Abstract
Background
The antidepressant effect of physical exercise has been reported in several clinical and animal studies. Since serotonin, norepinephrine, and dopamine play a central role in depression, it is possible that the beneficial effects of physical exercise are mediated via monoamine pathways. This study investigates the effects of voluntary wheel running on the excitability of monoamine neurons.
Materials and Methods
Male Sprague-Dawley rats were used in the study. Voluntary wheel running (VWR) rats were housed in individual cages with free access to a running wheel, while control animals were housed in standard laboratory cages. After three weeks, the rats were anesthetized, and in vivo electrophysiological recordings were taken from dorsal raphe nucleus serotonin neurons, locus coeruleus norepinephrine neurons, and ventral tegmental dopamine neurons.
Results
VWR stimulated activity in serotonin, but not in norepinephrine or dopamine neurons. Subsequently, acute administration of the selective serotonin reuptake inhibitor escitalopram in control rats led to complete suppression of serotonin neurons; this suppression was reversed by subsequent administration of selective antagonist of serotonin-1A receptors, WAY100135. Escitalopram induced only partial inhibition of serotonin neurons in the VWR rats while WAY100135 increased the firing activity of serotonin neurons above the baseline value.
Conclusions
The beneficial effect of physical exercise on mood is mediated, at least in part, via activation of serotonin neurons. Physical exercise can potentiate the response to selective serotonin reuptake inhibitors by increasing the basal firing activity and diminishing selective serotonin reuptake inhibitor-induced inhibition of serotonin neurons.
Keywords: Voluntary wheel running (VWR) rats, serotonin (5-HT), norepinephrine, dopamine, selective serotonin reuptake inhibitors (SSRIs)
Significance Statement
The aim of our study is to test the hypothesis that voluntary wheel running (VWR) in rats modifies the excitability of monoamine-secreting neurons. To the best of our knowledge, this is the first study to directly examine the effect of physical exercise, alone or in combination with an antidepressant drug, on brain monoamine-secreting neurons in vivo. We found that (1) VWR led to selective tonic activation of serotonin (5-HT) neurons; and (2) the inhibitory effect of acute administration of escitalopram on 5-HT neuronal firing activity in VWR rats was less pronounced than in controls. Our conclusions are that antidepressant-like effect of physical exercise is mediated, at least in part, by stimulation of 5-HT neurons, and regular physical exercise can potentiate and facilitate the response to antidepressant drugs in patients with depression.
Introduction
The beneficial effect of physical exercise on mood has been accepted since antiquity: mens sana in corpore sano (Satire X of the Roman poet Juvenal, II AD) and recent studies have demonstrated that regular physical exercise reduces the risk of developing Alzheimer and Parkinson diseases (Paillard et al., 2015). Additional benefits include the stimulation of memory and cognitive functioning (Ikudome et al., 2016), decreased anxiety, and improved emotional wellbeing (McMahon et al., 2016). The beneficial effect of physical exercise in depression has been investigated in several clinical studies as well and summarized in the recent review by Kvam et al. (2016). Seven open-label studies (Martinsen et al., 1985; Doyne et al., 1987; Veale et al., 1992; Singh et al., 1997; Pinchasov et al., 2000; Pilu et al., 2007; Hemat-Far et al., 2012) and 4 blind studies (Dunn et al., 2005; Singh et al., 2005; Blumenthal et al., 2012a; Salehi et al., 2014) demonstrated significantly decreased depressive symptoms in patients involved in physical exercise compared with sedentary controls. Furthermore, the efficacy of physical exercise was comparable with that of psychotherapy (Gary et al., 2010) and pharmacotherapy (Blumenthal et al., 2012a, 2012b).
Animal models of physical exercise, such as voluntary wheel running (VWR) in rats, are essential in investigating neural mechanisms mediating enhanced mood effects from physical exercise. There is evidence that VWR in rats has anxiolytic and antidepressant effects that mimic the antidepressant effects of physical exercise observed in human studies. A recent study demonstrated that VWR rats had reduced immobility during the forced swim test compared with sedentary controls (Chen et al., 2016). Earlier studies also reported that VWR diminished stress-induced depressive-like behavior in rats; including the immobility in the forced swim test, avoidance in the elevated T-maze test, and latency in the shuttle box test (Greenwood et al., 2003; Lapmanee et al., 2012).
Observations on central neurotransmission suggest involvement of glutamate, serotonin (5-HT), norepinephrine (NE), and dopamine (DA) systems in the behavioral effects induced by the VWR. Greenwood et al. (2003) found that VWR activity in rats reversed stress-induced suppression of c-fos gene expression and increased serotonin-1A (5-HT1A) receptor expression in the dorsal raphe nucleus (DRN), and a more recent study highlighted increased extracellular 5-HT and NE levels in the hippocampus of VWR rats (Wang et al., 2013).
More recently, Chen et al. (2016) reported increased cortical DA levels in VWR rats. It was also found that intra-cortical injection of the glucocorticoid receptor antagonist RU486 diminished the stimulatory effect of VWR on DA levels, and the same administration of the D2 receptor antagonist haloperidol completely suppressed the antidepressant-like effect of VWR. Other studies reported increased expression of genes encoding selected subunits of the main ionotropic glutamate receptors in the ventral tegmental area (VTA) of VWR rats (Makatsori et al., 2003; Schwendt et al., 2003). It is therefore possible that physical exercise alters the effect of glutamate and stress hormones on DA neurotransmission.
While the above-mentioned results support the crucial role of 5-HT, NE, and DA neurotransmission in the behavioral effect of physical exercise, the effect of exercise on the excitability of monoaminergic neurons has not been directly examined. Therefore, this study employed in vivo single-unit extracellular electrophysiology to investigate the impact of rat VWR on the firing activity of DRN 5-HT neurons, locus coeruleus (LC) NE neurons, and VTA DA neurons. We also assessed the influence of the selective serotonin reuptake inhibitor (SSRI) escitalopram on the firing activity of 5-HT neurons in VWR and control rats.
Materials and Methods
Animals
Adult male Sprague-Dawley rats weighting 200 to 250 g at the beginning of the experiments were ordered from Velaz s.r.o. The animals were housed under standard laboratory conditions with free access to food and water. A constant light-dark cycle was maintained with light on at 6:00 am and off at 6:00 pm. Temperature was maintained at 23±2°C and humidity at 60±5%. All experimental procedures were approved by the Animal Health and Animal Welfare Division of the State Veterinary and Food Administration of the Slovak Republic (permission no Ro 331/16–221) and they conformed to Directive 2010/63/EU on the protection of animals used for scientific purposes.
VWR
The animals were randomly assigned to the control and VWR groups, with VWR method as previously described (Makatsori et al., 2003; Ondkova et al., 2010). While control rats were housed in pairs in standard laboratory cages, VWR rats were housed for 3 weeks in individual 35-×20-×15-cm plastic cages with free access to the stainless-steel activity wheel (35-cm diameter, Techniplast Gazzada). VWR rat running time, distance covered, and average speed were monitored daily, using the U4 Digital Cycling Computers (Echowell, Taipei, Taiwan).
Twenty-eight control and 24 VWR rats were used in this study. Of 28 control rats, 7, 6, and 7 were used for the assessment of spontaneous firing activity of 5-HT, NE, and DA neurons, respectively; 8 were used to assess the effect of escitalopram on the excitability of 5-HT neurons. Of 24 control rats, 5, 5, and 6 were used for the assessment of spontaneous firing activity of 5-HT, NE, and DA neurons, respectively; 5 were used to assess the effect of escitalopram on the excitability of 5-HT neurons.
Electrophysiology
In vivo electrophysiological experiments were performed as described in our previous publications (Dremencov et al., 2007a, 2007b, 2009). Animals were anesthetized by chloral hydrate (400 mg/kg, i.p.) and mounted in a stereotaxic frame (David Kopf Instruments). Rat body temperature was maintained between 36°C and 37°C with a heating pad (Gaymor Instruments). Catheters were inserted to the lateral tail vein for later injection of escitalopram and WAY100135.
The scalp was then opened and a 3-mm hole drilled in the skull for insertion of electrodes into the DRN (7.8–8.3 mm posterior to the bregma and 4.5–7.0 mm ventral to the brain surface), LC (8.0–8.3 mm posterior to the bregma, 1.2–1.4 mm lateral to the midline, and 5.5–7.5 mm ventral to the brain surface), and VTA (4.5–5.5 mm posterior to the bregma, 0.6–0.8 mm lateral to the midline, and 7.0–8.5 mm ventral to the brain surface) as shown in Paxinos and Watson (2007). Since the electrode insertion into one brain area can affect the excitability of neurons in other brain areas, the firing activities of 5-HT neurons of the DRN, NE neurons of the LC, and DA neurons of the VTA were measured in separate animals.
Glass- ipettes with a fine tip approximately 1 μm in diameter were pulled using the programmable horizontal puller (DMZ-Universal, Zeitz-Instruments) and filled with 2 M NaCl solution. Electrode impedance ranged from 4 to 6 MΩ. The pipettes were lowered into the DRN, LC, and VTA by hydraulic micro-positioner (David Kopf Instruments), and signals were amplified by HEKA EPC-10 amplifier and recorded by Patchmaster software package (HEKA Elektronik-Dr Schulze).
Serotonin, NE, and DA neurons were identified by their firing patterns (5-HT neurons: regular firing rate of 0.5–2.5 Hz and positive action potential of 0.8–1.2 ms; NE neurons: regular firing rate of 0.5–5.0 Hz, positive action potential of 0.8–1.2 ms and characteristic bursts discharges in response to a nociceptive pinch of the contralateral hind paw; DA neurons: slow irregular firing rate of 0.5–10 Hz, mixed single spike and burst firing, tri-phasic action potentials with a dominant positive component, a minor one over 2.5-millisecond duration and a “notch” often present on the initial rising phase and a minimum 1.1-millisecond duration from action potential initiation to the negative trough).
Drugs
Escitalopram was received as a gift from Lundbeck A/S, Valby, Denmark, and dissolved in saline (0.9% NaCl) at 0.1 mg/mL concentration. WAY100135 was purchased from Sigma-Aldrich s.r.o. and dissolved in saline at 0.1 mg/mL concentration. When a 5-HT neuron was identified, its basal firing activity was recorded for 2 minutes and 0.1 mg/kg escitalopram was then administered via the tail vein catheter. After 2 minutes, WAY100135 was administered i.v. in a 0.1-mg/kg dosage. The doses of escitalopram and WAY100135 were chosen according to a previous study (El Mansari et al., 2005), which reported that i.v. administration of 0.1 mg/kg of escitalopram completely inhibited 5-HT neurons in the DRN of Sprague-Dawley rats, and subsequent administration of 0.1 mg/kg WAY100135 reversed the escitalopram-induced inhibition of 5-HT neurons.
Statistical Analysis
One-way ANOVA for repeated measures (RM ANOVA), for factor time, followed by Bonferoni posthoc test, was used to compare the daily running distances and times in VWR rats.
In the experiments aiming to compare spontaneous 5-HT, NE, and DA neuronal firing activity in control and VWR rats, multiple neurons per animal were recorded. Two-tailed Student’s t test was used to compare the average spontaneous activity of 5-HT, NE, and DA neurons in control and VWR rats. In experiments aiming to compare the effect of escitalopram on 5-HT neurons in control and VWR rats, one 5-HT neuron per animal was recorded.
For the comparison of the effects of escitalopram and WAY100,135 on 5-HT neurons in control and VWR rats the averaged basal firing rates (prior to escitalopram administration) of 5-HT neurons for each group of animals (control and VWR) were considered to be 100%. The values of the firing activity of each individual neuron after escitalopram administration and after subsequent WAY100135 administration were calculated as a percentage of the average basal activity of the corresponding group. Two-way RM ANOVA was used to determine the effect of the time (baseline, after escitalopram administration, and after subsequent WAY100135 administration), group (control and WVR), and time × group interaction on the firing activity of 5-HT neurons.
Results
Running Characteristics of VWR Rats
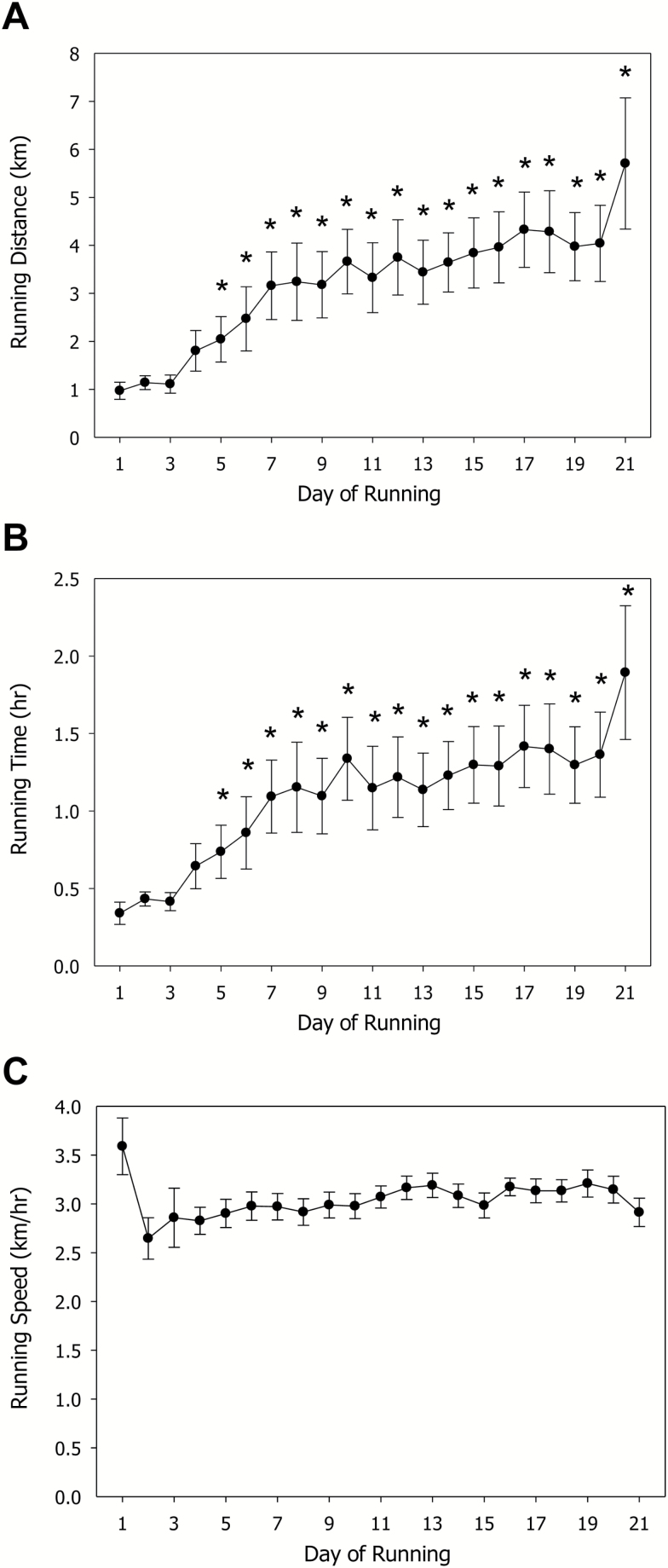
The daily running distance was 0.97±0.18km on day 1 and this gradually increased to 5.70±1.37km by day 21. One-way RM ANOVA demonstrated a significant effect of time (F=14.44, P<.001) and the Bonferoni posthoc test revealed significant (P<.001) difference between running distances on day one and days 5 to 21 (Figure 1A). Daily running time was 0.34±0.07 h on the first day with gradually increases to 1.90±0.43 h by day 21. One-way RM ANOVA demonstrated significant effect of time (F=11.69, P<.001) and the Bonferoni posthoc test revealed significant (P<.001) difference between running times on day 1 and days 5–21 (Figure 1B). Their average velocity was 3.04±0.15 km/h, and this did not change over the time course (Figure 1C).
Figure 1.
Characteristics of voluntary wheel running (VWR). (A) Daily running distances. (B) Running time. (C) Average running speed. *P<.05 in comparison with day 1 value (Bonferroni posthoc test).
Effect of the VWR on Spontaneous Activity of 5-HT, NE, and DA Neurons
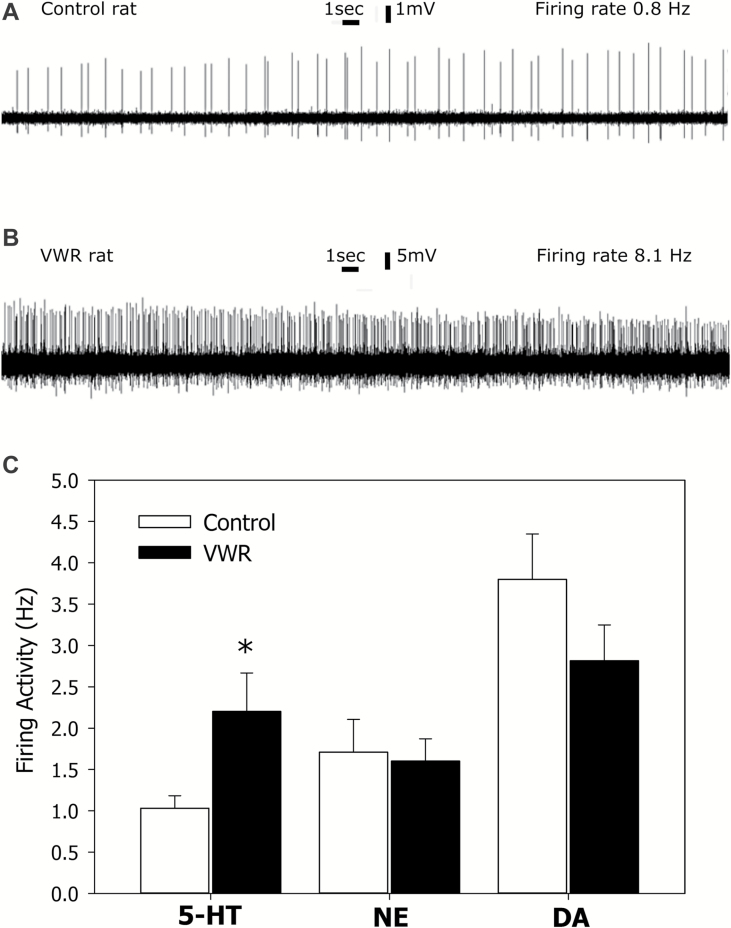
The mean firing frequencies of 5-HT, NE, and DA neurons in control rats were 1.03±0.15 (73 neurons from 7 rats), 1.71±0.40 (17 neurons from 6 rats), and 3.80±0.55 Hz (37 neurons from 7 rats), respectively. The mean firing frequencies of 5-HT, NE, and DA neurons in VWR rats were 2.20±0.43 (71 neurons from 8 rats), 1.60±0.27 (37 neurons from 5 rats), and 2.82±0.043 Hz (52 neurons from 6 rats), respectively. VWR led to tonic activation of 5-HT (214±45% of controls, P<.05, 2-tailed Student’s t test), but not in NE (P=.82) and DA (P=.16) neurons (Figure 2).
Figure 2.
The effect of voluntary wheel running (VWR) on serotonin (5-HT), norepinephrine (NE), and dopamine (DA) neuron spontaneous firing activity. (A) Representative recording from a spontaneously active 5-HT neuron from the dorsal raphe nucleus of a control rat. (B) Representative recording from a spontaneously active 5-HT neuron from the dorsal raphe nucleus of a VWR rat. (C) Average firing rates of 5-HT, NE, and DA neurons in control and VWR rats. *P<.05, 2-tailed Student’s t test. Note: Since an extracellular recording technique was used, the amplitude of the action potentials on the panels A and B does not correspond to the actual membrane potential of the neurons.
Effect of Acute Escitalopram on 5-HT Neuronal Firing Activity in Control and VWR Rats
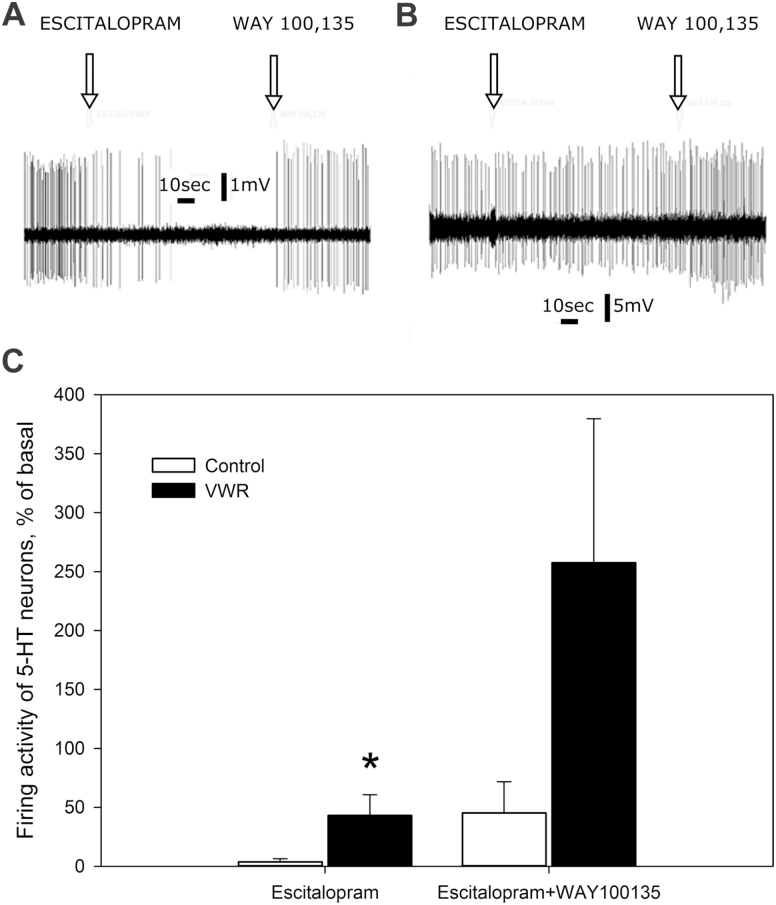
Acute 0.1 mg/kg i.v. administration of escitalopram to control rats almost completely suppressed 5-HT neurons (4±3% of baseline, 8 neurons from 8 rats) (Figure 3A, C). Subsequent administration of WAY100135 (0.1 mg.kg, i.v.) reversed this escitalopram-induced suppression to 45±26% of the baseline value. In contrast, escitalopram-induced inhibition of 5-HT neurons was less in VWR rats (43±13% of the baseline, 5 cells from 5 rats) (Figure 3), and WAY100135 induced a higher increase than in controls (258±122% of the baseline) (Figure 3B, C). Two-way RM ANOVA demonstrated the following significant main effects for time (F=4.52, P<.05); group (F=7.70, P<.05) and also time×group interaction (F=3.87, P<.05). Meanwhile, the Bonferroni posthoc test revealed significant difference in the degree of escitalopram-induced inhibition of 5-HT neurons between control and VWR rats (P<.05). Even though the Bonferroni posthoc test failed to reveal statistically significant difference between the values, the WAY100135-induced increase in 5-HT levels in VWR rats was 5.5 times greater than in controls.
Figure 3.
The effect of acute administration of escitalopram on the serotonin (5-HT) neuronal firing activity in control and voluntary wheel running (VWR) rats. (A) Effects of escitalopram and WAY 100135 on a representative 5-HT neuron from the dorsal raphe nucleus of a control rat. (B) Effects of escitalopram and WAY 100135 on a representative 5-HT neuron from the dorsal raphe nucleus of a VWR rat. (C) The effect of escitalopram on the average firing rates of 5-HT neurons in control and VWR rats, expressed as percent of baseline (mean±SEM); *P<.05 (Bonferroni posthoc test). Note: Since an extracellular recording technique was used, the amplitude of the action potential on the panels A and B does not correspond to the actual membrane potentials of the neurons.
Discussion
Rats placed in cages equipped with the exercise wheel became engaged in voluntary running behavior. Moreover, running time and distance increased daily to a plateau of 1.5 h and 4 km by day 8 to 9 of running wheel exposure. These are similar results to those in our previous studies (Makatsori et al., 2003; Bakos et al., 2007; Ondkova et al., 2010). This study demonstrates significantly greater spontaneous activity of 5-HT neurons in the DRN of VWR rats than in controls. In contrast, the firing activity of NE neurons of the LC and DA neurons of the VTA were not affected by VWR. In addition, the suppression of 5-HT neuronal activity induced by acute administration of the SSRI escitalopram was significantly diminished in VWR rats compared with controls, and the stimulatory effect of the subsequent administration of the selective agonist of 5-HT receptors WAY100135 on 5-HT neuronal firing activity in VWR rats tended to be greater than in controls.
Our results demonstrate that the average excitability of 5-HT neurons was significantly greater in VWR rats than in controls. Interestingly, an increase in hippocampal 5-HT levels in VWR rats was also observed in the in vivo microdialysis study of Wang et al. (2013). However, Greenwood et al. (2003) reported that while VWR did not activate 5-HT neurons per se, it reversed stress-induced inhibition of these neurons. The difference between our results and those of Greenwood and colleagues is likely due to the different techniques determining excitability of 5-HT neurons. Specifically, we used direct in vivo single-unit electrophysiological assessment of 5-HT neuronal firing activity in contrast to those authors’ indirect determination of 5-HT neuron activation by c-Fos gene expression measurement.
It is well established that 5-HT plays a central role in the pathophysiology and treatment of depression. Almost all known antidepressant drugs stimulate 5-HT transmission, either directly or indirectly (Dremencov, 2009; Pavlovicova et al., 2015). Accordingly, tryptophan depletion and the subsequent decrease in 5-HT bioavailability leads to the onset of the symptoms of depression (Delgado et al., 1994, 1999; Franklin et al., 2015). Since increased excitability of 5-HT neurons leads to the increased synaptic levels of 5-HT in the brain, it is possible that the anxiolytic and antidepressant effect of physical exercise, reported in previous studies in animal models (Greenwood et al., 2003; Lapmanee et al., 2012; Chen et al., 2016) and human subjects (Kvam et al., 2016) is mediated, at least in part, via increased excitability of 5-HT neurons in the DRN.
There is evidence that physical exercise in animals stimulates synaptic plasticity. Patten et al. (2013) reported that VWR enhances synaptic plasticity in the hippocampus of normal rats (Patten et al., 2013), and Bjornebekk et al. (2010) recorded the same effect in the Flinder Sensitive Line rats, a genetic animal model of depression. Since 5-HT plays a critical role in the regulation of the synaptic plasticity (Fernandez et al., 2017), it is possible that the enhancing effect of the VWR on the hippocampal plasticity is mediated, at least in part, via the stimulation of the excitability of 5-HT neurons.
While our study revealed no VWR effect on excitability of LC NE neurons or VTA DA neurons, Wang et al. (2013) reported increased levels of NE in the hippocampus, and Chen et al. (2016) reported increased levels of DA in the cortex. It is therefore likely that VWR increases brain NE and DA levels via a mechanism involving the alteration of synaptic release, reuptake, and/or metabolism of these neurotransmitters rather than attenuation of excitability of NE- and DA-secreting neurons. Consistent with this, Zangen et al. (2001) and Dremencov et al. (2004) found local administration of 5-HT to the nucleus accumbens (NAcc) increased extracellular DA levels, and Dremencov et al. (2006) have suggested the 5-HT-induced rise in NAcc DA levels is mediated via activation of 5-HT3 receptors on DA neuron terminals and does not involve stimulation of excitability of DA neurons.
We found that acute escitalopram administration almost completely inhibited 5-HT neurons in control rats and subsequent WAY100135 injection reversed this inhibition, similarly to the findings of el Mansari et al. (2005). In VWR rats, escitalopram induced only a partial inhibition of 5-HT neurons, and subsequent administration of WAY100135 increased the firing activity of 5-HT neurons above the basal values. It is therefore possible that the increased excitability of 5-HT neurons in VWR rats is the result, at least in part, of desensitization of 5-HT1A autoreceptors and/or 5-HT transporters (SERT) expressed on the cell bodies of 5-HT neurons of the DRN.
Indeed, Dey (1994) reported that chronic swimming exercise in rats leads to reduced behavioral responses to the administration of the 8-OH-DPAT, an agonist of 5-HT1A receptor. However, Greenwood et al. (2005) reported increased levels of 5-HT1A mRNA in VWR rats. A further possible mechanism underlying decreased escitalopram-induced inhibition of these neurons is decreased 5-HT1B autoreceptor and/or 5-HT transporter (SERT) expression at the nerve terminal (Greenwood et al., 2005). Since α1-adrenoceptors have a tonic stimulatory effect on 5-HT neuronal firing activity (Dremencov et al., 2007a), it is also possible that VWR-induced upregulation of α1-adrenergic receptors (Greenwood et al., 2005) contributes to increased 5-HT excitability. Interestingly, the increased stimulatory effect of WAY100135 on 5-HT neuronal firing activity observed in our study is similar to the stronger effect of the different 5-HT1A receptor antagonist, WAY100635, on the excitability of 5-HT neurons in heterozygote SERT+/- mice pretreated with escitalopram (Guiard et al., 2012). This supports the suggestion that physical exercise leads to SERT desensitization (Greenwood et al., 2005).
A limitation of the present study is single housing of VWR rats. The social isolation is a well-known stressor, and it may interfere with some but not all changes induced by the VWR (Shetty and Sadananda, 2017). It was shown that social isolation affected synaptic plasticity and leads to increased spontaneous activity of hippocampal CA1 pyramidal neurons (Gómez-Galán et al., 2016). Since in individually housed rats an antidepressant-like behavioral effect of VWR was reported in previous studies (Greenwood et al., 2003), it is not likely that increased excitability of 5-HT neurons in the DRN of the VWR rats was caused by the social isolation and not by the VWR.
We have demonstrated that VWR in rats leads to the tonic activation of mean spontaneous activity of DRN 5-HT neurons, and it is therefore likely that the increased 5-HT neuronal firing activity mediates, at least in part, the antidepressant and anxiolytic effects of physical exercise. VWR also leads to the diminished inhibitory effect of escitalopram on 5-HT neuronal firing activity. Moreover, numerous studies have reported that the inhibition of 5-HT neuronal firing after acute or subchronic administration of SSRIs is responsible, at least in part, for the delayed onset of the therapeutic effect of SSRIs and also for the lack of adequate response to SSRI treatment in some patients (Romero et al., 1996; Blier et al., 1998; Pineyro and Blier, 1999; Dremencov, 2009; Pavlovicova et al., 2015). It is therefore hypothesized that regular physical exercise can facilitate and potentiate the response to SSRIs in patients with depression. However, further studies are necessary to examine the effects of VWR, SSRIs, and their combination on anxiolytic and antidepressant-like behavior and to directly test our hypothesis.
Statement of Interest
Escitalopram was provided by Lundbeck A/S, Valby, Denmark.
Acknowledgments
This work was supported by the Slovak Academy of Sciences (SAS) Scholarship Award, by the Scientific Grant Agency of Ministry of Education of Slovak Republic and SAS (grant VEGA-2/0024/15), and partially by the Slovak Research and Development Agency (grant APVV-15-0388).
References
- Bakos J, Hlavacova N, Makatsori A, Tybitanclova K, et al. (2007) Oxytocin levels in the posterior pituitary and in the heart are modified by voluntary wheel running. Regul Pept 1–3:96–101. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. (2010) The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus 7:820–828. [DOI] [PubMed] [Google Scholar]
- Blier P, Pineyro G, el Mansari M, Bergeron R, et al. (1998) Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann NY Acad Sci 861:204–216. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, O’Connor C, Keteyian S, et al. (2012a) Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA 5:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, et al. (2012b) Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol 12:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Nakagawa S, Kitaichi Y, An Y, et al. (2016) The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology 69:1–9. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, et al. (1994) Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry 51:865–874. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, et al. (1999) Tryptophan-depletion challenge in depressed patients treated withdesipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry 46:212–220. [DOI] [PubMed] [Google Scholar]
- Dey S. (1994) Physical exercise as a novel antidepressant agent: possible role of serotonin receptor subtypes. Physiol Behav 2:323–329. [DOI] [PubMed] [Google Scholar]
- Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, et al. (1987) Running versus weight lifting in the treatment of depression. J Consult Clin Psychol 5:748–754. [DOI] [PubMed] [Google Scholar]
- Dremencov E, Gispan-Herman I, Rosenstein M, Mendelman A, et al. (2004) The serotonin-dopamine interaction is critical for fast-onset action of antidepressant treatment: in vivo studies in an animal model of depression. Prog Neuropsychopharmacol Biol Psychiatry 1:141–147. [DOI] [PubMed] [Google Scholar]
- Dremencov E, Weizmann Y, Kinor N, Gispan-Herman I, et al. (2006) Modulation of dopamine transmission by 5HT2C and 5HT3 receptors: a role in the antidepressant response. Curr Drug Targets 2:165–175. [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. (2007a) Distinct electrophysiological effects of paliperidone and risperidone on the firing activity of rat serotonin and norepinephrine neurons. Psychopharmacology (Berl) 1:63–72. [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. (2007b) Noradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brain. Biol Psychiatry 5:671–678. [DOI] [PubMed] [Google Scholar]
- Dremencov E. (2009). Aiming at new targets for the treatment of affective disorders: an introduction. Curr Drug Targets 11:1050–1051. [DOI] [PubMed] [Google Scholar]
- Dremecov E, El Mansari M, Blier P. (2009) Effects of sustained serotonin reuptake inhibiTion on the firing of dopamine neurons in the rat ventral tegmental area. J Psychiatry Neurosci 34:223–229. [PMC free article] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, et al. (2005) Exercise treatment for depression: efficacy and dose response. Am J Prev Med 1:1–8. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sanchez C, Chouvet G, Renaud B, et al. (2005) Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain. Neuropsychopharmacology 7:1269–1277. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Muzerelle A, Scotto-Lomassese S, Barik J, et al. (2017). Constitutive and acquired serotonin deficiency alters memory and hippocampal synaptic plasticity. Neuropsychopharmacology 42:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M, Hlavacova N, Babic S, Pokusa M, et al. (2015). Aldosterone signals the onset of depressive behaviour in a female rat model of depression along with SSRI treatment resistance. Neuroendocrinology 102:274–287. [DOI] [PubMed] [Google Scholar]
- Gary RA, Dunbar SB, Higgins MK, Musselman DL, et al. (2010) Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res 2:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Galán M, Femenía T, Åberg E, Graae L, et al. (2016) Running opposes the effects of social isolation on synaptic plasticity and transmission in a rat model of depression. PLoS One 11:e0165071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, et al. (2003) Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci 7:2889–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, et al. (2005) Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry 5:559–568. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Mansari ME, Murphy DL, Blier P. (2012) Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: an electrophysiological and neurochemical study. Int J Neuropsychopharmacol 3:349–361. [DOI] [PubMed] [Google Scholar]
- Hemat-Far A, Shahsavari A, Mousavi SR. (2012) Effects of selected aerobic exercises on the depression and concentrations of plasma serotonin in the depressed female students aged 18 to 25. J Appl Res 12:47–52. [Google Scholar]
- Ikudome S, Mori S, Unenaka S, Kawanishi M, et al. (2016) Effect of long-term body-mass-based resistance exercise on cognitive function in elderly people. J Appl Gerontol. doi:10.1177/0733464815625834 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kvam S, Kleppe CL, Nordhus IH, Hovland A. (2016) Exercise as a treatment for depression: a meta-analysis. J Affect Disord 202:67–86. [DOI] [PubMed] [Google Scholar]
- Lapmanee S, Charoenphandhu N, Krishnamra N, Charoenphandhu J. (2012) Anxiolytic-like actions of reboxetine, venlafaxine and endurance swimming in stressed male rats. Behav Brain Res 1:20–28. [DOI] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, et al. (2003) Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology 5:702–714. [DOI] [PubMed] [Google Scholar]
- Martinsen EW, Medhus A, Sandvik L. (1985) Effects of aerobic exercise on depression: a controlled study. Br Med J (Clin Res Ed) 291:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EM, Corcoran P, O’Regan G, Keeley H, et al. (2016) Physical activity in European adolescents and associations with anxiety, depression and well-being. Eur Child Adolesc Psychiatry [DOI] [PubMed] [Google Scholar]
- Ondkova S, Bakos J, Macejova D, Jezova D, et al. (2010) Changes in retinoic acid receptor status, 5’-deiodinase activity and neuroendocrine response to voluntary wheel running. Gen Comp Endocrinol 2:304–308. [DOI] [PubMed] [Google Scholar]
- Paillard T, Rolland Y, de Souto Barreto P. (2015) Protective effects of physical exercise in alzheimer’s disease and parkinson’s disease: a narrative review. J Clin Neurol 3:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Sickmann H, Hryciw BN, Kucharsky T, et al. (2013) Long-term exercise is needed to enhance synaptic plasticity in the hippocampus. Learn Mem 11:642–647. [DOI] [PubMed] [Google Scholar]
- Pavlovicova M, Lacinova L, Dremencov E. (2015) Cellular and molecular mechanisms underlying the treatment of depression: Focusing on hippocampal G-protein-coupled receptors and voltage-dependent calcium channels. Gen Physiol Biophys 34:353–366. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2007). The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Pilu A, Sorba M, Hardoy MC, Floris AL, et al. (2007). Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchasov BB, Shurgaja AM, Grischin OV, Putilov AA. (2000) Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res 1:29–42. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P. (1999) Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev 3:533–591. [PubMed] [Google Scholar]
- Romero L, Bel N, Artigas F, de Montigny C, et al. (1996) Effect of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology 4: 349–360. [DOI] [PubMed] [Google Scholar]
- Salehi I, Hosseini SM, Haghighi M, Jahangard L, et al. (2014) Electroconvulsive therapy and aerobic exercise training increased BDNF and ameliorated depressive symptoms in patients suffering from treatment-resistant major depressive disorder. J Psychiatr Res 57:117–124. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Duncko R, Makatsori A, Moncek F, et al. (2003) Involvement of glutamate neurotransmission in the development of excessive wheel running in Lewis rats. Neurochem Res 3-4:653–657. [DOI] [PubMed] [Google Scholar]
- Shetty RA, Sadananda M, (2017). Brief social isolation in the adolescent Wistar-Kyoto rat model of endogenous depression alters corticosterone and regional monoamine concentrations. Neurochem Res 42:1470–1477. [DOI] [PubMed] [Google Scholar]
- Singh NA, Clements KM, Fiatarone MA. (1997) A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci 1:M27–35. [DOI] [PubMed] [Google Scholar]
- Singh NA, Stavrinos TM, Scarbek Y, Galambos G, et al. (2005) A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci 6:768–776. [DOI] [PubMed] [Google Scholar]
- Veale D, Le Fevre K, Pantelis C, de Souza V, et al. (1992) Aerobic exercise in the adjunctive treatment of depression: a randomized controlled trial. J R Soc Med 9:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen X, Zhang N, Ma Q. (2013) Effects of exercise on stress-induced changes of norepinephrine and serotonin in rat hippocampus. Chin J Physiol 5:245–252. [DOI] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet DH, Yadid G. (2001) Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 4:434–439. [DOI] [PubMed] [Google Scholar]