., 1987); (4) efficacy on functional capacity assessed with the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory 19- or 23-item Scale (Galasko et al., 1997) or the Disability Assessment for Dementia (Gélinas et al., 1999); (5) mortality; (6) AEs defined as the proportion of patients experiencing any AE during the study; and (7) serious adverse events (SAEs) defined as the proportion of patients experiencing one or more SAEs during the clinical trial. We preferred intention-to-treat analysis data to per-protocol data. Furthermore, for efficacy outcomes, we preferred change scores to endpoint scores, and these to response rates.
The following covariates were considered: number of study sites (single vs multi-site); lead-in period (yes vs no); placebo lead-in period (yes vs no); type of ChEIs; dose (low vs high); dosage (fixed vs flexible); length of intervention (weeks); age (years); gender (percent women); baseline cognitive function; neuropsychiatric symptom severity; and functionality. Dose was labelled as “high” when it was equal or greater than the mean point between the highest and lowest authorized dose and “low” when it was lower than the mean point (e.g., since the authorized dose of galantamine is 8–24 mg, the mean point dose was 16 mg). Given that several scales were used for determining cognitive function, neuropsychiatric symptom severity, and functionality, we standardized baseline scores as the percent of scale maxima. This means reexpressing the score as if the scale ranged from 0 to 100.
Statistical Analysis
Odds ratio (OR) and 95% CI were calculated for dichotomous outcomes and standardized mean difference (SMD) for continuous ones using Cohen’s d. A SMD of 0.2 was considered small, 0.5 moderate, and ≥0.8 large (Cohen, 1998). In studies with multiple comparisons, for example, 2 different pharmacological interventions being compared with one placebo group, we analyzed each intervention separately by dividing the number of patients and events in the placebo group by 2 to avoid overcounting. In addition, for efficacy results, OR were subsequently reexpressed as SMD to allow further combinations of continuous and dichotomous outcomes (Higgins et al., 2011b). Change scores, endpoint scores, and response rates were all used, since combining change and endpoint scores has been shown to be valid (Da Costa et al., 2013) and also the combination of continuous and binary data (Higgins et al., 2011b). Heterogeneity was assessed using the uncertainty factor I2, which measures the percentage of the variance of the observed results (Thorlund et al., 2012). We combined, both the OR and SMD, by means of a model of random effects (DerSimonian et al., 1986). This model allows both the within-study and between-study heterogeneities to be taken into account. In addition, we used meta-regressions to control the heterogeneity on discontinuation, efficacy, and safety outcomes, introducing possible heterogeneity-explaining variables. Due to the greater flexibility of the Bayesian estimation, a consequence of its hierarchical strategy, we chose to do the meta-analysis and the meta-regressions by means of a Bayesian framework. In summary, first of all, the initial uncertainty about the effect measures being meta-analyzed (i.e., OR and SMD), and on extent of among-study variation, was expressed through prior distributions. Secondly, we combined prior distributions with the so-called likelihood (i.e., the current data to meta-analysed in the random effects models) to obtain posterior distribution for the quantities of interest (again, OR and SMD). Finally, we summarized the posterior distributions by point estimates and credible intervals (analogous to the classical confidence intervals). As is known, in Bayesian analysis the choice of the prior distribution may have a considerable impact on the results. For this reason, in this paper we used penalizing complexity priors. These priors are invariant to re-parameterizations and have robustness properties (Simpson et al., 2015). Among the advantages of the Bayesian meta-analysis with respect to the classical (or frequentist) meta-analysis are: this approach is considered the most suitable for accounting model uncertainty, both in the parameters and in the specification of the models; only under the Bayesian approach is it possible to model both variability with relatively sparse data, and within the Bayesian approach, it is easy to specify more complex scenarios. All analyses were conducted using the free software R (version 3.2.3) (R Core Team, 2016) through the INLA library (R Foundation, 2016). Sensitivity analyses were performed by repeating the analysis after excluding RPCCTs that were deemed to have high risk of bias and by using a frequentist approach, with Revman (The Nordic Cochrane Centre, 2014). Publication bias was assessed with Egger’s test for asymmetry (Egger et al., 1997) and funnel plots (Sterne et al., 2001).
Results
Study Design, Intervention, and Patient Characteristics
Forty-three RPCCTs were included (supplementary Figure 1 for the flow diagram and supplementary Table 2 for the reference of the included trials). As 15 studies investigated different doses or formulations of the same ChEI, we analyzed 60 drug-placebo comparisons. Study design, intervention, and patient characteristics are reported in Table 1 and supplementary Table 3. Regarding the study design, most studies were multicentre (88.1%) and about one-quarter (25.6%) had a lead-in period, the majority of these being a placebo lead-in period (90.9%). More than one-half of studies used the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Association (88.4%) or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (51.2%) diagnostic criteria. A high proportion of studies (69.8%) report that patients with dementias other than AD were excluded while only in one (AD2000 Collaborative Group, 2004), patients with vascular dementia were eligible. Thirty-eight studies (88.4 %) had a commercial sponsorship.
Table 1.
Studies, Intervention, and Patients Characteristics and Risk of Bias of Included RPCCTs
| Studies | |
|---|---|
| Number of studies | 43 |
| Number of drug-placebo comparisons | 60 |
| Number of patients/study (median) | 268 |
| Multi-site studies (%) | 88.1 |
| Lead-in period (%) | 25.6 |
| Placebo lead-in period (%) | 90.9 |
| Interventiona | |
| Donepezil (%) | 45.0 |
| Galantamine (%) | 26.7 |
| Rivastigmine (%) | 28.3 |
| Dose (%)b | |
| Low | 27.3 |
| High | 72.7 |
| Dosage (%) | |
| Fixed | 60.0 |
| Flexible | 40.0 |
| Length (mean) | 25.1 |
| 12–24 weeks (%) | 23.3 |
| ≥24–36 weeks (%) | 68.4 |
| ≥36 weeks (%) | 8.3 |
| Patients | |
| Number of patients | 16,106 |
| Age (years) | 74.5 |
| Women (%) | 63.4 |
| Cognitive function (mean)c | 57.7 |
| Neuropsychiatric symptom severity (mean)c | 13.5 |
| Functionality (mean)c | 62.2 |
| High risk of biasd | |
| Discontinuation outcomes | 0 |
| Efficacy cognitive function | 22.0 |
| Efficacy global change | 25.0 |
| Efficacy neuropsychiatric symptoms | 21.1 |
| Efficacy functional capacity | 33.3 |
| Mortality | 17.3 |
| Any AE | 23.5 |
| SAE | 16.7 |
Abbreviations: AE, adverse event; SAE, serious adverse event.
aProportion of drug-placebo comparisons.
bHigh, mean daily dose of donepezil >7.5 mg, galantamine >16 mg, and rivastigmine >5.5 mg; Low, mean daily dose of donepezil <7.5 mg; galantamine <16 mg, and rivastigmine <5.5 mg.
cAs a percentage of scale maxima (0–100).
dProportion of comparisons with high risk of bias for each outcome.
Regarding the interventions, donepezil was studied in 23 studies involving 27 drug vs placebo comparisons with 5755 patients, galantamine in 11 studies (16 drug vs placebo comparisons) with 6251 patients, and rivastigmine in 9 studies (17 drug vs placebo comparisons) with 4100 patients included. Most RPCCTs investigated high ChEIs doses and used a fixed dosage. The mean treatment length was 25 weeks and ranged from 12 to 54.
A total of 16106 patients with AD were enrolled and 9555 received ChEIs and 6551 placebo. The mean age was 74.5 years and almost two-thirds of patients were women (63.4%). On average, patients showed a moderate cognitive impairment. Neuropsychiatric symptoms severity was mild and functionality impairment was moderate.
No study was deemed to have a high risk of bias for discontinuation outcomes. For the other study outcomes, between 17% and 33% of drug-placebo comparisons were scored “high risk of bias” (supplementary Table 4; supplementary Figures 2 and 3). The most common reason for scoring high risk of bias was attrition bias due to a high withdrawal rate or between-group differences in the discontinuation rate.
Discontinuation, Efficacy, and Safety Outcomes
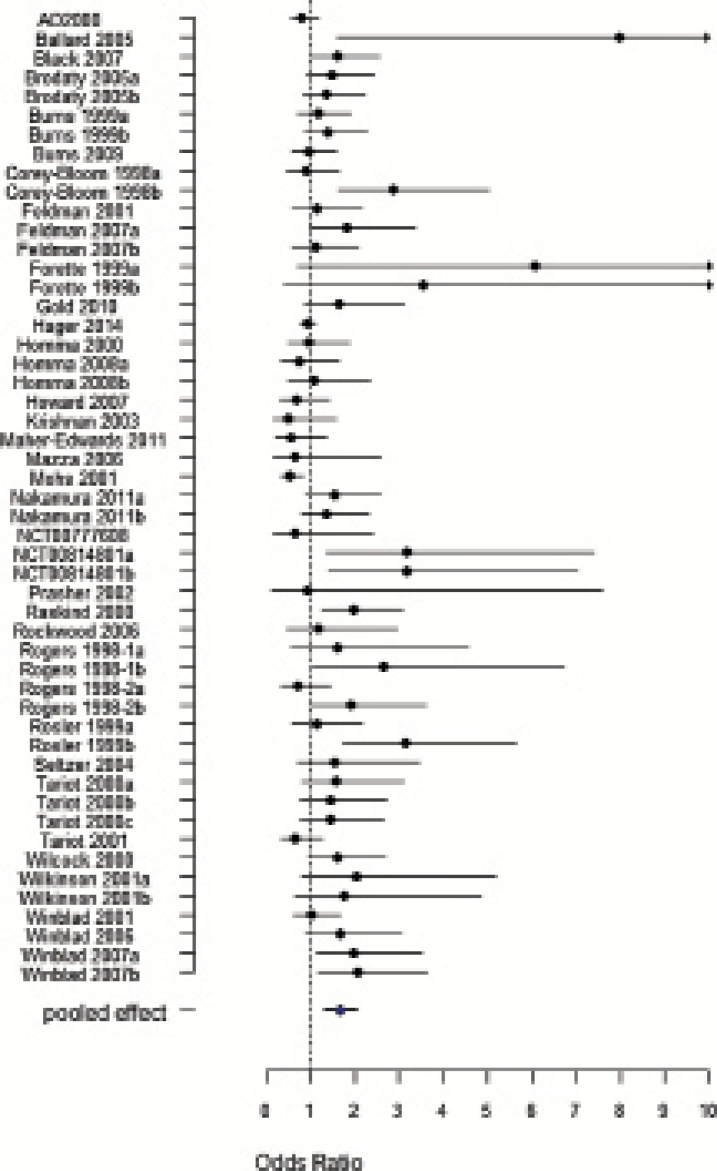
The results of the effect of ChEIs vs placebo on study outcomes are presented in Table 2 (For raw data analyzed, see supplementary Tables 5–14). All-cause discontinuation was higher with ChEIs than with placebo (OR = 1.66, 95%CI 1.30, 2.03) (Figure 1). Discontinuation due to AEs was also higher with ChEIs (OR=1.75, 95%CI 1.45, 2.05) (supplementary Figure 4), while discontinuation due to LoE was lower (OR = 0.56, 95%CI 0.34, 0.78) (supplementary Figure 5).
Table 2.
Effect of ChEIs on Discontinuation, Efficacy, and Safety Outcomes in Patients with Alzheimer’s Disease
| Outcome | n | Effect size (95% CI) | I2 (%) |
|---|---|---|---|
| Discontinuation | |||
| All-cause discontinuation | 51 | OR = 1.66 (1.30, 2.03) | 51.7 |
| Discontinuation due to AEs | 44 | OR = 1.75 (1.45, 2.05) | 0 |
| Discontinuation due to LoE | 12 | OR = 0.56 (0.34, 0.78) | 0 |
| Efficacy | |||
| Cognitive function | 41 | SMD = 0.38 (0.28, 0.47) | 41.1 |
| Global change | 32 | SMD = 0.28 (0.22, 0.34) | 0 |
| Neuropsychiatric symptoms | 19 | SMD = 0.03 (-0.04, 0.09) | 0 |
| Functional capacity | 18 | SMD = 0.16 (0.11, 0.20) | 0 |
| Safety | |||
| Mortality | 19 | OR = 0. 65 (0.47, 0.83) | 0 |
| Proportion patients AEs | 34 | OR = 1.69 (1.46, 1.93) | 0 |
| Proportion patients SAEs | 32 | OR = 1.10 (0.84, 1.35) | 0 |
Abbreviations: AE, adverse event; LoE, Lack of efficacy; OR, odds ratio.
Figure 1.
Forest plot meta-analysis pooled effect for all-cause discontinuation of 51 drug-placebo comparisons. Odds Ratio value > 1 placebo more favourable than ChEIs.
ChEIs were more efficacious than placebo for reducing cognitive symptoms (SMD = 0.38, 95%CI 0.28, 0.47) (supplementary Figure 6). Similarly, ChEIs slightly improved the global symptoms (SMD = 0.28, 95%CI 0.22, 0.34) (supplementary Figure 7). However, these drugs did not improve neuropsychiatric symptoms (SMD = 0.03, 95%CI -0.04, 0.09) (supplementary Figure 8). A very small effect was found on functional capacity (SMD = 0.16, 95%CI 0.11, 0.20) (supplementary Figure 9). The type of scale used to evaluate the efficacy did not affect the results of any efficacy outcome (Table 3).
Table 3.
Meta-Regression Analyses of Study Design-, Intervention- and Patient-Related Characteristics Associated with Discontinuation, Efficacy, and Safety Outcomes
| All-cause discontinuation | Discontinuation due to Aes | Discontinuation due to LoE | Cognitive function | Global change | Neuropsychiatric symptoms | Functional capacity | Mortality | Proportion patients AEs | Proportion patients SAEs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | Constant (95%CI) | |
| ROR (95%CI) | ROR (95%CI) | ROR (95%CI) | Diff SMD (95%CI) | Diff SMD (95%CI) | Diff SMD (95%CI) | Diff SMD (95%CI) | ROR (95%CI) | ROR (95%CI) | ROR (95%CI) | |
| Study site | ||||||||||
| Single site (ref.) | 2.550 (1.251, 3.846) |
3.096 (1.104, 5.081) |
0.001 (-0.822, 0.824) |
0.087 (-0.268, 0.441) |
(NA) | -0.170 (-0.443, 0.103) |
(NA) | (NA) | (NA) | (NA) |
| Multi-site | -0.965 (-2.315, 0.385) |
-1.381 (-3.390, 0.630) |
0.600 (-0.253, 1.451) |
0.313 (-0.056, 0.681) |
(NA) | 0.207 (-0.073, 0.488) |
(NA) | (NA) | (NA) | (NA) |
| Lead-in period | ||||||||||
| No (ref.) | 1.785 (1.346, 2.223) |
1.885 (1.536, 2.233) |
0.675 (0.437, 0.913) |
0.387 (0.267, 0.507) |
0.314 (0.247, 0.381) |
-0.019 (-0.111, 0.073) |
0.159 (0.086, 0.232) |
0.564 (0.341, 0.787) |
1.846 (1.558, 2.133) |
1.190 (0.896, 1.484) |
| Yes | -0.407 (-1.200, 0.382) |
-0.491 (-1.147, 0.164) |
-0.428 (-0.889, 0.032) |
-0.032 (-0.246, 0.181) |
-0.114 (-0.234, 0.006) |
0.086 (-0.041, 0.213) |
-0.003 (-0.101, 0.095) |
0.248 (-0.131, 0.626) |
-0.400 (-0.865, 0.065) |
-0.344 (-0.907, 0.218) |
| Placebo lead-in period | ||||||||||
| No (ref.) | 1.755 (1.321, 2.189) |
1.885 (1.536, 2.233) |
0.675 (0.437, 0.913) |
0.388 (0.269, 0.505) |
0.314 (0.247, 0.381) |
-0.016 (-0.103, 0.071) |
0.159 (0.086, 0.232) |
0.576 (0.359, 0.793) |
1.846 (1.558, 2.133) |
1.190 (0.896, 1.484) |
| Yes | -0.330 (-1.138, 0.478) |
-0.491 (-1.147, 0.164) |
-0.428 (-0.889, 0.032) |
-0.038 (-0.256, 0.180) |
-0.114 (-0.234, 0.006) |
0.089 (-0.037, 0.215) |
-0.003 (-0.101, 0.095) |
0.242 (-0.151, 0.635) |
-0.400 (-0.865, 0.065) |
-0.344 (-0.907, 0.218) |
| Intervention | ||||||||||
| Donepezil (ref.) | 1.071 (0.610, 1.531)* † |
1.549 (1.096, 2.001) |
0.333 (-0.292, 0.959) |
0.383 (0.230, 0.536) |
0.410 (0.326, 0.494)* |
-0.076† (-0.157, 0.004) |
0.177 (0.073, 0.282) |
0.626 (0.372, 0.881) |
1.662 (1.294, 2.030) |
1.189 (0.844, 1.534) |
| Galantamine | 0.650 (-0.119, 1.418) † |
0.181 (-0.525, 0.887) |
0.171 (-0.596, 0.937) |
-0.087 (-0.319, 0.146) |
-0.247 (-0.363, -0.131)* |
0.181 (0.063, 0.298)* † |
-0.039 (-0.163, 0.084) |
0.054 (-0.376, 0.484) |
-0.035 (-0.556, 0.484) |
-0.219 (-0.779, 0.341) |
| Rivastigmine | 1.658 (0.871, 2.445)* † |
0.544 (-0.196, 1.283) |
0.303 (-0.389, 0.994) |
0.079 (-0.166, 0.323) |
-0.135 (-0.251, -0.020)* |
0.171 (0.032, 0.310)* † |
0.008 (-0.140, 0.155) |
0.056 (-0.532, 0.643) |
0.257 (-0.414, 0.928) |
-0.162 (-0.971, 0.645) |
| Dose | ||||||||||
| Low (ref.) | 1.225 (0.577, 1.873) |
1.483 (0.962, 2.004) |
0.507 (0.105, 0.907) |
0.385 (0.207, 0.563) |
0.302 (0.196, 0.408) |
0.066 (-0.065, 0.197) |
0.175 (0.089, 0.260) |
0.630 (0.263, 0.996) |
1.446 (1.064, 1.828) |
0.853 (0.425, 1.280) |
| High | 0.628 (-0.151, 1.406) |
0.390 (-0.245, 1.024) |
0.081 (-0.410, 0.572) |
-0.013 (-0.228, 0.202) |
-0.034 (-0.162, 0.094) |
-0.054 (-0.206, 0.099) |
-0.027 (-0.131, 0.078) |
0.028 (-0.398, 0.453) |
0.382 (-0.094, 0.857) |
0.365 (-0.158, 0.888) |
| Dosage | ||||||||||
| Fixed (ref.) | 1.378 (0.918, 1.837) |
1.701 (1.307, 2.095) |
0.553 (0.113, 0.992) |
0.330 (0.210, 0.449) |
0.269 (0.193, 0.345) |
0.015 (-0.068, 0.099) |
0.152 (0.092, 0.211) |
0.555 (0.314, 0.796) |
1.682 (1.396, 1.968) |
1.110 (0.813, 1.407) |
| Flexible | 0.699 (-0.024, 1.422) |
0.108 (-0.505, 0.720) |
0.011 (-0.503, 0.524) |
0.138 (-0.068, 0.342) |
0.022 (-0.098, 0.142) |
0.03 (-0.107, 0.168) |
0.017 (-0.086, 0.120) |
0.219 (-0.147, 0.585) |
0.035 (-0.468, 0.538) |
-0.051 (-0.620, 0.517) |
| Length (weeks) | ||||||||||
| Intercept | 2.320 (1.242, 3.395) |
2.589 (1.709, 3.468) |
0.689 (-0.164, 1.540) |
0.188 (-0.086, 0.462) |
0.191 (-0.026, 0.408) |
-0.025 (-0.565, 0.515) |
0.190 (-0.009, 0.389) |
0.265 (-0.186, 0.715) |
1.810 (1.367, 2.252) |
0.817 (0.111, 1.523) |
| -0.026 (-0.067, 0.014) |
-0.033 (-0.065, 0.000) |
-0.005 (-0.036, 0.026) |
0.008 (-0.003, 0.018) |
0.004 (-0.005, 0.012) |
0.002 (-0.021, 0.025) |
-0.001 (-0.009, 0.006) |
0.014 (-0.001, 0.029) |
-0.008 (-0.035, 0.020) |
0.011 (-0.015, 0.037) |
|
| Age (years) | ||||||||||
| Intercept | 0.453 (-1.919, 2.821) |
6.701 (0.804, 12.536) |
-1.986 (-8.233, 4.321) |
0.070 (-0.536, 0.676) |
1.172 (-0.393, 2.734) |
-0.640 (-1.364, 0.085) |
-0.197 (-1.520, 1.124) |
1.638 (-1.702, 4.961) |
1.982 (0.691, 3.269) |
7.648 (4.794, 10.474) |
| 0.017 (-0.016, 0.049) |
-0.066 (-0.144, 0.012) |
0.035 (-0.051, 0.119) |
0.004 (-0.004, 0.012) |
-0.012 (-0.033, 0.009) |
0.009 (-0.001, 0.018) |
0.005 (-0.013, 0.022) |
-0.013 (-0.056, 0.031) |
-0.004 (-0.022, 0.014) |
-0.088 (-0.125, -0.050)* |
|
| Women (%) | ||||||||||
| Intercept | 2.7779 (1.701, 3.855) |
2.845 (1.902, 3.786) |
0.605 (0.035, 1.174) |
0.217 (-0.136, 0.570) |
0.310 (0.113, 0.505) |
0.021 (-0.553, 0.595) |
0.077 (-0.515, 0.668) |
0.505 (-0.993, 2.001) |
2.454 (1.625, 3.281) |
2.270 (1.011, 3.527) |
| -0.019 (-0.036, -0.002)* † |
-0.018 (-0.033, -0.003)* † |
-0.001 (-0.01, 0.009) |
0.003 (-0.003, 0.008) |
-0.001 (-0.004, 0.003) |
0 (-0.001, 0.018) |
0.001 (-0.007, 0.010) |
-0.002 (-0.020, 0.024) |
-0.013 (-0.026, 0.001) |
-0.019 (-0.038, 0.001) |
|
| Cognitive function (mean) | ||||||||||
| Intercept | 2.348 (1.396, 3.300) |
0.693 (-0.158, 1.542) |
3.643 (-1.472, 8.669) |
0.377 (0.077, 0.677) |
0.154 (-0.029, 0.338) |
-0.048 (-0.175, 0.079) |
0.159 (0.047, 0.271) |
0.572 (-0.109, 1.251) |
1.696 (1.108, 2.283) |
1.294 (0.648, 1.939) |
| -0.013 (-0.030, 0.004) |
0.019 (0.004, 0.034)* † |
-0.048 (-0.128, 0.318) |
0.000 (-0.005, 0.005) |
0.002 (-0.001, 0.005) |
0.002 (-0.001, 0.004) |
0 (-0.002, 0.002) |
0.001 (-0.011, 0.014) |
0 (-0.011, 0.011) |
-0.004 (-0.016, 0.008) |
|
| Neuropsychiatric symptoms severity (mean) | ||||||||||
| Intercept | 1.823 (1.405, 2.241) |
1.846 (1.060, 2.186) |
0.426 (0.177, 0.675) |
0.399 (0.286, 0.512) |
0.290 (0.222, 0.358) |
-0.027 (-0.143, 0.090) |
0.138 (0.066, 0.210) |
0.759 (0.459, 1.058) |
1.669 (1.427, 1.969) |
1.194 (0.890, 1.498) |
| -0.034 (-0.083, 0.015) |
-0.022 (-0.061, 0.017) |
0.019 (-0.001, 0.039) |
-0.005 (-0.018, 0.008) |
-0.002 (-0.009, 0.005) |
0.004 (-0.004, 0.013) |
0.002 (-0.003, 0.007) |
-0.06 (-0.035, 0.022) |
-0.003 (-0.031, 0.026) |
-0.011 (-0.040, 0.020) |
|
| Functionality (mean) | ||||||||||
| Intercept | 1.767 (1.330, 2.204) |
1.761 (1.390, 2.186) |
0.381 (0.075, 0.686) |
0.375 (0.251, 0.498) |
0.300 (0.221, 0.379) |
-0.073 (-0.169, 0.023) |
0.162 (0.033, 0.292) |
0.722 (0.424, 1.019) |
1.677 (1.363, 1.991) |
1.280 (1.012, 1.548) |
| -0.005 (-0.017, 0.007) |
-0.001 (-0.01, 0.009) |
0.005 (-0.001, 0.011) |
0 (-0.003, 0.003) |
-0.001 (-0.003, 0.001) |
0.002 (0.001, 0.004)* † |
0 (-0.002, 0.002) |
0 (-0.007, 0.007) |
0 (-0.007, 0.007) |
-0.006 (-0.014, 0.001) |
|
| Type of scalea | ||||||||||
| Intercept (ref.) | (NA) | (NA) | (NA) | 0.420 (0.311, 0.528) |
0.275 (0.211, 0.339) |
-0.058 (-0.253, 0.138) |
-0.054 (-0.150, 0.042) |
(NA) | (NA) | (NA) |
| (NA) | (NA) | (NA) | -0.198 (-0.430, 0.034) |
0.023 (-0.139, 0.185) |
0.064 (-0.076, 0.204) |
0.232 (0.091, 0.373) |
(NA) | (NA) | (NA) | |
Abbreviations: AEs, adverse events; LoE, lack of efficacy; ROR, risk of odd ratios; Diff SMD, difference of standardized mean differences; NA, not applicable; SAEs, serious adverse events.
aType of scale used to evaluate the efficacy. Cognitive function MMSE or ADAS-Cog, global change CIBIC-Plus or CGI, neuropsychiatric symptoms NPI or BEHAVE-AD, functional capacity ADCS-ADL or DAD.
*Statistically significant effect (P < .05).
†Covariates included in multivariate analysis.
Thirty-eight studies provided information on mortality in a suitable way for meta-analysis. Two hundred and fifty-two patients died, mortality being slightly lower with ChEIs than with placebo (OR= 0.65, 95%CI 0.47, 0.83) (supplementary Figure 10). Most patients experienced AEs and the rate was higher for ChEIs than for the placebo group (OR=1.69 95%CI 1.46, 1.93) (supplementary Figure 11). No statistically significant differences in SAEs were found between ChEIs and placebo (OR=1.10 95%CI 0.84, 1.35) (supplementary Figure 12).
Meta-Regression Analysis: Effect of Covariates
The effects of study design-, intervention-, and patient-related covariates on study outcomes are presented in Table 3. Bi-variant meta-regression analysis showed that the gender and type of ChEI modified the effect on all-cause discontinuation outcome. However, in the multivariate analysis (supplementary Table 15), only the type of ChEI was independently associated with the effect on all-cause discontinuation. In this analysis, donepezil showed a better outcome than rivastigmine, and no statistically significant differences were found between galantamine and donepezil. Discontinuation due to AEs was negatively associated with the proportion of women and positively with cognitive function. These effects did not remain statistically significant in the multivariate analysis.
The type of ChEI was also associated with the effect on global symptomatology of AD, with donepezil showing a higher efficacy on global change than galantamine or rivastigmine. The efficacy of ChEIs on neuropsychiatric symptoms was modified by baseline functional capacity and the type of ChEI, but only the latter remained statistically significant in the multivariate analysis: galantamine and rivastigmine were found to be slightly more efficacious than donepezil. Regarding safety, SAEs were negatively correlated with age.
No covariate analysed in this study had a statistically significant effect on discontinuation due to LoE, efficacy on cognitive function, efficacy on functional capacity, the proportion of patients with AEs, and mortality.
Sensitivity Analysis and Publication Bias
The sensitivity analyses yielded similar findings to the primary ones with two exceptions. When the primary analyses were repeated using a frequentist approach, the effect of ChEIs on discontinuation due to LoE was not significant. Conversely, ChEIs were more efficacious than placebo on neuropsychiatric symptoms in this analysis (supplementary Table 16).
No evidence of asymmetry was found for the majority of study outcomes (supplementary Figures 13–22). For all-cause discontinuation and neuropsychiatric symptoms, the funnel plots were asymmetrical but not suggestive of publication bias, because they did not have a gap in the bottom corner where small studies with negative results are expected to lay. Egger’s test for these outcomes was statistically significant.
Discussion
The present study found that a large number of RPCCTs have studied the efficacy and safety of ChEIs. Overall, ChEIs showed a modest efficacy on cognitive function and global symptomatology, nonclinically significant efficacy on functional capacity, and no evidence of efficacy on neuropsychiatric symptoms in patients with mild-moderate AD. Furthermore, our results could indicate that the modest improvement of AD symptoms does not compensate the frequent AEs of these drugs, as all-cause discontinuation rate was higher with ChEIs than with placebo. It is likely that since patients with AD are elderly persons with a high rate of comorbid disorders and receive concomitant interventions, the administration of ChEIs is poorly tolerated, leading to discontinuation for this reason. Our findings expand and complement those of previous studies (Birks, 2012; Di Santo et al., 2013; Tan et al., 2014) and, like the NICE assessment (Kmietowicz, 2005), would support that ChEIs have an unclear risk-benefit ratio. However, this study also suggests that this outcome varies depending on intervention and patient-related characteristics.
Firstly, we found that, while donepezil, galantamine, and rivastigmine show similar safety, they seem to differ in their efficacy and their effect on all-cause discontinuation. In addition, our results suggest that donepezil can be slightly more efficacious on the global symptomatology of AD than galantamine or rivastigmine. Furthermore, donepezil and galantamine can have a better outcome on all-cause discontinuation than rivastigmine, which was the only drug that showed a higher rate of all-cause discontinuation than placebo. In fact, some studies suggest that these two drugs have neuroprotective effects that could result in a delayed progression of the disease as shown in some clinical studies (Raskind et al., 2004; Hashimoto et al., 2005).
We also found that mortality was slightly lower in patients taking ChEIs than those taking placebo. This finding was unexpected, as safety warnings alert of a possible increase in mortality while using galantamine (Loy et al., 2006). To the best of our knowledge, this is the first time that a study comparing ChEIs vs placebo showed a beneficial effect on mortality. Only some observational studies have pointed to this possibility in the past (Wattmo et al., 2014; Zhu et al., 2013). We could not determine the causes of death and we cannot elucidate the mechanism by which ChEIs reduce mortality in patients with AD. Nevertheless, ChEIs may reduce cardiovascular-related deaths, which account for the second cause of death in patients with AD (Nordström et al., 2013; Monacelli et al., 2014). Future studies should address the specific causes of death in RPCCTs of ChEIs in order to draw firm conclusions.
Regarding ChEIs’ safety, we found patients’ age to be negatively associated with SAEs. A possible explanation is that as the prevalence of comorbidities and their severity increases with age, the same can happen with the incidence of SAEs in the placebo group, thereby hiding a statistically significant difference of SAEs between the ChEIs and placebo groups.
Our results could have clinical consequences. Generally, the clinical guidelines do not make a clear recommendation on which ChEIs should be used as first-line treatment. Our findings would support donepezil as the ChEIs of choice for several reasons: it shows better results on withdrawals for any reason and better efficacy on global symptomatology than galantamine and rivastigmine, although donepezil is slightly worse for neuropsychiatric symptoms. However, before a clinical recommendation can be made, these results should be confirmed in rigorously performed comparative clinical trials (Hogan et al., 2004).
Limitations and Strengths
This study has several limitations. Firstly, biased RPCCTs can also bias the results of our meta-analysis. However, biased RPCCTs do not seem to affect the results of our meta-analysis, as shown by the sensitivity analyses. These yield similar results to the primary ones after excluding those studies deemed to have a high risk of bias. No clear evidence of publication bias was found. The finding that ChEIs were not efficacious for reducing neuropsychiatric symptoms in our study contrasts with those of Wang et al., 2015. Methodological differences in the statistical analysis may explain these apparently discrepant findings. Limitations affecting the meta-regression analysis must also be born in mind. Firstly, ecological bias should be considered because our findings derive from aggregated data. Secondly, the effect of covariates associated with study outcomes can be confounded with that of other covariates not included in the analyses. And thirdly, as multiple comparisons have been performed, it is not possible to rule out that the differences have been found by chance. Finally, limitations concerning the external validity include the difficult extrapolation of our results to clinical practice, where patients present differences in their characteristics compared with patients included in RPCCTs due to strict inclusion criteria. These criteria exclude patients with common comorbid conditions such as psychiatric disorders and cardiovascular diseases (Leinonen et al., 2015). Furthermore, the length of trials is relatively short in comparison with the chronic course of the disease and its treatment in real-life patients with AD. In addition, no information on the efficacy of ChEIs in relevant clinical outcomes was included in these trials.
In relation to strengths, this study is the largest systematic review and meta-analysis performed in the context of AD. It included 43 RPCCTs, 16106 patients, and 60 drug-placebo comparisons. Furthermore, we have investigated all-cause discontinuation as a primary outcome to evaluate the benefit-risk relationship. This outcome is objective and not affected by attrition bias. Besides, it is the first systematic review and meta-analysis to determine the effect of ChEIs on mortality. Moreover, it is the first study to use a Bayesian methodology and to evaluate the association between study design-, intervention-, and patient-related characteristics in ChEIs discontinuation, efficacy and safety for AD. The Bayesian analysis has the advantage over the most frequent one in that prior information is incorporated into the analysis using a flexible method, which can lead to more precise and reliable results.
Conclusions
This study found mixed results. While ChEIs show a poor risk-benefit relationship, as indicated by small symptom improvement, and a higher all-cause discontinuation than placebo, a reduction in mortality was also found, which could renew interest in clinical research on ChEIs. Nevertheless, the clinical relevance of reduction in mortality accompanied by only a small improvement in symptoms is uncertain. Finally, intervention- and patient-related factors, but not study design, were found to slightly modify the effect of ChEIs in patients with AD. However, the clinical relevance of this is arguable.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by Universitat de Girona (grant nos. IFUdG2015/17, MPCUdG2016/ref50). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- AD2000 Collaborative Group (2000) Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 363:2105–2115. [DOI] [PubMed] [Google Scholar]
- Birks J. (2006) Cholinesterase inhibitors for Alzheimer’s disease (review). Cochrane Database Syst Rev 1:CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. (2012) Cholinesterase inhibitors for Alzheimer’s disease (review). Cochrane Database Syst Rev 1:CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnström HR, Englund EM. (2009) Cause of death in patients with dementia disorders. Eur J Neurol 16:488–492. [DOI] [PubMed] [Google Scholar]
- California Workgroup on Guidelines for Alzheimer’s Disease Management (2008) Guideline for Alzheimer’s disease management. Los Angeles: Alzheimer’s Disease and Related Disorders Association. [Google Scholar]
- Castells X, Ramos-Quiroga JA, Rigau D, Bosch R, Nogueira M, Vidal X, Casas M. (2011) Efficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysis. CNS Drugs 25:157–169. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Zhou X, Giovane C, Hetrick SE, Qin B, Whittington C, Coghill D, Zhang Y, Hazell P, Leucht S, Cuijpers P, Pu J, Cohen D, Ravindran AR, Liu Y, Michael KD, Yang L, Liu L, Xie P. (2016) Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet 388:881–890. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis in the behavioural sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associated. [Google Scholar]
- Corey-Bloom J, Anand R, Veach J. (1998) A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1:55–65. [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314. [DOI] [PubMed] [Google Scholar]
- Cunill R, Castells X, Tobias A, Capellà D. (2015) Pharmacological treatment of attention deficit hyperactivity disorder with co-morbid drug dependence. J Psychopharmacol 29:15–23. [DOI] [PubMed] [Google Scholar]
- Cunill R, Castells X, Tobias A, Capellà D. (2016) Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology 233:187–197. [DOI] [PubMed] [Google Scholar]
- Da Costa BR, Nüesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, Guyat GH, Jüni P. (2013) Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. J Clin Epidemiol 66:847–855. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M. (2013) A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimer’s Dis 35:349–361. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley NC, Affoo RH, Martin RE. (2015) A systematic review and meta-analysis examining pneumonia-associated mortality in dementia. Dement Geriatr Cogn Disord 39:52–67. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Rest 12:189–198. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress 66:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. (1997) An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 11:S33–39. [PubMed] [Google Scholar]
- Gélinas I, Gauthier L, McIntyre M, Gauthier S. (1999) Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Therep 53:471–481. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) CGI Clinical Global Impressions. In: ECDEU assessment manual for psychopharmacology (Department of Health, Education and Welfare), pp218–222. Rockville: National Institute of Mental Health. [Google Scholar]
- Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. (2005) Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry 162:676–682. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S.(2011a) The Cochrane Collaboration tool for assessing risk of bias. In: Cochrane handbook for systematic reviews of interventions version 5.1.0 (Higgins JPT, Green S, eds). Copenhagen: The Cochrane Collaboration. [Google Scholar]
- Higgins JPT, Green S.(2011b) How to include multiple groups from one study. In: Cochrane handbook for systematic reviews of interventions version 5.1.0 (Higgins JPT, Green S, eds). Copenhagen: The Cochrane Collaboration. [Google Scholar]
- Hogan DB, Goldlist B, Naglie G, Patterson C. (2004) Comparison studies of cholinesterase inhibitors for Alzheimer’s disease. Lancet Neurol 3:622–626. [DOI] [PubMed] [Google Scholar]
- Jeon WJ, Dean B, Scarr E, Gibbons A. (2015) The role of muscarinic receptors in the pathophysiology of mood disorders: a potential novel treatment? Curr Neuropharmacol 13:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmietowicz Z. (2005) NICE proposes to withdraw Alzheimer’s drugs from NHS. BMJ 330:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, Loulou MM, Einarson TR. (2003) Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 169:557–564. [PMC free article] [PubMed] [Google Scholar]
- Leinonen A, Koponen M, Hartikainen S. (2015) Systematic review: representativeness of participants in RCTs of acetylchonesterase inhibitors. PLoS One 100(5):e0124500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy C, Schneider L, Loy C, Schneider L. (2006) Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev 1:CD0017471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monacelli F, Rosa G. (2014) Cholinesterase inhibitors: cardioprotection in Alzheimer’s disease. J Alzheimers Dis 42:1071–1077. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2011) Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. London: NICE. [Google Scholar]
- Nordström P, Religa D, Wimo A, Winblad B, Eriksdotter M. (2013) The use of cholinesterase inhibitor and the risk of myocardial infarction and death: a nationwide cohort study in subjects with Alzheimer’s disease. Eur Heart J 34:2585–2591. [DOI] [PubMed] [Google Scholar]
- Pérez-Mañá C, Castells X, Torrens M, Capellà D, Farre M. (2013) Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst Rev 9:CD009695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabins PV, Blacker D, Barry WR, Rummans T, Schneider LS, Tariot PN, Blass DM. (2010) Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Washington: American Psychiatric Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Truyen L, Kershaw P, Damaraju CV. (2004) The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Arch Neurol 61:252–256. [DOI] [PubMed] [Google Scholar]
- R Core Team (2016) The R Project for Statistical Computing. Vienna: R Foundation. [Google Scholar]
- Regional Health Council (2011) Dementia. Diagnosis and treatment. Milan: Regione Toscana, Consiglio Sanitario Regionale. [Google Scholar]
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. (1987) Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry 48:S9–15. [PubMed] [Google Scholar]
- R Fundation (2016) R-INLA Project. Vienna: R Fundation. [Google Scholar]
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT, Donepezil Study Group (1998) Donepezil improves cognition and global function in Alzheimer Disease. Arch Intern Med 158:1021–1031. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. (1984) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141:1356–1364. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. (1997) Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative. Alzheimer Dis Assoc Disord 11:S22–32. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Correll CU. (2014) Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry 53:174–187. [DOI] [PubMed] [Google Scholar]
- Simpson DP, Rue H, Martins TG, Riebler A, Sørbye SH. (2015). Penalising model component complexity: a principled, practical approach to constructing priors. Statistical Science Arxiv:1403.4630. [Google Scholar]
- Sterne JA, Egger M. (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54:1046–1055. [DOI] [PubMed] [Google Scholar]
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, Hammad TA, Temple R, Rochester G. (2009) Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 339:b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup TS, Mcevoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. (2003) The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 29:15–31. [DOI] [PubMed] [Google Scholar]
- Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L. (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 41:615–631. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre (2014) Review Manager Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. [Google Scholar]
- Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, Gluud C, Devereaux PJ, Wetterslev J. (2012) Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One 7:e39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ. (2012) Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology 37:851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu JT, Wang HF, Meng XF, Wang C, Tan CC, Tan L. (2015) Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 86:101–109. [DOI] [PubMed] [Google Scholar]
- Wattmo C, Londos E, Minthon L. (2014) Response to cholinesterase inhibitors affects lifespan in Alzheimer’s disease. BMC Neurol 14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK, Lilienfeld S, Gaens E. (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. BMJ 321:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015) Dementia. Geneva: WHO. [Google Scholar]
- Zhu CW, Livote EE, Scarmeas N, Albert M, Brandt J, Blacker D, Sano M, Stern Y. (2013) Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dement 9:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.