Abstract
Febrifugine is the active principal isolated 50 years ago from the Chinese herb chang shan (Dichroa febrifuga Lour), which has been used as an antimalarial in Chinese traditional medicine for more than 2,000 years. However, intensive study of the properties of febrifugine has been hindered for decades due to its side effects. We report new findings on the effects of febrifugine analogs compared with those of febrifugine extracted from the dry roots of D. febrifuga. The properties of the extracted febrifugine were comparable to those obtained from the standard febrifugine provided by our collaborators. A febrifugine structure-based computer search of the Walter Reed Chemical Information System identified 10 analogs that inhibited parasite growth in vitro, with 50% inhibitory concentrations ranging from 0.141 to 290 ng/ml. The host macrophages (J744 cells) were 50 to 100 times less sensitive to the febrifugine analogs than the parasites. Neuronal (NG108) cells were even more insensitive to these drugs (selectivity indices, >1,000), indicating that a feasible therapeutic index for humans could be established. The analogs, particularly halofuginone, notably reduced parasitemias to undetectable levels and displayed curative effects in Plasmodium berghei-infected mice. Recrudescence of the parasites after treatment with the febrifugine analogs was the key factor that caused the death of most of the mice in groups receiving an effective dose. Subcutaneous treatments with the analogs did not cause irritation of the gastrointestinal tract when the animals were treated with doses within the antimalarial dose range. In summary, these analogs appear to be promising lead antimalarial compounds that require intensive study for optimization for further down-selection and development.
Plasmodium falciparum and P. vivax are dominant pathogens of the most severe parasitic disease, malaria, which causes 300 million to 500 million clinical cases and more than 3,000 deaths each day worldwide (34). Control of the disease mostly relies on drug therapies, but most currently available antimalarials have been used for decades, with the rapid emergence of widespread drug resistance (23). The urgent need for treatments for malaria is largely dependent on the development of novel antimalarials from new and existing lead compounds. Several compounds identified decades ago, such as tryptanthrin and fosmidomycin, have been rediscovered and have been found to have remarkable antimalarial activities and other functions in clinical medicine (27). Likewise, febrifugine {(2′R,3′S)-3-[3-(3-hydroxy-2-piperidinyl) acetonyl]-4(3H) quinazolinone} is the active principle extracted from a Chinese medicinal plant, chang shan (Dichroa febrifuga Lour), that has been prescribed in traditional Chinese medicine for more than 2,000 years. The medical use of chang shan was described in an ancient Chinese pharmacopoeia (17) for the treatment of malaria and stomach cancer and as an expectorant, emetic, and febrifuge, with side effects of nausea and vomiting (10). Febrifugine was extracted (7) and was later identified as a quinazoline derivative with the molecular structure C16H21O3N3 (15). The purified febrifugine displayed potent antimalarial activity and was 100 times as active as quinine against P. lophurae in duck models and 50 times as active as quinine against P. cynomolgi infection in rhesus monkeys (12, 13). Severe gastrointestinal injury, however, was also observed in a chicken model when the chickens were overdosed (8, 29). Clinical studies of both the crude extract and isolated forms of febrifugine conducted in Yunnan Province, People's Republic of China, from the 1940s through the 1960s showed that it had excellent antipyretic and antiparasitic effects, similar to those of quinine (7, 16). Unfortunately, the emetic effects and gastrointestinal irritation caused by the compound rendered it insignificant for further investigation. During the 1960s, a series of febrifugine derivatives, including halofuginone {7-bromo-6-chloro-3-[(3-hydroxy-2-piperidinyl)-2-oxopropyl]-4(3H)-quinazolinone} (28), were synthesized by the U.S. Army Medical Research Command with the aim of identifying novel antimalarials with clinical profiles better than those of febrifugine. Some of these febrifugine derivatives were briefly screened in studies with mice right after the derivatives were submitted to the Walter Reed Army Institute of Research (WRAIR) Chemical Information System (CIS). None of the screened compounds was found to be curative for P. berghei-infected mice (2). Further study of these derivatives discontinued a few decades ago because of funding shortages and a lack of appropriate research approaches to support detailed in vivo and in vitro measurements.
Recently, state-of-the-art biomedical technology has proven that chemical modification of febrifugine decreases its toxicity, while its antiparasitic efficacy remains unchanged (11, 31). For example, it was found that the administration of febrifugine analogs did not cause changes to body or liver weights, irritation of the gastrointestinal tract, or alteration of the levels of hepatic enzyme markers in infected mice (6, 20). Febrifugine altered the production of nitric oxide and tumor necrosis factor alpha in mouse macrophages (19). These studies indicate not only that febrifugine is a plausible antimalarial lead for the search for new analogss but also that it possesses unique antimalarial mechanisms. We therefore selected a group of febrifugine analogs that have been stored in WRAIR CIS for years to reevaluate their potential antimalarial activities and possible toxicities. The synthesis and chemical characterization of the analogs were partially published before (2). This report summarizes the new data that we obtained from studies of the antimalarial properties of the febrifugine analogs.
(The compounds described in this report are all included in an invention disclosure [MRMC docket no. WRAIR 2002-42] and a utility patent application entitled Antimalarial Activities and Therapeutic Properties of Febrifugine Analogues [document no. 60/390,334] that have been filed with the U.S. Patent and Trademark Office on 20 June 2002 and 20 June 2003, respectively, by S. Jiang, T. H. Hudson, and W. K. Milhous.)
MATERIALS AND METHODS
Febrifugine isolation.
The dry roots of D. febrifuga were purchased from a herbal store (Kwok Shing Trading Co., Inc., New York, N.Y.). A modified method (19) was used for the extraction of febrifugine. Briefly, 5 kg of the dry roots was ground, followed by maceration in 14 liters of methanol at room temperature for a week. After filtration, the solvent was evaporated to give approximately 153 g of crude methanol extract, which was then suspended in 130 ml of 0.1 M HCl. The HCl suspension was partitioned with 400 ml of CHCl3 three times, and the aqueous HCl portion collected was adjusted to pH 9.5 with NaOH, followed by CHCl3 extraction and evaporation to obtain 12 g of alkaloids. The alkaloidal portion was separated by chromatography on a silica gel 60 column (70 to 230 mesh; Merck), and the febrifugines were eluted with CHCl3 and methanol in proportions of 6:1 and 4:1.
Confirmation of isolated febrifugine.
The isolated febrifugine was analyzed by nuclear magnetic spectrum (NMR) analysis in an Hitachi R-3000 spectrometer, and chemical shifts were recorded in δ units. The 1H-NMR spectrum of febrifugine indicated that the purity was >99%. The NMR signal patterns, chemical shifts, mass spectra, melting point, and optical rotation of the isolated febrifugine fully agreed with previously reported values (14, 31). Elemental analysis of C, H, and N also gave satisfactory results.
Parasite culture.
The chloroquine-sensitive D6 strain and the chloroquine-resistant W2 strain of P. falciparum were cultivated in RPMI 1640 medium with 6% human erythrocytes supplemented with 10% human serum (33). The parasites were cultured in an atmosphere of 5% CO2, 5% O2, and 90% N2 at 37°C.
In vitro drug susceptibility assay.
Febrifugine and its analogs were tested in a cell-based in vitro drug susceptibility assay to determine if they were capable of interrupting Plasmodium cell metabolism and growth. A semiautomated microdilution technique (3) was used to assess the sensitivities of the parasites to the compounds selected. The incorporation of [3H]hypoxanthine into the parasites was measured as a function of the compound concentration to determine 50% inhibitory concentrations (IC50s).
In vitro toxicity assay.
The compounds selected were tested for their in vitro toxicities for two mammalian cell lines as described previously (9, 30).
In vivo efficacy test.
In conducting the research described in this report, the investigators adhered to the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council (21A), in accordance with the stipulations mandated for a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. A modified Thompson test was conducted for in vivo efficacy determination. Typically, 4- to 5-week-old ICR mice were housed in plastic cages with free access to water and food. P. berghei parasite-infected erythrocytes were obtained from donor mice. On experiment day 0, the donor mice were anesthetized and exsanguinated by cardiac puncture. The pooled blood from the donor mice was then diluted with normal mouse serum so that the concentration was 106 P. berghei-infected erythrocytes per inoculum (0.1 ml). The groups of experimental and control mice were inoculated with this parasitized blood on day 0. The tested mice were treated orally or subcutaneously once a day for 8 days from day 3 to day 10 with either candidate antimalarial drugs or vehicle alone, which served as a negative control. Each experimental group received a different dose, with up to five different doses of each compound tested. Blood for blood film analysis and body weights were obtained on the third and sixth days postinfection and then at weekly intervals through day 60. Films were Giemsa stained and examined microscopically to determine the levels of parasitemia. All mice were observed twice a day to assess their clinical signs, which were recorded. All treated mice with a negative smear on day 60 were considered cured.
Reagents.
Febrifugine standards were kindly provided by Yoshiteru Oshima, Tohoku University, Sendai, Japan; and halofuginone was kindly provided by Neal Farber, Collgard Biopharmaceuticals, Newton, Mass. Methanol, ethyl acetate, petroleum ether, and chloroform were obtained from Fisher Chemical Co. All other chemicals were obtained from Sigma Chemical Co., St. Louis, Mo. All cell culture media and supplement reagents were obtained from Gibco, Grand Island, N.Y. The [3H]hypoxanthine used for the in vitro drug susceptibility assay was purchased from Amersham, Piscataway, N.J.
RESULTS
A piperidinyl(acetonyl)quinazoline-based database search through WRAIR CIS identified 133 analogs, 35 of which were assayed to determine their in vitro antimalarial efficacies. Ten analogs possessed very potent antimalarial activities (Table 1) that have not been reported before. WR237645 (halofuginone), however, was the most active febrifugine analog against the parasites and had an IC50 similar to that reported previously (4). The IC50s of WR222048, WR139672, and WR092103 were less than 5 ng/ml for parasite strains W2 and D6, while the IC50s of WR221232, WR140085, WR090212, WR146115, and WR059421 ranged from 10 to 30 ng/ml (Table 1). WR088442 had the lowest potency, with an IC50 of about 200 ng/ml. Moreover, febrifugine analogs were slightly more active against chloroquine-sensitive strain D6 than chloroquine-resistance strain W2, implying that no substantial resistance to these compounds existed in the parasites.
TABLE 1.
In vitro antimalarial efficacies and cytotoxicities of febrifugine analogs
| Drugs | IC50 (ng/ml)a
|
Selectivity index
|
||||
|---|---|---|---|---|---|---|
| P. falciparum (W2) | P. falciparum (D6) | Neuronal (NG108) cells | Macrophages (J774) cells | NG108, W2 | J744, W2 | |
| Febrifugine | 0.53 ± 0.19 | 0.34 ± 0.08 | 63.5 ± 1.86 | 81 ± 2.13 | 119.81 | 152.83 |
| Halofuginone | 0.145 ± 0.02 | 0.12 ± 0.02 | 177.06 ± 52.5 | 132.25 ± 5.23 | 1,221.12 | 912.05 |
| WR222048 | 0.98 ± 0.26 | 0.82 ± 0.24 | 878.59 ± 198.49 | 498.84 ± 337.32 | 898.81 | 510.33 |
| WR139672 | 1.46 ± 0.27 | 1.23 ± 1.10 | 1,157.36 ± 118.38 | 392.16 ± 72.3 | 791.35 | 268.14 |
| WR092103 | 2.93 ± 0.34 | 2.38 ± 0.05 | 6,864.29 ± 977.14 | 3,605.13 ± 668.51 | 2,346.77 | 1,232.52 |
| WR221232 | 11.54 ± 3.94 | 9.45 ± 1.45 | 7,996.88 ± 1,879.21 | 612.95 ± 310.58 | 692.82 | 53.10 |
| WR140085 | 15.07 ± 2.61 | 12.94 ± 3.03 | 15,479.39 ± 4,184.15 | 425.46 ± 65.26 | 1,027.00 | 28.23 |
| WR090212 | 23.38 ± 1.81 | 18.95 ± 1.65 | 24,820.7 ± 2,193.24 | 2,973.31 ± 490.08 | 1,061.62 | 127.17 |
| WR146115 | 28.73 ± 6.41 | 21.22 ± 2.45 | 67,249.2 ± 15,897.91 | 6,110.63 ± 4,531.51 | 2,340.53 | 212.67 |
| WR059421 | 23.67 ± 2.3 | 17.44 ± 2.82 | 8,933.3 ± 3,186.45 | 492.12 ± 362.21 | 377.37 | 20.79 |
| WR088442 | 245.18 ± 32.77 | 192.8 ± 12.58 | 35,081.2 ± 12,748 | 7,090.2 ± 2,838.04 | 143.09 | 28.92 |
Values are means ± standard deviations. All data were collected from three independent experiments, with four replicates in each experiment.
Cytotoxicity assays with mammalian neuronal cell line NG108 and macrophage cell line J774 showed that these analogs were not toxic for the targeted host cell lines until their concentrations were about 100-fold higher than the effective antimalarial doses (Table 1). The neuronal cells appeared to be less sensitive to the analogs, with selectivity indices ranging from 120 to 2,346, while the macrophages were much more sensitive to the analogs, with selectivity indices ranging from 21 to 1,232. Febrifugine and halofuginone did not show remarkable specificities for the neuronal or macrophage cell lines. WR222048, WR139672, and WR092103 were less toxic than febrifugine. Interestingly, the macrophages were much more vulnerable than the neuronal cells to WR221232, WR140085, WR090212, WR146115, and WR059421, indicating that these five analogs specifically targeted the macrophages.
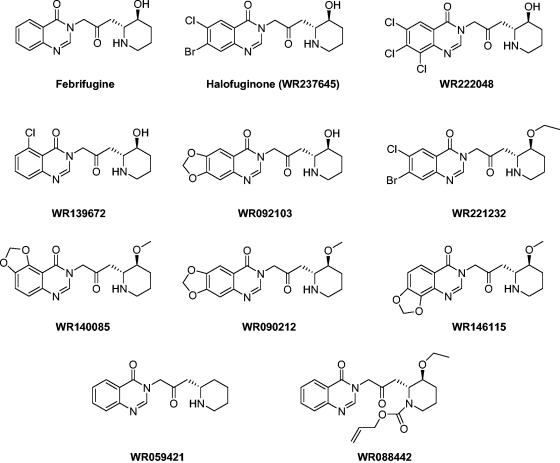
The chemical structures of febrifugine, halofuginone, and the other analogs are shown in Fig. 1. Analysis of the structure-activity relationships of these analogs indicated that the addition of chloride and bromide to positions 5, 6, 7, and 8 of the quinazoline ring not only kept the antimalarial efficacies of halofuginone, WR222048, and WR139672 unchanged but also lowered the cytotoxicity for the host cells, which resulted in a 10-fold increase in the selectivity indices compared to that for febrifugine. However, a modification on the piperidine ring of febrifugine apparently altered its antimalarial properties. The addition of a methyl or an ethyl group to the hydroxyl group of the piperidine ring, as in WR221232, WR140085, WR090212, and WR146115, or removal of the hydroxyl group, as in WR059421, caused more than 20-fold decreases in the antimalarial activities of the compounds compared to that of febrifugine. The decreased activity was more explicit when both the nitrogen atom and the hydroxyl group of the piperidine ring were modified, as shown for WR088442. A similar phenomenon was also found for the other 25 analogs that were screened but that are not listed in Table 1 due to their weak or undetectable antimalarial activities. Moreover, the replacement of the piperidine ring with other groups or modification of the acetonyl group connecting the quinazoline and piperidine rings resulted in the complete loss of antimalarial activity, as learned from the other 25 analogs. Analysis of the structure-activity relationships of the febrifugine analogs tested further supports the proposition that the nitrogen atom and the hydroxyl group of the piperidine ring and the 4-quinazolinone moiety of febrifugine are necessary for antimalarial activity (11, 31).
FIG. 1.
Molecular structures of 10 febrifugine analogs selected from the WRAIR CIS.
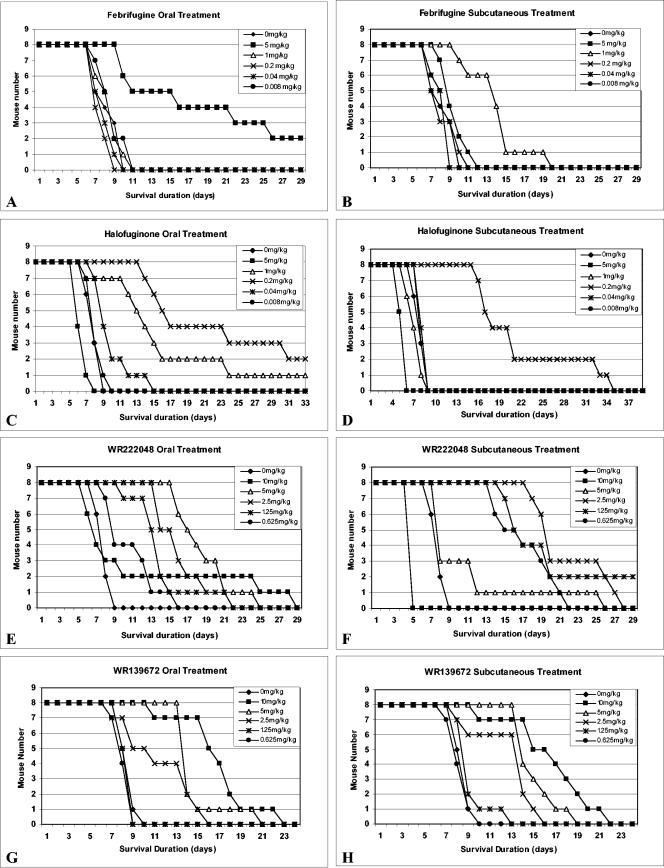
Febrifugine and the three most potent analogs, including halofuginone, WR222048, and WR139672, were selected for assessment of their in vivo efficacies and toxicities in P. berghei-infected ICR mice. As shown in Fig. 2, all four compounds clearly extended the survival times of the parasite-infected mice. Oral treatment with 5 mg of febrifugine per kg of body weight partially cured the parasite-infected mice, and two mice in this dose group survived for 60 days after the treatment (Fig. 2A). Subcutaneous treatment with febrifugine failed to cure the infected mice in all dose groups, although the infected mice treated with 1 mg of febrifugine per kg survived up to 20 days after infection, while all other mice, including those in the control group, died before day 11 (Fig. 2B). Subcutaneous administration of 5 mg of febrifugine per kg was toxic to the mice.
FIG. 2.
Effects of oral and subcutaneous treatments with febrifugine, halofuginone, WR222048, and WR139672 in P. berghei-infected mice.
Halofuginone was 10 times more efficacious against P. berghei than febrifugine. Oral treatment with halofuginone at doses of 0.2 and 1 mg/kg had an apparent curative effect for the infected mice (Fig. 2C). The subcutaneous administration of 0.2 mg of halofuginone per kg likewise extended the survival times of the infected mice, but none of the mice was cured (Fig. 2D). The mice in the 5-mg/kg dose groups died before the completion of treatment with the drug either orally or subcutaneously, indicating that halofuginone is lethal to mice at doses equal to and greater than 5 mg/kg. Subcutaneous treatment with halofuginone appeared to be more toxic to mice than oral treatment, because most of the mice treated with 1 mg of halofuginone per kg subcutaneously died before the completion of the 8-day treatment (Fig. 2D).
Unlike other febrifugine analogs, subcutaneous treatment with WR222048 appeared to be more potent against P. berghei than oral treatment. Two mice in the group treated with WR222048 at 1.25 mg/kg subcutaneously were cured (Fig. 2F), while the other doses also extended the mouse survival times. However, subcutaneous administration of 10 mg of WR222048 per kg killed all mice tested, and oral treatment with 10 mg of WR222048 per kg caused the death of more than 50% of the mice tested before completion of the treatment, suggesting that the lethal dose of WR222048 for mice is about 10 mg/kg.
Compound WR139672 had no curative effect for the infected mice at doses up to 10 mg/kg, but it apparently extended the survival times of the infected mice at doses ranging from 2.5 to 10 mg/kg (Fig. 2G and H). No apparent difference between oral and subcutaneous treatments with WR139672 was found in terms of antimalarial efficacy and host toxicity. In this study this compound did not show lethal toxicity for mice, even at a dose up to 10 mg/kg.
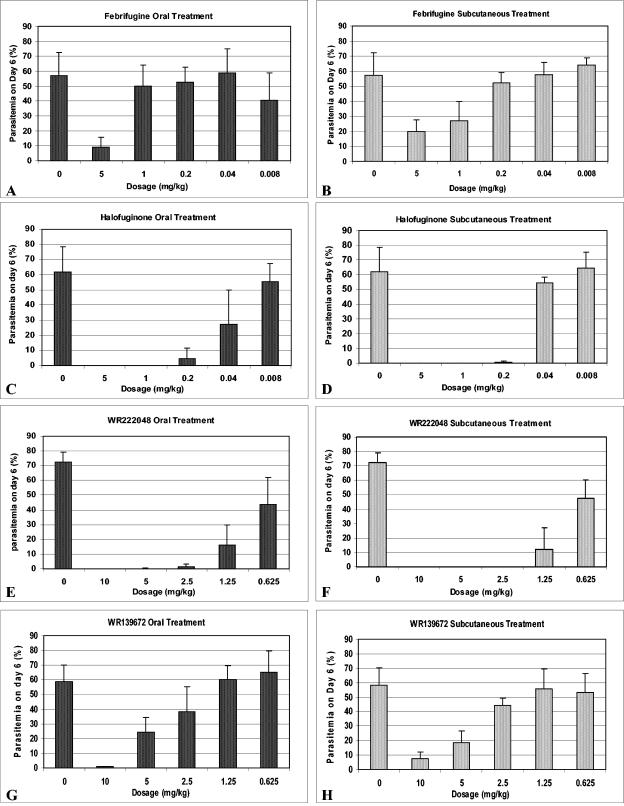
The level of parasitemia of the infected mice reached an average of 1.2% on day 3 after parasite inoculation. On day 6, after 3 days of oral or subcutaneous treatment with febrifugine at a dose of 5 mg/kg, the parasitemia was controlled to levels below 20%, while doses below 5 mg/kg did not noticeably inhibit the proliferation of the parasites (Fig. 3A and B). Oral treatment with 1 mg of halofuginone per kg cleared the blood-stage parasites from the infected mice (Fig. 3C). Subcutaneous treatment with halofuginone at a dose of 0.2 mg/kg inhibited parasite growth, with parasitemia controlled down to a level of 0.2% (Fig. 3D). WR222048 displayed in vivo antimalarial functions different from those displayed by febrifugine and halofuginone. Subcutaneous treatment with WR222048 was more active against parasite growth than oral treatment, as shown in Fig. 3E and F. A dose of 2.5 mg of WR222048 per kg completely blocked the proliferation of erythrocyte-stage parasites, while a dose of 1.25 mg/kg decreased the level of parasitemia down to 10%. WR139672 had an effect similar to that of febrifugine, but a higher dosage (up to 10 mg/kg) was required to block the growth of P. berghei in infected mice (Fig. 3G and H).
FIG. 3.
Parasitemia profiles of P. berghei-infected mice after treatment with febrifugine analogs once per day for 3 days. The error bar on each histogram represents the standard deviation.
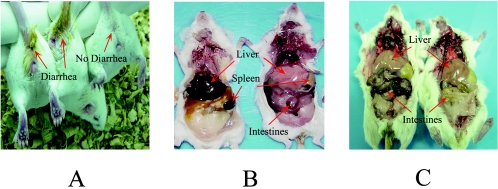
The side effects of febrifugine and its analogs detected in the mice mainly included diarrhea and gastrointestinal hemorrhage when they were overdosed with these drugs. As shown in Fig. 4, mice treated orally with toxic doses of halofuginone displayed watery defecation. Necropsy analysis showed symptomatic differences between the mice killed by malarial parasites and mice killed as a result of the toxic effects of the drugs tested. The livers and spleens of Plasmodium-killed mice were apparently enlarged and had a dark purple color, indicating the heavy growth of the parasites (Fig. 4B, left mouse), while the livers and spleens of the mice killed by lethal doses of febrifugine remained normal in size and had a pale pink color, but their intestines were apparently subjected to hemorrhage and serious injury (Fig. 4B, right mouse). The toxic effects of the oral and subcutaneous treatments were also different. Oral treatment with halofuginone at toxic doses caused severe gastrointestinal lesions and hemorrhage (Fig. 4C, left mouse), but the subcutaneous treatment did not induce the gastrointestinal tract injury (Fig. 4C, right mouse).
FIG. 4.
Necropsy observations for effects of parasite infection and side effects from febrifugine and halofuginone treatments in test mice. (A) Mice treated with toxic doses of halofuginone developed diarrhea, as indicated by the arrows; (B) different lethal effects caused by malarial parasites (left) and toxic dose of febrifugine (right); (C) gastrointestinal tract injury in a mouse due to the oral administration of 5 mg of halofuginone per kg (left) compared with that in a mouse due to subcutaneous treatment (right).
DISCUSSION
Febrifugine has been identified as a natural antimalarial compound for more than a half century. Reevaluation of the properties of febrifugine by advanced biomedical research approaches has brought a number of new discoveries since the 1990s. Our study of the febrifugine analogs has indicated that the mechanisms of antimalarial action of febrifugine appear to be unique. Febrifugine is an excellent compound that can be used to block the proliferation of malarial parasites. However, we found that febrifugine failed to stop the recrudescence of the parasites, which was the main cause of death of the infected mice after the discontinuation of drug treatment. This phenomenon is also the major reason that febrifugine was excluded from the list of active antimalarials in the past. Nevertheless, we have found that the mice that were cured in the in vivo tests sustained and cleared the recrudescent parasites without any subsequent treatment. The mice experienced heavy loads of recrudescent parasites and appeared to be very sick about 10 days after the treatment stopped. A week later, however, they mysteriously eliminated the parasites from their bodies and completely recovered from the sickness. We are absolutely clueless about how the mice survived the recrudescence and recovered from the illness. It is likely that febrifugine analogs and their metabolites may stay in the mice and remain potent against P. berghei for a much longer time. It could also be possible that the innate immunity of the tested mice was enhanced by infection with the parasites and thereafter enabled the recrudescent malarial parasites to be eradicated from the mice.
It has been well documented that febrifugine causes emetic effects and gastrointestinal tract irritation. Because mice do not possess a vomiting reflex, the emetic effects could not be observed in tests with mice. However, the mice treated subcutaneously in our tests did not display apparent diarrhea or gastrointestinal tract hemorrhage, similar to data reported previously (6). This phenomenon implies that the diarrhea and intestinal hemorrhage caused by febrifugine analogs are acquired merely through contact with the host gastrointestinal tract. Hence, the development of febrifugine analogs for injection may be a plausible approach not only for the elimination of side effects but also for the treatment of patients with severe malaria. To better understand the antimalarial functions and side effects of febrifugine, efficacy and toxicity data should be obtained in studies with primate models. Moreover, febrifugine pharmacokinetic and pharmacodynamic measurements may also provide information about the febrifugine metabolites and their durations in the host, which have never been studied before. Finally, novel derivatives need to be synthesized by modification of febrifugine to obtain better leads with greater therapeutic indices.
Halofuginone (WR237645), originally synthesized in the late 1960s (28) as a potential antimalarial agent, is a Food and Drug Administration-approved feed additive that has been used as an anticoccidiosis agent in the poultry industry for more than 20 years. Recently, halofuginone has been identified as a specific inhibitor of collagen type I gene expression (5, 25). The rediscovery of halofuginone has led to intensive preclinical studies and rapid development of the drug for the control of many diseases involved in excessive collagen synthesis, such as pulmonary fibrosis (21), liver cirrhosis (24), peritendinous fibrous adhesions after surgery (22), wound repair (1), and injury-induced arterial intimal hyperplasia (18). A phase I clinical trial of halofuginone for the treatment of scleroderma is under way, and a phase II clinical trial of Tempostatin (halofuginone hydrobromide) for the treatment of recurring bladder carcinoma has just started in the United Kingdom. The National Cancer Institute selected halofuginone for a rapid development program for cancer therapy (26). Our new data on the aspects of the antimalarial properties of halofuginone in vivo and in vitro strongly suggest that it should be selected for further development as a novel antimalarial treatment. Halofuginone is soluble in the aqueous phase, which allows the drug to be delivered in physiological saline both orally and intravenously for the treatment of severe malaria. In addition, the information already obtained from clinical studies on other diseases, mechanism-of-action studies on collagen type I gene inhibition, and toxicity studies with animal models will greatly facilitate the process of development of halofuginone as a new antimalarial drug in the future.
The WR139672 and WR222048 analogs were synthesized and first inventoried in the WRAIR CIS in 1967 and 1975, respectively. Their chemical structures considerably resemble that of their parental compound, febrifugine. WR222048 has three chlorides at positions 6, 7, and 8 of the quinazoline moiety, while WR139672 has only one chloride, at position 5 of the quinazoline moiety. Therefore, both compounds retain potent antimalarial activities and share mechanisms of action in vivo similar to those of febrifugine and halofuginone. In order to thoroughly understand the modes of action and the properties of WR139672 and WR222048, it would be worthwhile to conduct advanced lead optimization and validation for the compounds.
WR092103, WR140085, WR090212, and WR146115 are a group of methylenedioxy analogs of febrifugine synthesized and inventoried in the WRAIR CIS in 1967. These compounds were found to be very active against P. berghei and less toxic in vivo than their parent compound (2). Our data showed that these analogs are indeed able to inhibit the growth of the parasites in vitro and are also less toxic to host neuronal cells. However, the macrophage cells were very sensitive to these compounds, with a narrow selective window, implying potential toxicity to the host. This finding indicates that methylenedioxy analogs are not suitable for further lead optimization.
Acknowledgments
We are grateful to Yoshiteru Oshima for generosity in providing us with the febrifugine standards from Tohoku University. We thank Neal Farber, Lior Zelikovich, and Liat Lomnitski from Collgard Biopharmaceuticals for providing us with halofuginone. We thank Lucia Gerena, Miriam Lopez-Sanchez, and Opawale Oluwasikemi from the Department of Parasitology, WRAIR, for help with the in vitro drug susceptibility and cytotoxicity assays. Our particular thanks are extended to Dennis S. Blunt for corrections of the syntax of the text.
REFERENCES
- 1.Abramovitch, R., H. Dafni, M. Neeman, A. Nagler, and M. Pines. 1999. Inhibition of neovascularization and tumor growth, and facilitation of wound repair, by halofuginone, an inhibitor of collagen type I synthesis. Neoplasia 1:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien, P. L., and C. C. Cheng. 1970. Structural modification of febrifugine. Some methylenedioxy analogs. J. Med. Chem. 13:867-870. [DOI] [PubMed] [Google Scholar]
- 3.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geary, T. G., A. A. Divo, and J. B. Jensen. 1983. An in vitro assay system for the identification of potential antimalarial drugs. J. Parasitol. 69:577-583. [PubMed] [Google Scholar]
- 5.Halevy, O., A. Nagler, F. Levi-Schaffer, O. Genina, and M. Pines. 1996. Inhibition of collagen type I synthesis by skin fibroblasts of graft versus host disease and scleroderma patients: effect of halofuginone. Biochem. Pharmacol. 52:1057-1063. [DOI] [PubMed] [Google Scholar]
- 6.Hirai, S., H. Kikuchi, H. Kim, K. Begum, Y. Wataya, H. Tasaka, Y. Miyazawa, K. Yamamoto, and Y. Oshima. 2003. Metabolites of febrifugine and its synthetic analogue by mouse liver S9 and their antimalarial activity against Plasmodium malarial parasite. J. Med. Chem. 46:4351-4359. [DOI] [PubMed] [Google Scholar]
- 7.Jang, C. S., F. Y. Fu, C.Y. Wang, K.C. Huang, G. Lu, and T. C. Chou. 1946. Chang shan, a Chinese antimalarial herb. Science 103:59. [DOI] [PubMed] [Google Scholar]
- 8.Jang, C. S., F. Y. Fu, K.C. Huang, and C. Y. Wang. 1948. Pharmacology of chang shan (Dichroa febrifuga), a Chinese antimalarial herb. Nature 161:400-401. [DOI] [PubMed] [Google Scholar]
- 9.Jiang, S., S. T. Prigge, L. Wei, L., Y. Gao, T. H. Hudson, L. Gerena, J. B. Dame, and D. E. Kyle. 2001. New class of small nonpeptidyl compounds blocks Plasmodium falciparum development in vitro by inhibiting plasmepsins. Antimicrob. Agents Chemother. 45:2577-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keys, J. D. 1981. Chinese herbs. Charles E. Tuttle Company, Rutland, Vt.
- 11.Kikuchi, H., H. Tasaka, S. Hirai, Y. Takaya, Y. Iwabuchi, H. Ooi, S. Hatakeyama, H. S. Kim, Y. Wataya, and Y. Oshima. 2002. Potent antimalarial febrifugine analogues against the plasmodium malaria parasite. J. Med. Chem. 6:2563-2570. [DOI] [PubMed] [Google Scholar]
- 12.Koepfli, J. B. 1947. An alkaloid with high antimalarial activity from Dichroa febrifuga. J. Am. Chem. Soc. 69:1837. [DOI] [PubMed] [Google Scholar]
- 13.Koepfli, J. B., J. A. Brockman, Jr., and J. Moffat. 1950. Structure of febrifugine and isofebrifugine. J. Am. Chem. Soc. 72:3323. [Google Scholar]
- 14.Koepfli, J. B., J. F. Mead, and J. A. Brockman, Jr. 1948. Alkaloids of Dichroa febrifuga. I. Isolation and degradative studies. J. Am. Chem. Soc. 70:1048-1054. [DOI] [PubMed] [Google Scholar]
- 15.Kuehl, K. A., Jr., C. F. Spencer, and K. Folkers. 1948. Alkaloids of Dichroa febrifuga Lour. J. Am. Chem Soc. 70:2091-2093. [DOI] [PubMed] [Google Scholar]
- 16.Lei, S. H., and G. Bodeker. 2003. Changshan (Dechroa febrifuga)—ancient febrifuge and modern antimalarial: lessons for research from a forgotten tale. .In M. Willcox, G. Bodeker, and P. Rasaoanaivo (ed.), Traditional medicinal plants and malaria. Harwood Press, Taylor & Francis Publishing, London, United Kingdom.
- 17.Li, S. 1593. Ben Cao Gang Mu (Systema Materia Medica). [Reprint, China Archive Press, Beijing, China, 1999.]
- 18.Liu, K., S. Sekine, Y. Goto, K. Iijima, I. Yamagishi, K. Kondon, M. Matsukawa, and T. Abe. 1998. Halofuginone inhibits neointimal formation of cultured rat aorta in a concentration-dependent fashion in vitro. Heart Vessels 13:18-23.9923561 [Google Scholar]
- 19.Murata, K., F. Takano, S. Fushiya, and Y. Oshima. 1998. Enhancement of NO production in activated macrophages in vivo by an antimalarial crude drug, Dichroa febrifuga. J. Nat. Prod. 61:729-733. [DOI] [PubMed] [Google Scholar]
- 20.Murata, K., F. Takano, S. Fushiya, and Y. Oshima. 1999. Potentiation by febrifugine of host defense in mice against Plasmodium berghei NK65. Biochem. Pharmacol. 58:1593-1601. [DOI] [PubMed] [Google Scholar]
- 21.Nagler, A., N. Firman, R. Feferman, S. Cotev, M. Pines, and S. Shoshan. 1996. Reduction in pulmonary fibrosis in vivo by halofuginone. Am. J. Respir. Crit. Care Med. 154:1082-1086. [DOI] [PubMed] [Google Scholar]
- 21a.National Research Council, Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 22.Nyska, M., A. Nyska, E. Rivlin, S. Porat, M. Pines, S. Shoshan, and A. Nagler. 1996. Topically applied halofuginone, an inhibitor of collagen type I transcription, reduces peritendinous fibrous adhesions following surgery. Connect. Tissue Res. 34:97-103. [DOI] [PubMed] [Google Scholar]
- 23.Olliaro, P. 2001. Mode of action and mechanisms of resistance for antimalarial drugs. Pharmacol. Ther. 89:207-219. [DOI] [PubMed] [Google Scholar]
- 24.Pines, M., V. Knopov, O. Genina, I. Lavelin, and A. Nagler. 1997. Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J. Hepatol. 27:391-398. [DOI] [PubMed] [Google Scholar]
- 25.Pines, M., and A. Nagler. 1998. Halofuginone: a novel antifibrotic therapy. Gen. Pharmacol. 30:445-450. [DOI] [PubMed] [Google Scholar]
- 26.Pines, M. I., Vlodavsky, and A. Nagler. 2000. Halofuginone: from veterinary use to human therapy. Drug Dev. Res. 50:371-378. [Google Scholar]
- 27.Ridley, R. G. 1999. Planting the seeds of new antimalarial drugs. Science 285:1502-1503. [DOI] [PubMed] [Google Scholar]
- 28.Ryley, J. F., and M. J. Betts. 1973. Chemotherapy of chicken coccidiosis. Adv. Pharmacol. Chemother. 10:221-293. [DOI] [PubMed] [Google Scholar]
- 29.Siegfried, J. 1986. Quinazoline alkaloids. The Alkaloides. 29:99-140. [Google Scholar]
- 30.Skehan, P., R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, and M. R. Boyd. 1990. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82:1107-1112. [DOI] [PubMed] [Google Scholar]
- 31.Takaya, Y., H. Tasaka, T. Chiba, K. Uwai, M. Tanitsu, H. S. Kim, Y. Wataya, M. Miura, M. Takeshita, and Y. Oshima. 1999. New type of febrifugine analogues, bearing a quinolizidine moiety, show potent antimalarial activity against Plasmodium malaria parasite. J. Med. Chem. 42:3163-3166. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi, Y., M. Hattori, H. Abe, and T. Harayama. 1999. Synthesis of d/l-febrifugine and d/l-isofebrifugine. Synthesis 10:1814-1818. [Google Scholar]
- 33.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:674-675. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2001. Roll back malaria. Malaria at a glance. World Health Organization, Geneva, Switzerland.