Abstract
The ERG3 gene in Candida albicans was identified as a gene whose mRNA level was higher in the cph1/cph1 efg1/efg1 double mutant than in the wild-type cells. Further study showed that Efg1, but not Cph1, negatively regulated ERG3. Mutations in EFG1 consistently increased the susceptibility of the cells to antifungal agents.
Candida albicans is the most frequently isolated fungal pathogen in humans and has caused morbidity in seriously debilitated and immunocompromised hosts (2). The incidence of drug resistance has increased with the increased usage of antifungal agents (9, 14). However, the mechanisms of drug resistance are not well-known. The cells of C. albicans can switch from the unicellular yeast form into either one of the two distinct filamentous forms, cells with pseudohyphae or hyphae. Mutations in EFG1 affect hyphal formation and also reduce the virulence of C. albicans in a mouse model (5). Here we show that in addition to virulence, Efg1 is also involved in drug resistance by negatively regulating ERG3 of the ergosterol biosynthesis pathway in C. albicans.
In this study, the wild-type strain (SC5314) (3) and the cph1/cph1 efg1/efg1 double mutant (HLC54) (5) of C. albicans were harvested after the cells were incubated in medium containing 10% serum at 37°C for 4 h. Total RNAs were then isolated from the cells and subjected to suppression subtractive hybridization (1). By this method, we obtained a total of 340 plasmids containing cDNA fragments of candidate genes for which the levels of mRNA expression were higher in the cph1/cph1 efg1/efg1 double mutant than in the wild-type strain. The plasmids were then digested with HaeIII, and their restriction patterns were analyzed by gel electrophoresis and recorded by a charge-coupled device imaging system.
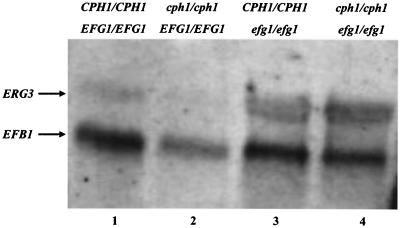
To facilitate categorization of the candidate genes, the recorded images were then subjected to analysis by an automatic imaging-processing software system that converts each set of restriction bands into a sequence of integers. Identical restriction patterns produce the same sequence of integers. Representative candidates from each category were sequenced and compared to sequences in databases to reveal the identities of the genes. Northern blotting and real-time PCR were then used to determine the levels of expression of selected candidates in order to assess the result of the suppression subtractive hybridization. The level of expression of ERG3 was indeed higher in the efg1/efg1 and cph1/cph1 efg1/efg1 strains than in the wild-type and cph1/cph1 strains (Fig. 1). Further quantitative analysis by real-time PCR showed that the expression of ERG3 was increased approximately 2.5-fold in the efg1/efg1 mutant strain, but not in the cph1/cph1 mutant strain, after cells were incubated in medium with 10% serum at 37°C for 4 h. Thus, ERG3 is one of the genes identified in this study whose expression is negatively regulated by Efg1 in C. albicans.
FIG. 1.
Efg1 negatively regulates ERG3. Approximately 10 μg of total RNA isolated from each strain was subjected to Northern blot analysis. EFB1 mRNA was used as the loading control. Lane 1, CPH1/CPH1 EFG1/EFG1 (SC5314); lane 2, cph1/cph1 EFG1/EFG1 (Can16); lane 3, CPH1/CPH1 efg1/efg1 (HLC52); lane 4, cph1/cph1 efg1/efg1 (HLC54).
The predominant target of the azole-based drugs is lanosterol demethylase, the product of ERG11 (4, 7, 16). Modifying the target enzyme is a major mechanism contributing to drug resistance in clinical isolates of C. albicans (10, 15). For instance, altering specific steps in the ergosterol biosynthesis pathway has been documented as a compensatory mechanism for azole resistance. Treatment with azoles results in accumulating 14α-methylergosta-8,24-dien-3,6-diol, the toxic product from the activity of the sterol Δ5,6-desaturase encoded by the ERG3 gene (10), a gene upstream of ERG11 in the pathway. Thus, mutations in ERG3 can suppress toxicity by blocking the production of 14α-methylergosta-8,24-dien-3,6-diol, which would then lead to resistance to azoles (6). Furthermore, accumulation of ergosta-7,22-dienol due to loss of the sterol Δ5,6-desaturase activity has been observed in two clinical isolates of C. albicans with resistance to azoles (8). Recently, it was reported that null mutations in ERG3 do cause fluconazole resistance (12).
The Etest method (13) was applied to determine the susceptibility to fluconazole of the wild-type strain and the efg1/efg1 mutant (Fig. 2A) with the fluconazole (0.016 to 256 μg/ml) drug strip (AB Biodisk, Solna, Sweden). Cells grown on the synthetic dextrose (SD) (0.67% yeast nitrogen base without amino acids but with 2% dextrose and 2% agar) plate overnight were homogenized in a 0.85% NaCl aqueous solution to reach a final concentration of 5 × 106 cells/ml. A sterile swab was dipped into the homogenized suspension and used to swab the entire agar surface of an SD plate evenly. The Etest strips were then applied to the plate when the excess moisture was absorbed completely. After incubation, the wild-type cells showed the extensive trailing phenotype, and the MIC of fluconazole to the efg1/efg1 mutant was 4 μg/ml.
FIG. 2.
Mutations in EFG1 increase the susceptibility to antifungal agents. A. Etest. The CaNDT80/CaNDT80 (SC5314) strain (wild type) (a) and efg1/efg1 (HLC52) mutant (b) were grown on SD medium. The results were photographed after the cells were incubated at 30°C for 2 days. B. Agar dilution assay. Cells were grown on medium containing 4% serum without drug (a) or with 5 μg of fluconazole per ml (b), 5 μg of miconazole per ml (c), or 1 μg of voriconazole per ml (d). The results were photographed after the cells were incubated at 30°C for 1 day. In each panel, wild-type cells are shown in the top row and efg1/efg1 mutant cells are shown in the bottom row.
The susceptibility to antifungal agents was further addressed by use of the agar dilution method (Fig. 2B). Cells were grown on medium containing 4% serum and with one of the following treatments: 0.1% dimethyl sulfoxide (DMSO) alone, 5 μg of fluconazole per ml and 0.1% DMSO, 5 μg of miconazole per ml and 0.1% DMSO, or 1 μg of voriconazole per ml and 0.1% DMSO. Cells of different strains were diluted to reach an optical density at 600 nm of 2 (approximately 2 × 107 cells/ml). Cells from each strain (0.5 μl) were then spotted onto plates containing different drugs with a replica device (Oxoid, Inc., Nepean, Ontario, Canada), along with 10-fold serial dilutions of the cells. Both the wild-type and efg1/efg1 cells could grow on medium containing DMSO and 4% serum without drug (Fig. 2B, panel a). However, compared with the wild-type cells, fewer efg1/efg1 mutant cells grew on medium containing fluconazole (Fig. 2B, panel b), miconazole (Fig. 2B, panel c), or voriconazole (Fig. 2B, panel d). The data from both Etest and the agar dilution assay have shown that mutations in EFG1 increased the susceptibility to antifungal agents (Fig. 2).
Efg1 is known to be required for the virulence of C. albicans in a mouse model (5), but its role in drug resistance has not been demonstrated until this study. Other groups have suggested that there may be genes capable of regulating both drug susceptibility and virulence. For example, it has been suggested that calcineurin A is involved in antifungal tolerance, cell morphogenesis, and virulence in C. albicans (11). However, in a mouse model, the loss of virulence of a C. albicans mutant lacking the gene encoding the calcineurin A subunit may be attributed to the fact that calcineurin is essential for C. albicans viability in medium containing serum. Thus, the mechanisms underlying the involvement of calcineurin A and Efg1 in virulence may be different. Our data have showed that Efg1, a protein known to be involved in virulence, also participates in regulating the expression of ERG3, a gene responsible for drug resistance in C. albicans.
REFERENCES
- 1.Diatchenko, L., S. Lukyanov, Y. F. Lau, and P. D. Siebert. 1999. Suppression subtractive hybridization: a versatile method for identifying differentially expressed genes. Methods Enzymol. 303:349-380. [DOI] [PubMed] [Google Scholar]
- 2.Edwards, E. J. J. 1990. Candida species, p. 1943-1958. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 3.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 4.Lamb, D. C., D. E. Kelly, W. H. Schunck, A. Z. Shyadehi, M. Akhtar, D. J. Lowe, B. C. Baldwin, and S. L. Kelly. 1997. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J. Biol. Chem. 272:5682-5688. [DOI] [PubMed] [Google Scholar]
- 5.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 6.Lupetti, A., R. Danesi, M. Campa, M. Del Tacca, and S. Kelly. 2002. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8:76-81. [DOI] [PubMed] [Google Scholar]
- 7.Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt, W. Luyten, M. Borgers, F. C. Ramaekers, F. C. Odds, and H. V. Bossche. 1999. Contribution of mutations in the cytochrome P450 14α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 145:2701-2713. [DOI] [PubMed] [Google Scholar]
- 8.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and The SENTRY Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY Antimicrobial Surveillance Program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 12.Sanglard, D., F. Ischer, T. Parkinson, D. Falconer, and J. Bille. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 47:2404-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapia, C., E. Leon, and E. Palavecino. 2003. Antifungal susceptibility of yeasts by Etest. Comparison of 3 media. Rev. Med. Chil. 131:299-302. (In Spanish with English summary.) [PubMed] [Google Scholar]
- 14.Vanden Bossche, H., D. W. Warnock, B. Dupont, D. Kerridge, G. S. Sen, L. Improvisi, P. Marichal, F. C. Odds, F. Provost, and O. Ronin. 1994. Mechanisms and clinical impact of antifungal drug resistance. J. Med. Vet. Mycol. 32(Suppl. 1):189-202. [DOI] [PubMed] [Google Scholar]
- 15.White, T. C. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob. Agents Chemother. 41:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]