Abstract
A novel antienterococcal peptide was prepared by fusing the enterococcal cCF10 pheromone to the channel-forming domain of colicin Ia, forming Enterococcus faecalis pheromonicin (PMC-EF). This peptide was bactericidal against vancomycin-resistant Enterococcus faecalis (VRE) organisms. Electron microscopy and vital dyes confirmed increased membrane permeability. All mice made bacteremic with VRE strains survived when they were treated with PMC-EF, while all controls died.
A major problem in the treatment of bacterial infections has been the emergence of antibiotic resistance. This is true for Enterococcus faecium and to a lesser extent for Enterococcus faecalis, which have developed resistance to many antibiotics, including vancomycin (12, 16). We have shown that a staphylococcal pheromone fused to a channel-forming peptide killed staphylococci in culture and animal infections (14). Here, we report on an application of that strategy to vancomycin-resistant E. faecalis (VRE) strains.
Escherichia coli cells can secrete colicins, which possess channel-forming properties bactericidal only to E. coli. Colicin Ia introduces a lethal 175-residue C-terminal channel into cell membranes of targeted E. coli (6, 8, 10, 13, 15, 17). Our plan was to fuse the C terminus of colicin Ia to cCF10, a 7-residue pheromone produced by enterococci (2, 8). This pheromone traverses recipient cell walls and interacts with a corresponding prgZ component of the cell membrane (1, 3, 4).
Mutagenesis of PMC-EF.
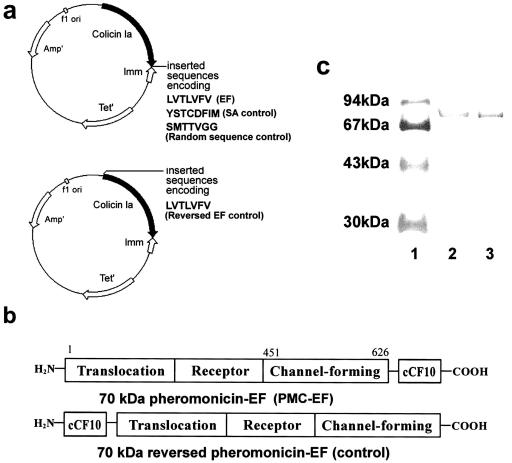
We constructed the LVTLVFV amino acid sequence of pheromone cCF10 to follow position I626 of colicin Ia by double-stranded oligonucleotide mutagenesis (QuickChange kit; Stratagene) by using a Promega pSELECT-1 plasmid containing the colicin Ia gene (P. Gosh, University of California at San Francisco) to form E. faecalis pheromonicin (PMC-EF). We used the 5′-3′ oligonucleotide containing the desired LVTLVFV mutation (in bold) GCGAATAAGTTCTGGGGTATTCTGGTTACCCTTGTGTCCGTGTAAATAAAATATAAGACAGGC. Controls consisted of a random peptide of the same length as cCF10 and an unrelated staphylococcal pheromone fused at the N terminus instead of the C terminus (reversed PMC-EF) (Fig. 1a). Plasmids were transfected into TG1 E. coli cells and purified (7, 9).
FIG. 1.
Structure of PMC-EF. (a, upper panel) Shown is an 8.3-kbp colicin Ia plasmid used for site-directed mutagenesis and subsequent preparation of PMC-EF consisting of the colicin Ia plasmid with insertion of a cCF10 (EF) pheromone gene, PMC-SA with insertion of an unrelated staphylococcal pheromone (YSTCDFIM), or insertion of a random (SMTTVGG) peptide gene following amino acid position I626. (a, lower panel) Shown is a construct with insertion of cCF10 before amino acid position S1 of colicin Ia to form the reversed PMC-EF control. (b) Domain diagram of the PMC-EF construct with the cCF10 pheromone at the C terminus and the reversed PMC-EF construct with cCF10 pheromone at the N terminus. (c) Sodium dodecyl sulfate-15% polyacrylamide gel of PMC-EF purified by gradient elution from a carboxymethyl column: lane 1, molecular weight markers; lane 2, PMC-EF; and lane 3, PMC-SA.
In vitro bactericidal assays.
We grew vancomycin-sensitive E. faecalis ATCC 29212 and vancomycin-resistant E. faecalis ATCC 700802 (American Type Culture Collection, Manassas, Va.) as described previously (3). Shenyang Biotechnic Co. (Shanghai, People's Republic of China) synthesized cCF10.
Wild-type colicin Ia from TG1 cells transfected with colicin Ia plasmid, PMC-EF from TG1 cells transfected with reversed PMC-EF, a random protein with an SMTTVGG peptide introduced at the C terminus of colicin Ia, penicillin, and vancomycin served as controls. Turbidimetric measurements and colony counting were performed (5).
Electron microscopy and immunolabeling.
We incubated VRE cells with PMC-EF, vancomycin, or growth medium alone for 2 h, then stained them with 1% phosphotungstic acid, and observed them under an electron microscope (Jeol JEM-100SX; Jeol, Akishima, Japan) at a magnification of ×15,000 to ×40,000.
We incubated VRE and BAA-42 methicillin-resistant Staphylococcus aureus cells with PMC-EF, PMC-EF plus free cCF10, vancomycin, or growth medium alone for 40 min, then fixed the cells in phosphate-buffered saline-paraformaldehyde, incubated specimens with rabbit anti-colicin Ia antibody (1:10 in 3% skim milk) (Babco Berkeley Antibody Co., Richmond, Calif.) followed by goat anti-rabbit antibody labeled with 5-nm-diameter gold particles (1:100 in 3% skim milk) (Wuhan Boster Biol. Tech. Co., Wuhan, People's Republic of China), and fixed the cells in phosphate-buffered saline-glutaraldehyde. Specimens were fixed in OsO4 and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and then observed with a Hitachi H-600 electron microscope at 80 kV (11).
The domains of the fusion peptide PMC-EF are shown in Fig. 1a and b. Figure 1c shows a polyacrylamide gel of the enterococcal chimeric protein PMC-EF (Fig. 1c, lane 2) and a control chimeric protein containing pheromone from S. aureus (PMC-SA) (Fig. 1c, lane 3).
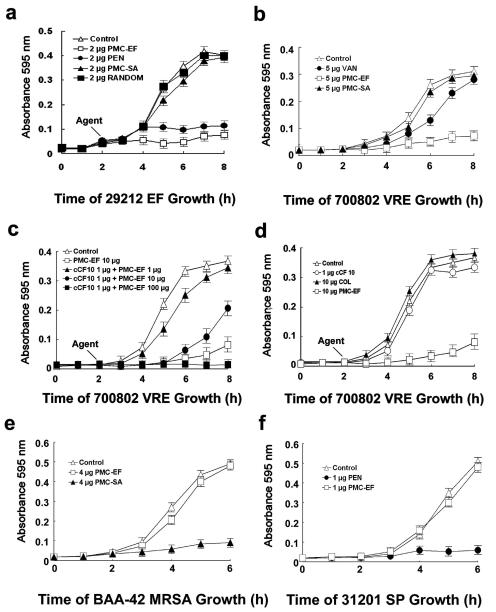
Exposure of E. faecalis ATCC 29212 nonresistant cells to PMC-EF inhibited growth by 90% (Fig. 2a), while penicillin G inhibited growth by 80%. Control peptides with an unrelated 8-residue staphylococcal pheromone or a random 7-residue peptide linked at the C terminus of colicin Ia had no effects. Vancomycin (5 μg/ml) slowed initial growth of VRE cells (Fig. 2b). However, later there was no significant difference compared to untreated controls. In contrast, 5 μg of PMC-EF/ml inhibited VRE cell growth by 85%. Addition of free cCF10 pheromone (Fig. 2c) blocked PMC-EF inhibition of VRE cell growth in a concentration-dependent manner. Incubation of VRE with either free cCF10 or wild-type colicin Ia alone (Fig. 2d) did not inhibit cell growth. In contrast, PMC-EF resulted in less than 10% growth inhibition when administered to S. aureus or Streptococcus pneumoniae (Fig. 2e and f). Exposure of S. aureus cells to PMC-SA, a control chimeric protein containing pheromone from S. aureus, caused an 80% growth inhibition (Fig. 2e) (14). Withdrawal of PMC-EF failed to restore growth (data not shown).
FIG. 2.
Measurement of bactericidal activity of PMC-EF against E. faecalis by in vitro cell growth assays. The following additions were made to the media: no addition (Control), penicillin G (PEN), vancomycin (VAN), wild-type colicin Ia (COL), free cCF10 pheromone alone (cCF10), staphylococcal colicin fusion protein (PMC-SA), colicin Ia with C-terminal random 7-residue peptide (RANDOM), and enterococcal colicin fusion protein (PMC-EF). E. faecalis 29212, a vancomycin-sensitive E. faecalis strain, was incubated with all additives at 2 μg/ml (a) or all additives at 5 μg/ml (b). (c) Free cCF10 pheromone (1 μg/ml) was added to compete with increasing concentrations of PMC-EF against ATCC 700802 E. faecalis cells. (d) Growth of ATCC 700802 VRE cells treated with 1 μg of free cCF10/ml or 10 μg of wild-type colicin Ia or PMC-EF/ml. (e) Growth of S. aureus ATCC BAA-42 with 4 μg of PMC-EF or PMC-SA (an unrelated staphylococcal chimeric protein)/ml. (f) Growth of S. pneumoniae 31201 with 1 μg of PMC-EF or penicillin G/ml.
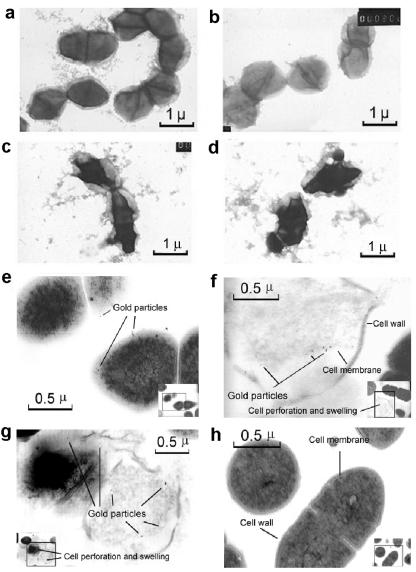
Electron microscopy of VRE cells incubated with vancomycin (Fig. 3b) showed a morphological appearance like that of untreated cells (Fig. 3a). In contrast, after incubation with PMC-EF, 60% of VRE cells per field were distorted (Fig. 3c and d) (less than 7% per field were distorted in controls). Free cCF10 blocked adsorption of gold-labeled antibody on cytoplasmic membranes of enterococcal cells treated with PMC-EF (Fig. 3h). As shown in Fig. 3f and g, some VRE cells exposed to PMC-EF developed ruptured cell walls.
FIG. 3.
Electron micrographs of ATCC 700802 VRE cells. (a) Untreated cells. (b) Vancomycin-treated cells (5 μg/ml). (c and d) PMC-EF-treated cells (5 μg/ml). (a to d) Negative staining. Magnification, ×15,000. Cells were incubated and stained with rabbit anticolicin antibody and gold-labeled goat anti-rabbit antibody. (e to g) VRE cells were treated with PMC-EF (20 μg/ml). (h) Cells treated with PMC-EF (20 μg/ml) and competed with free cCF10 (1 μg/ml). Magnification, ×30,000 to ×40,000. Insets show full-field views.
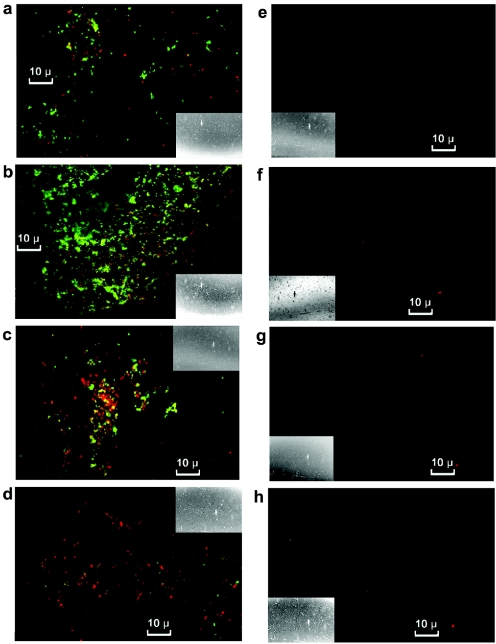
Initially after exposure to PMC-EF (Fig. 4a), the number of VRE cells stained (green) by fluorescein isothiocyanate (FITC)-labeled antibody exceeded that stained (red) with propidium iodide, a vital dye. The ratio of red to green VRE cells increased with time (Fig. 4a to d). The number of dual-labeled cells increased to a maximum at 0.5 h (Fig. 4b) and then declined, while cells with propidium iodide staining decreased, and FITC staining was almost absent by 2 h (Fig. 4d). There was no significant propidium staining and no FITC labeling of untreated cells (Fig. 4e to h).
FIG. 4.
Cells treated with PMC-EF (a to d) or medium alone (e to h) stained with propidium iodide and FITC-labeled antibody and examined under a confocal microscope. The following times elapsed after additions: 0.1 h, panels a and e; 0.5 h, panels b and f; 1 h, panels c and g; and 2 h, panels d and h. Insets show full-field views.
In a mouse VRE sepsis model, all mice treated with PMC-EF survived, while all untreated mice died within 3 days. None of the E. faecalis-infected mice survived with vancomycin (data not shown).
The emergence of vancomycin-resistant enterococci has been the result of increased use of vancomycin in enterococcal infections (12, 16). In addition, conjugal transfer of the vanA operon from enterococci has resulted in vancomycin resistance in other bacteria (12, 16). Multidrug resistance is now common among those pathogens (12, 16). Successful treatment of antibiotic-resistant bacteria will require novel agents whose mechanisms of action are different from those of conventional agents.
We conclude that a chimeric peptide containing pheromone and channel-forming domains can be targeted to antibiotic-resistant bacteria, resulting in substantial bactericidal effects. Furthermore, because a variety of pheromones are known, fusion peptides could be tailored to particular bacteria and provide novel “narrow-spectrum” antibiotics.
Acknowledgments
This work was supported by National Nature Science Foundation of China grants 39725009, 39770243, and 30276820 and National Basic Research Program of China grant G19990540001 to X.-Q.Q.; a grant from the National Institute of Diabetes and Digestive and Kidney Disorders (DK-42182) to G.Y.W.; and the Herman Lopata Chair in Hepatitis Research to G.Y.W.
REFERENCES
- 1.Buttaro, B. A., M. H. Antiporta, and G. M. Dunny. 2000. Cell-associated pheromone peptide (cCF10) production and pheromone inhibition in Enterococcus faecalis. J. Bacteriol. 182:4926-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunny, G. M., and B. A. B. Leonard. 1997. Cell-cell communication in gram-positive bacteria. Annu. Rev. Microbiol. 51:527-564. [DOI] [PubMed] [Google Scholar]
- 3.Haas, W., B. Shepard, and M. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 415:84-87. [DOI] [PubMed] [Google Scholar]
- 4.Hancock, L., and M. Perego. 2002. Two-component signal transduction in Enterococcus faecalis. J. Bacteriol. 184:5819-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual, p. 71. Cold Spring Harbor Laboratory Press, Woodbury, N.Y.
- 6.Jacob, F., L. Siminovitch, and E. Wollman. 1952. Sur la biosynthése d'une colicine et sur son mode d'action. Ann. Inst. Pasteur 83:295-315. [PubMed] [Google Scholar]
- 7.Jakes, K. S., C. K. Abrams, and A. Finkelstein. 1990. Alteration of the pH-dependent ion selectivity of the colicin E1 channel by site-directed mutagenesis. J. Biol. Chem. 265:6984-6991. [PubMed] [Google Scholar]
- 8.Kienker, P. K., X. Q. Qiu, S. L. Slatin, and A. Finkelstein. 1997. Transmembrane insertion of the colicin Ia hydrophobic hairpin. J. Membr. Biol. 157:27-37. [DOI] [PubMed] [Google Scholar]
- 9.Madigan, M. T., J. M. Martinko, and J. Parker. 1997. Brock biology of microorganisms, 8th ed., p. 151-179. Prentice Hall, Inc., London, United Kingdom.
- 10.Mankovich, J. A., C. H. Hsu, and J. Konisky. 1986. DNA and amino acid sequence analysis of structural and immunity genes of colicins Ia and Ib. J. Bacteriol. 168:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongodin, E., O. Bajolet, and J. Hinnrasky. 2000. Cell wall-associated protein A as a tool for immunolocalization of Staphylococcus aureus in infected human airway epithelium. J. Histochem. Cytochem. 48:523-533. [DOI] [PubMed] [Google Scholar]
- 12.Paulsen, I. T., L. Banerjei, and G. S. Myers. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 13.Qiu, X. Q., K. S. Jakes, and P. K. Kienker. 1996. Major transmembrane movement associated with colicin Ia channel gating. J. Gen. Physiol. 107:313-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu, X. Q., H. Wang, and X. F. Lu. 2003. An engineered multidomain bactericidal peptide as a model for targeted antibiotics against specific bacteria. Nat. Biotechnol. 21:1480-1485. [DOI] [PubMed] [Google Scholar]
- 15.Schein, S. J., B. L. Kagan, and A. Finkelstein. 1978. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature 276:159-163. [DOI] [PubMed] [Google Scholar]
- 16.Shankar, N., A. Baghdayan, and M. Gilmore. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746-750. [DOI] [PubMed] [Google Scholar]
- 17.Slatin, S. L., X. Q. Qiu, and K. S. Jakes. 1994. Identification of a translocated protein segment in a voltage-dependent channel. Nature 371:158-161. [DOI] [PubMed] [Google Scholar]