Abstract
Drugs of abuse, including cocaine, amphetamine (AMPH), and heroin, elevate extracellular dopamine (DA) levels in the brain, thereby altering the activity/plasticity of reward circuits and precipitating addiction. The physiological release of DA occurs through the calcium-dependent fusion of a synaptic vesicle with the plasma membrane. Extracellular DA is cleared by uptake through the Na+/Cl--dependent DA transporter (DAT). In contrast, the substrate AMPH induces nonvesicular release of DA mediated by DAT. Extracellular AMPH is generally believed to trigger DA efflux through DAT by facilitating exchange for cytosolic DA. Here, in outside-out patches from heterologous cells stably expressing DAT or from dopaminergic neurons, by using ionic conditions in the patch pipette that mimic those produced by AMPH stimulation, we report that AMPH causes DAT-mediated DA efflux by two independent mechanisms: (i) a slow process consistent with an exchange mechanism and (ii) a process that results in rapid (millisecond) bursts of DA efflux through a channel-like mode of DAT. Because channel-like release of DA induced by AMPH is rapid and contains a large number of DA molecules, with a single burst of DA on par with a quantum of DA from exocytotic release of a vesicle, this burst mode of release may play a role in the synaptic actions and psychostimulant properties of AMPH and related compounds. Unlike AMPH, the endogenous substrate DA, when present on both sides of the plasma membrane, inhibits this channel-like activity, thereby suggesting that the DAT channel-like mode cannot accumulate DA against a concentration gradient.
Keywords: patch clamp, amperometry
Dopamine (DA) transporter (DAT) is a member of a gene family that includes norepinephrine transporter (NET), serotonin transporter (SERT), and γ-aminobutyric acid transporter 1 (GAT-1) (1–3). These neurotransmitter transporters are thought to play an important role in the reuptake (3, 4) and release (5, 6) of neurotransmitters. Their mechanism of transport is often described by using an alternating access model (7). In this model, the binding of cargo (DA, Na+, and Cl-) to an extracellularly oriented transporter induces a conformational rearrangement to an intracellularly oriented transporter from which the cargo is released into the cytosol, thereby completing the transport process. Consistent with this model, the recent crystal structure of the nonhomologous secondary transporter lactose permease (8) showed the transporter in what was inferred to be the “inward-facing state” with substrate bound within a cytoplasmic vestibule and no access route to the “extracellular” space.
In the case of DAT, DA transport generates an electrical current because of the net movement of positive charge into the cell (9). DA efflux induced by the psychostimulant amphetamine (AMPH) is believed to result from the ability of AMPH to reverse this inward transport process, in that the inward transport of AMPH by DAT increases the number of “inward-facing” transporter binding sites and thereby increases the rate of outward transport of DA through an exchange process (10) (11). Curiously, however, modulators and mutations have been identified that differentially affect AMPH-induced DA efflux and DA uptake. For example, Khoshbouei et al. (12) recently reported that human DAT (hDAT) N-terminal phosphorylation plays a critical role in AMPH-induced DA efflux but not in DA uptake. These data suggest that AMPH-induced DA efflux mediated by the reversal of DAT is more complex than a simple exchange process.
Analysis of the electrical currents generated by the homologous NET (13–15), SERT (16), GAT-1 (17), and, recently, Caenorhabditis elegans DAT-1 (18) has revealed low-probability, channel-like events that correspond to a rapid, inward flux of ions and/or substrate molecules (14). Here, we report that AMPH induces channel-like activity of the hDAT that supports the efflux of DA. Remarkably, unlike the psychostimulant AMPH, the endogenous substrate DA does not induce channel-like activity by DAT.
Materials and Methods
Cell Culture. A yellow fluorescent protein (YFP)-tagged hDAT (YFP-hDAT) was constructed as described in ref. 19, and stably transfected pools of EM4 cells, an HEK 293 cell line stably transfected with macrophage scavenger receptor to increase their adherence (20), were transfected with the YFP-DAT as described in ref. 21 and in detail in Supporting Text, which is published as supporting information on the PNAS web site.
Electrophysiology and Confocal Microscopy. Parental or stably transfected cells were plated at a density of 105 per 35-mm culture dish. Before electrical recordings, attached cells were washed twice with the bath solution containing 130 mM NaCl, 10 mM Hepes, 1.5 mM CaCl2, 0.5 mM MgSO4, 1.3 mM KH2PO4, and 34 mM dextrose (pH 7.35). Cells were visualized with an Eclipse TE300 inverted microscope and a PCM2000 confocal imaging system, both from Nikon. Images were acquired by using 488-nm excitation with a 510-nm long-pass filter. Cells with easily visualized plasma membrane fluorescence (high YFP-hDAT expression) were selected for analysis. A programmable puller (model P-2000, Sutter Instruments, Novato, CA) was used to fabricate quartz recording pipettes with a resistance of 7 MΩ. The electrode is in the cell-detached outside-out configuration (Fig. 5, which is published as supporting information on the PNAS web site), with the inner membrane exposed to the pipette solution containing 90 mM KCl, 30 mM NaCl, 2.0 mM MgCl2, 0.1 mM CaCl2, 1.1 mM EGTA, 10 mM Hepes, and 30 mM dextrose (pH 7.35) plus 100 μM or 2 mM DA. We previously showed that AMPH increases intracellular Na+ to ≈30 mM, and that this increase was essential for its induction of DA efflux (22). In addition, the Km of intracellular sodium for DA efflux was ≈40 mM, depending on the voltage (22). In the outside-out patch configuration, AMPH cannot increase intracellular Na+, which is clamped by the pipette solution. Therefore, we included 30 mM Na in the pipette, a concentration similar to that produced by AMPH in unclamped cells expressing DAT (22), thus making this the most physiological condition that we could choose for these experiments. The Km for intracellular DA for efflux has been determined to be ≈1.5 mM (12). In addition, in chromaffin cells, basal concentrations of intracellular catecholamine have been found to be as high as 50 μM, and AMPH increases these basal levels 6-fold. The 2 mM DA in the patch pipette to facilitate measurement of DA efflux by the amperometric electrode is in the range of the Km for DA and is in the same order of magnitude as the physiological concentration measured in chromaffin cells (23). We also performed recordings of DA efflux mediated by DAT channel-like activity with 100 μM DA in the pipette, and the results were qualitatively similar. Thus, the conditions that we have used for our studies (30 mM Na+ plus 100 μM or 2 mM DA)are in the range of the physiological concentrations induced by AMPH.
The whole-cell and amperometric currents recorded from DA neurons were obtained by using an experimental configuration and current analysis described by Khoshbouei et al. (22). We used the external and internal solutions described by Ingram et al. (24) but replacing isoosmotically 30 mM KCl for NaCl.
hDAT currents were recorded by using an Axopatch 200B (Axon Instruments, Foster City, CA) with a low-pass Bessel filter set at 1,000 Hz. The Axopatch 200B was used to vary the membrane potential. Data were stored digitally on a computer (Dell, Round Rock, TX) running pclamp8 software (Axon Instruments) and were analyzed offline. The basic experimental protocol consisted of two voltage steps of 8-sec duration separated by a return to the baseline for a minimum of 4 sec. This protocol was performed before drug application (control) and was repeated after 3 min of AMPH application to the bath as well as after subsequent addition of cocaine for 3 min to the bath. We consistently used the same 8 sec in each condition relative to both the drug application and the voltage step; no qualitative differences were observed between the two 8-sec steps under any of the conditions.
The AMPH-induced increase in intracellular Na+ was recorded in dissociated mouse midbrain DA neurons cultured on 35-mm glass-bottom dishes for ≈7–10 days before the experiment. Intracellular sodium was measured with sodium green-AM (Molecular Probes) as described in ref. 22 and in Supporting Text.
Amperometry. A carbon fiber (amperometric electrode) measures catecholamine flux through oxidation/reduction reactions (25). The outside-out membrane-excised patch was held ≈1 μm from the carbon fiber, which was held at +700 mV for DA oxidation (14, 25, 26). The carbon fiber electrode did not affect voltage clamping of the patch membrane. A second Axopatch 200B was used to maintain an amperometric electrode potential, and the data were recorded and stored digitally on a Dell computer running pclamp8 software and were analyzed offline. Amperometric currents were low-pass-filtered as indicated above. In whole-cell experiments, cocaine reduces DA release and DAT electrical activity in hDAT cells (22). Therefore, hDAT-mediated DA flux from the excised patch was defined by its cocaine sensitivity (10 μM). We stepped the voltage and recorded the patch current and the oxidative current simultaneously by using the basic experimental protocol described above under Electrophysiology and Confocal Microscopy.
Unlike the usual amperometric calibration, which requires conversion to concentration, we report the current directly without considering the effective volume. Thus, our requirements are a defined baseline, and our data represent a lower limit to the DA flux, because some transmitter may be lost to the bulk solution. The relationship between the oxidative current and the number of DA molecules reacted is given by Faraday's law: Q = zeM, where Q is the total charge involved in the redox reaction, M is the number of molecules reacted, and z is the number of electronic charges (e) transferred per reacted molecule. To calculate the number of DA molecules reacted with the carbon fiber, we used z = 2 (26).
Data Analysis. To measure the properties of the DAT channel-like activity, an amplitude histogram analysis of the hDAT electrical currents was performed as described in ref. 13. The upward spikes in Figs. 1A and 2A are representative of DAT channel-like activity and were blocked by perfusion of the outside of the patch with either 10 μM cocaine or 5 μM mazindol. The cocaine/mazindol sensitivity of these recordings indicates the hDAT origin of the channel activity.
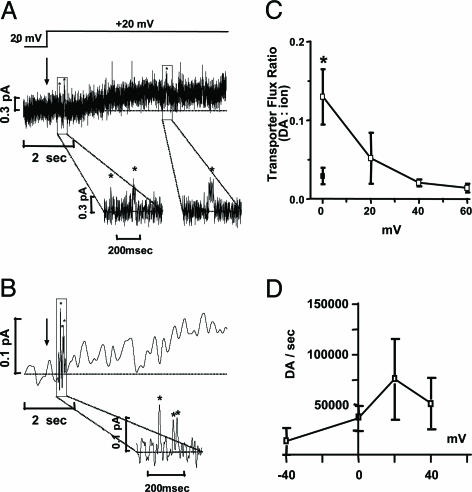
Fig. 1.
AMPH-induced hDAT-mediated DA efflux occurs via transporter-like and channel-like pathways. (A) Current trace of AMPH-induced hDAT transporter-like activity after a voltage step from -20 to +20 mV (see arrow). The AMPH-induced outward hDAT ionic current was defined as the current recorded in the presence of AMPH (10 μM) minus the current recorded after bath application of cocaine (10 μM) with AMPH still present. (Inset) DAT-mediated channel-like activity recorded from the same membrane patch (shown as asterisks). (B) Amperometric trace of AMPH-induced hDAT transporter-like DA efflux after a voltage step from -20 to +20 mV (see arrow, trace filtered at 2 Hz). The hDAT-mediated DA efflux was activated by bath application of 10 μM AMPH and was defined as the oxidation current recorded with AMPH minus the current recorded after bath application of cocaine (10 μM) with AMPH still present. (Inset) DAT-mediated channel-like efflux of DA (asterisks) recorded from the same membrane patch. (C) Transporter flux ratio of DA molecules to ions for voltage steps from 0 to +60 mV with 10 μM AMPH in the bath solution (□). We compared the flux ratio measured under control conditions (▪) (0 mV, no AMPH, cocaine subtracted) with that measured in the presence of AMPH. Data are shown as mean values ± SEM and were calculated 6–7 sec after the voltage step. *, = P < 0.05, by t test against control conditions. (D) Transporter-like efflux (DA molecules per sec) as measured in outside-out patches for voltage steps from -40 to +40 mV with 10 μM AMPH in the bath.
Fig. 2.
Substrate-regulated hDAT-mediated, outward channel-like activity. (A) Upper traces show sequential recordings of hDAT channel-like activity at +20 mV in control condition (CTR), after bath application of 10 μM AMPH (AMPH), and after bath application of 10 μM cocaine with AMPH still present (COC). Upward spikes signify outward channel-like current; see arrow. Lower traces show sequential recordings of hDAT channel-like activity at +20 mV in CTR, after bath application of 10 μM DA (DA), and after bath application of 10 μM cocaine with DA still present (COC). The fraction of the time that the hDAT channel is in the open state (NPo) and current (i) were calculated for each condition between 0 and 60 mV and reported in Fig. 2B and Fig. 6, which is published as supporting information on the PNAS web site. (B) The hDAT-channel NPo increases after AMPH application, compared with the CTR condition for each voltage tested (n = 4–6 for each voltage). *, significantly different from CTR, P < 0.05, paired t test.
Fig. 1 was designed to allow analysis of both transporter-like and channel-like activity. Therefore, for both the outside-out patch currents and amperometric currents, we subtracted the traces in the presence of cocaine plus AMPH from the traces in the presence of AMPH. The cocaine-plus-AMPH trace was filtered at 2 Hz to obtain the average nonspecific current not attributable to DAT and to minimize the increase of the noise of the resulting trace after subtraction. In patches in which nonspecific channels were observed (i.e., not blocked by cocaine), the patches were discarded and not analyzed further.
For Fig. 2, the amplitude histograms were obtained from 8 sec of data filtered at 1 kHz. We used a Gaussian subtraction procedure to extract the DA-induced hDAT channel current from the raw amplitude histogram (13). The mean of the outside-out current trace was first set arbitrarily to zero. We then constructed a Gaussian distribution that represented background noise centered at zero. To construct the Gaussian distribution, we used the left limb of the raw histogram (negative side of the background noise) and reflected it about the origin that is the center of the background noise, to reveal its positive side. This theoretical curve was then subtracted from the total histogram. After this procedure, the left limb of the histogram will vanish. However, if the right limb is asymmetrical with respect to the left, the procedure will reveal an underlying component. Even small asymmetries may be extracted from the total histogram by using this method. With this procedure, it is possible to determine single-channel current (i) at various voltages i(V)s and therefore the conductance as well as the fraction of time the channel is in the open state (NPo).
Results and Discussion
To monitor the activity of DATs in isolated membrane patches from DAT-expressing cells, we used the patch-clamp technique in the outside-out configuration (13, 14). In this configuration, the intracellular side of the plasma membrane faces the pipette solution, whereas the extracellular side faces the bath solutions (Fig. 5 Left). For these studies, to visually select DAT-expressing cells, we used either HEK 293 cells stably expressing YFP-tagged hDAT (11) or acutely dissociated mouse midbrain DA neurons expressing red fluorescent protein driven by a tyrosine hydroxylase promoter (27). The membrane potential of the patch vesicle was stepped to voltages between -20 and +60 mV (a range of voltages in which DA efflux is observable) (11, 22, 28). The dependence of the electrical activity and DA release on hDAT was confirmed by their absence in nontransfected cells (data not shown, n = 5) and their sensitivity to cocaine.
Fig. 1A shows AMPH-induced outward hDAT electrical currents recorded from an excised membrane patch during a voltage step from -20 to +20 mV. The hDAT-mediated current was defined as the current recorded in the presence of 10 μM AMPH minus the nonspecific current recorded after the application of 10 μM cocaine with AMPH still present (19). This current has a slow activation and is distinct from the DAT channel-like behavior (see below); we defined it as “transporter-like behavior.” At +20 mV, the average hDAT-mediated transporter-like current stimulated by AMPH, calculated 6 sec after the voltage step, was 0.30 ± 0.13 pA (n = 4). Expanding the time scale of the current trace, however, revealed the presence of rapid outward channel-like events (Fig. 1A Insets) (see below).
The identity of the ions composing the hDAT transporter-like current cannot be resolved by patch-clamp recordings alone. To determine whether AMPH-induces DA efflux through this transporter-like pathway, we coupled patch clamp with amperometry to record hDAT ionic currents and DA efflux simultaneously (Fig. 5) (14). Briefly, a 5-μm carbon fiber held at +700 mV was positioned ≈1 μm from the outer leaflet of the membrane. If DA permeation contributes to the hDAT transporter-like electrical activity, we should simultaneously measure an upward drift in the patch trace (indicating ion/DA efflux) (Fig. 1A) as well as an upward amperometric current generated by the oxidation of DA effluxed onto the carbon fiber. Fig. 1B shows that hDAT slowly released DA during a voltage step from -20 to +20 mV. We defined the hDAT-mediated DA efflux by subtracting the nonspecific efflux measured after the application of 10 μM cocaine (with AMPH still present) from the oxidation current recorded in the presence of 10 μM AMPH. At +20 mV, the average amperometric current stimulated by AMPH (2 Hz), calculated 6 sec after the voltage step, was 0.034 ± 0.008 pA (n = 7). This slow release of DA through hDAT (Fig. 1B), in combination with the transporter-like electrical currents (Fig. 1A), represents a pathway for DA efflux fundamentally distinct from channel-like activity and may result from the facilitated reversal of the transport process by AMPH (transporter-like behavior). In marked contrast, low-pass filtering of the amperometric traces at 20 Hz revealed amperometric spikes within the signal noise that correspond to large amounts of DA rapidly crossing the plasma membrane and being oxidized (Fig. 1B Inset). These discrete DA efflux events correlate with hDAT channel-like activity (see below). This burst activity is observed at potentials more positive than 0 mV and is stimulated by AMPH, which has been shown to increase the firing activity of dopaminergic neurons (24). Therefore, it is possible that the train of action potentials induced by AMPH stimulates these burst events that contribute to the total AMPH-induced DA efflux.
By using radioactive tracers, it has been shown that hDAT carries ions and DA with a flux ratio that depends on the membrane voltage (28). As for this inward activity of DAT, we found that outward activity also carries DA and ions in a ratio (transporter flux ratio) that depends on the voltage across the membrane patch (Fig. 1C). At 0 mV with 10 μM AMPH in the bath solution, the transporter flux ratio of DA molecules calculated from the amperometric current to ions calculated from the patch-clamp currents (DA molecule/ion ratio calculated from the mean current, 6 sec after the voltage step) was 0.13 ± 0.04 or an average efflux rate of at least 1 DA molecule for every 8 ions (Fig. 1C). This ratio compares with a transporter flux ratio for uptake of 1 DA for every 6.8 ions measured by Sonders et al. at 0 mV (28).
We have shown previously that DA efflux is increased at positive potentials although these whole-cell measurements represented a combination of transporter-like and channel-like efflux (22). Transporter-like efflux, as measured in outside-out patches, also is increased at positive potentials (Fig. 1D). However, the increase in efflux plateaus at ≈+40 mV (Fig. 1D). That the flux ratio of DA to total ions decreases at increasingly positive potentials suggests that the increase in charge movement has a different voltage sensitivity than does DA efflux and continues to increase at more positive potentials. This increase in charge movement may result from increased ion binding at positive potentials or from the inclusion of a smaller substate of DAT channel-like activity that might be present in the noise of the trace of these “transporter-like” current measurements and thus did not emerge in our analysis (see below).
We next characterized the AMPH-induced, hDAT-mediated channel-like activity. In Fig. 2A, the channel openings (as indicated by upward spikes, arrow) were present in the control (CTR) condition, were increased after the application of 10 μM AMPH (AMPH), and were blocked after application of 10 μM cocaine with AMPH still present (COC). Remarkably, in parallel experiments, extracellular application of 10 μM DA failed to elicit this channel activity (Fig. 2A, bottom traces).
Amplitude histogram analysis of 8 sec of data was performed to calculate the hDAT single-channel amplitude (i) and the fraction of time that the hDAT channels were open (NPo). The hDAT channel conductance (between 0 and +60 mV) was 2.25 ± 0.21 pS and did not change after AMPH application (Fig. 6; CTR compared with AMPH, n = 4–6 for each voltage). In contrast, AMPH significantly increased the NPo of the hDAT channel, compared with CTR, at all voltages tested (Fig. 2B, n = 4–6 for each voltage). Consequently, AMPH stabilizes a channel-like state of hDAT, and because voltage and ion gradients were clamped, we attribute this activity to a direct action of AMPH on hDAT. In contrast, DA application (Fig. 2A, bottom traces) tended to decrease the hDAT channel NPo (at +20 mV, the NPo for CTR was 0.012 ± 0.003 vs. 0.004 ± 0.001 for the NPo for DA, n = 4). Whereas at +20 mV this effect did not reach statistical significance, at 0 mV, DA significantly decreased the hDAT channel NPo (NPo for CTR was 0.015 ± 0.003 vs. 0.0016 ± 0.001 for the NPo for DA, n = 3; P < 0.05, paired t test). Thus, these observations revealed a mechanistic distinction between AMPH and DA.
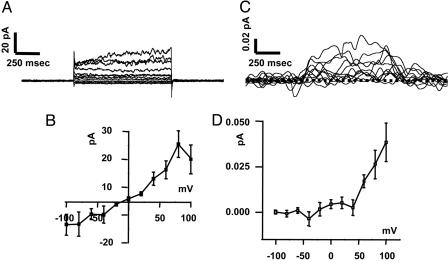
By using the patch-clamp technique in the whole-cell configuration with simultaneous amperometry (22), we next characterized the ability of AMPH to induce DA efflux in DA neurons with the intent of defining the experimental conditions to record single channel-like activity from a native preparation. With 10 μM AMPH in the bath solution, we recorded whole-cell (Fig. 3A) and amperometric currents (Fig. 3B) at different voltages. The steady-state whole-cell and amperometric currents recorded were then plotted against different voltages to obtain current/amperometric voltage relationships (Fig. 3 C and D, respectively). DA efflux increased exponentially at voltages more positive than -40 mV in a voltage-dependent manner very similar to DA efflux recorded in heterologous expression systems (22). In contrast to the exponential increase measured in the whole-cell configuration, the transporter-like efflux, as measured in outside-out patches, plateaus at ≈+40 mV (Fig. 1D). The increased efflux seen at positive potentials in the whole-cell configuration may be contributed, at least in part, by the DAT channel.
Fig. 3.
AMPH-induced DA effluxes and DAT currents in DA neurons. (A) DAT-mediated whole-cell currents recorded by stepping the membrane voltage from a holding potential of -60 mV to potentials between -100 and +100 mV. The internal solution of the whole-cell pipette contained 2 mM DA and 30 mM Na+. (C) Oxidation currents acquired concomitant to the whole-cell currents represented in A by voltage-clamping the cell between -100 and +100 mV with the whole-cell patch pipette. For voltage steps higher than -40 mV, the amperometric electrode recorded an oxidation current (positive) that increased during the voltage step, reaching a steady state. (B and D) Current (B) and amperometric (D) voltage relationships obtained from five different DA neurons. Data are presented as the mean ± SEM.
Therefore, to determine whether AMPH-induced DA efflux is sustained by the measured increase of NPo of an hDAT channel, we coupled patch clamp with amperometry to record hDAT channel-like activity and DA efflux simultaneously (Fig. 5) (14). As for the slower transporter-like activity described above, if DA permeation contributes to the hDAT channel-like electrical activity, we should simultaneously measure upward spikes in the patch trace (indicating ion/DA efflux) as well as upward amperometric currents generated by the oxidation of DA effluxed onto the carbon fiber.
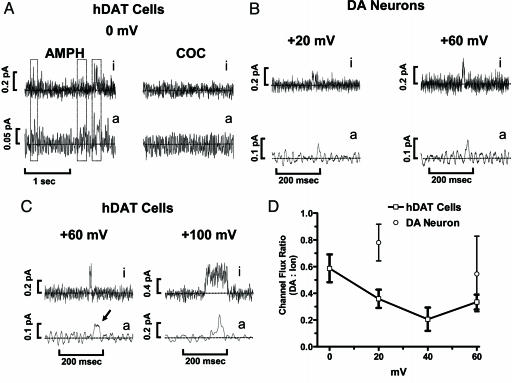
Recordings from membrane patches isolated from hDAT cells revealed temporally correlated hDAT channel-like activity (i) and discrete DA efflux events (a) (Fig. 4A, AMPH, r = 0.4), where r is the sample Pearson product moment correlation coefficient calculated as described in refs. 13 and 14. Application of 10 μM cocaine to the extracellular solution (with AMPH still present) inhibited these hDAT-mediated electrical events and significantly reduced r (r = -0.1). In the absence of Na+ in the patch pipette, AMPH did not stimulate amperometric spikes (n = 7, data not shown), demonstrating that intracellular Na+ is required for AMPH to stimulate DA efflux in the burst mode. Fig. 4C shows representative examples of simultaneous cocaine-sensitive hDAT channel-like activity and discrete DA efflux events recorded from several experiments with AMPH in the external solution. When the channel opens, the flux of a large number of ions is associated with the arrival of DA molecules at the carbon fiber (e.g., at +60 mV, 7,656 DA molecules, arrow). In the absence of simultaneous hDAT-channel openings, no amperometric spikes of this dimension were detected. Therefore, the AMPH-induced efflux of DA results in part from an AMPH-induced increase in the NPo of the hDAT channel. Similar results were observed with 100 μM DA (instead of 2 mM) in the patch pipette (data not shown). In these experiments, the AMPH-induced amperometric spikes were blocked by the application of 5 μM mazindol, further demonstrating that these spikes are DAT-mediated.
Fig. 4.
DA effluxes through an hDAT aqueous pore. The data shown are raw traces of channel-like activity and amperometric spikes; in each case, the channel activity was blocked at the end of the experiment by a 3-min application of cocaine to confirm that it was DAT-mediated. (A) hDAT-mediated channel activity (top trace, i) temporally correlates with discrete DA efflux events (bottom trace, a) at various voltages. All recordings were performed with 10 μM AMPH in the bath solution. The AMPH-induced hDAT channel-like efflux was blocked by bath solution of 10 μM cocaine with AMPH still present. To reduce background noise and highlight specific efflux events, the patch-clamp and amperometric recordings were filtered at 0.2 and 0.05 kHz, respectively. (B and C) Shown are recordings of individual efflux events generated by using excised membrane patches isolated from hDAT cells (C) or from excised membrane patches isolated from mouse DA neurons (B). Patch-clamp and amperometric recordings were filtered at 1 and 0.05 kHz, respectively. (D) The channel flux ratio between the number of DA molecules to ions that efflux when the hDAT channel opens is voltage-dependent in hDAT cells (□) and in DA neurons (○). The number of DA molecules and ions for each efflux event was calculated from the area under the respective discrete current events. Mean and SEM for 3–8 traces obtained from different experiments are shown.
Fig. 4B shows data from independent experiments in which the efflux of DA through the cocaine-sensitive DAT channel was recorded from excised membrane patches taken from acutely dissociated midbrain DA neurons. Like those recorded from hDAT cell membranes, neuronal DAT, expressed at endogenous levels, displays outward channel-like currents that are cocaine-sensitive, can be activated by AMPH, and are correlated in time with DA-efflux events.
The ratio between the number of DA molecules and the number of ions for the hDAT channel-like activity (channel flux ratio) can be calculated for each channel-like event (upward spikes in Fig. 4 B and C). Fig. 4D shows that, like the transporter flux ratio (Fig. 1C), the channel flux ratio is voltage-dependent. However, the channel flux ratio is substantially higher than the transporter flux ratio. For example, at 0 mV, the AMPH-induced hDAT channel conducts a ratio of DA molecules to ions of 0.59 ± 0.11 or an average efflux rate of ≈1 DA molecule for every 2 ions (four times larger than the corresponding transporter flux ratio). Similar results were observed with 100 μM DA in the patch pipette: in these experimental conditions, the AMPH-induced hDAT channel ratio (DA molecules to ions) was 0.53 ± 0.07 at 0 mV (n = 13) and 0.11 ± 0.06 at +60 mV (n = 9).
We demonstrate that AMPH induces DA efflux through hDAT both by transporter-like and channel-like conductance pathways. Because both pathways are present in the same excised membrane patch, the relative contribution of each pathway to total DA efflux can be directly calculated by taking the ratio of the integral of the burst to the integral of the entire 8-sec amperometric current. At +20 mV, the channel-like efflux contributed 10.5 ± 3.4% of the total DA released (n = 5); at 0 mV, the contribution was 12.3 ± 5.4%. However, the hDAT channel-like pathway releases this ≈10% of the total DA in very brief pulses (a few milliseconds, Fig. 4). Because DA synapses are ≈200 nm long and ≈15 nm wide, the diffusion of released DA outside the synapse takes only ≈50 msec (29). Thus, despite the fact that only 10% of total DA efflux in response to AMPH occurs by channel-like activity, this DA release mimics vesicular release (DA quantum) in both timing and magnitude (30). Because the sphere of influence at high-affinity receptors of a DA quantum (2,000–14,000 DA molecules) (30, 31) is estimated at between 20 and 100 DA synapses (31), this AMPH mechanism of DA release may play an important role in the synaptic actions and resulting psychostimulant properties of AMPH and related compounds.
In summary, AMPH induces hDAT-mediated DA release through two fundamentally different DAT-mediated mechanisms: (i) a slow, transporter-like pathway and (ii) a fast channel-like pathway. Although Carvelli et al. (18) recently demonstrated channel-like activity of DAT, they were unable to determine whether DA moves through the transporter channel. We show that the DA efflux-competent channel state of DAT is responsible for a significant fraction of DA released through DAT in response to AMPH. Remarkably, unlike AMPH, extracellular DA does not activate this hDAT channel-like behavior but instead inhibits it, and it is attractive to speculate that these subtle differences between AMPH and DA might underlie AMPH's psychoactive and/or addictive properties. DA and AMPH are both substrates of DAT, and both have been shown to induce DA efflux (32). Sitte et al. (32) also have shown that the greater electrical current induced by AMPH accounts for its greater ability to induce DA efflux. AMPH increases intracellular Na+ in hDAT cells (22) and in midbrain DA neurons (see Supporting Text). However, in outside-out patches, we controlled the intracellular Na+ concentration, and AMPH stimulated DAT-channel activity, but DA did not. Thus, although intracellular Na+ may play a role in the difference between AMPH and DA in the transporter-like mode of efflux, it cannot play a role in the different effects of AMPH and DA on the channel-like mode recorded in the outside out configuration. Therefore, our analysis suggests that the previously observed DA-induced efflux must result from transporter-like mode and not from channel-like mode. Substrates can regulate the interaction of neurotransmitter transporters with associated proteins (33), and such interactions could alter channel activity. For example, the soluble N-ethylmaleimide-sensitive factor attachment protein receptor syntaxin IA interacts with the N terminus of the norepinephrine transporter and reduces channel activity when perfused intracellularly (34). It is possible that AMPH and DA differentially affect the interaction of DAT with an associated protein, and this may account for the differences that we have observed in their effects on burst activity.
For both sodium and potassium channels, a number of drugs and toxins are known to act by preventing channel closure or inactivation (35). In the case of veratridine, for example, sodium channels remain open for very long times (35). The ATP-sensitive potassium channel opener pinacidil largely prevents the closure of the channel induced by cytoplasmic ATP (35). Moreover, palytoxin has been shown to stimulate channel activity of the Na,K-ATPase. Artigas and Gadsby (36) proposed that the Na/K pump is a modified ion channel with two gates and a central pore. There are exceptions, but in general, the gates of an ion channel work independently from the binding of ions in the permeation pathway. The gates of transporters and pumps, on the other hand, must be controlled with high selectivity by the binding and unbinding of ions and substrates. With DA on only one side of the membrane, intracellularly in the present study or extracellularly (18), a slippage of ions can occur in a channel-like mode because of a lack of coordination between the intracellular and extracellular gates. However, when DA is present on both sides of the plasma membrane, we observed a decrease in channel-like activity, which may result from the tight coordination of the two gates that allows DAT to accumulate DA against its concentration gradient. Our data show that DAT cannot accumulate DA in a channel-like mode against the DA gradient, at least in the outward direction. In contrast, AMPH, although similar in structure to DA, may act on DAT as palytoxin does on the NaK pump to increase the probability that both gates open simultaneously. This process may occur, as discussed above, through altered protein–protein interaction, but it also is possible that other downstream cellular modulators, such as Ca2+ (37) and activated PKC (38), may enhance the ability of AMPH to trigger DA efflux by promoting the stability of the channel-like state.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support provided by Drs. Valentina Savchenko, Dao-Qi Zhang, and Hidenobu Ohta. This work was supported by National Institutes of Health Grants MH58921 (to R.D.B.), MH57324 and DA11495 (to J.A.J.), DA13975 and DA14684 (to A.G.), and EY015815 (to D.G.M.).
Author contributions: R.D.B., J.A.J., and A.G. designed research; K.M.K., F.B., and H.K. performed research; D.G.M. contributed new reagents/analytic tools; K.M.K., F.B., H.K., and A.G. analyzed data; and K.M.K., R.D.B., D.G.M., J.A.J., and A.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPH, amphetamine; DA, dopamine; DAT, DA transporter; hDAT, human DAT; NET, norepinephrine transporter; SERT, serotonin transporter; GAT-1, γ-aminobutyric acid transporter 1; CTR, control; YFP, yellow fluorescent protein.
References
- 1.Giros, B. & Caron, M. G. (1993) Trends. Pharmacol. Sci. 14, 43-49. [DOI] [PubMed] [Google Scholar]
- 2.Blakely, R. D., De Felice, L. J. & Hartzell, H. C. (1994) J. Exp. Biol. 196, 263-281. [DOI] [PubMed] [Google Scholar]
- 3.Giros, B. (1996) Nature 379, 606-612. [DOI] [PubMed] [Google Scholar]
- 4.Neal, M. J. & Iversen, L. L. (1972) Nat. New Biol. 235, 217-218. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz, E. A. (1982) J. Physiol. 323, 211-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkenburger, B. H., Barstow, K. L. & Mintz, I. M. (2001) Science 293, 2465-2470. [DOI] [PubMed] [Google Scholar]
- 7.Jardetzky, O. (1966) Nature 211, 969-970. [DOI] [PubMed] [Google Scholar]
- 8.Abramson, J., Smirnova, I., Kasho, V., Verner, G., Kaback, H. R. & Iwata, S. (2003) Science 301, 610-615. [DOI] [PubMed] [Google Scholar]
- 9.Sonders, M. S. & Amara, S. G. (1996) Curr. Opin. Neurobiol. 6, 294-302. [DOI] [PubMed] [Google Scholar]
- 10.Sulzer, D., Chen, T. K., Lau, Y. Y., Kristensen, H., Rayport, S. & Ewing, A. (1995) J. Neurosci. 15, 4102-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahlig, K. M. & Galli, A. (2003) Eur. J. Pharmacol. 479, 153-158. [DOI] [PubMed] [Google Scholar]
- 12.Khoshbouei, H., Sen, N., Guptaroy, B., Johnson, L., Lund, D., Gnegy, M. E., Galli, A. & Javitch, J. A. (2004) PLoS Biol. 2, E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli, A., Blakely, R. D. & DeFelice, L. J. (1996) Proc. Natl. Acad. Sci. USA 93, 8671-8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli, A., Blakely, R. D. & DeFelice, L. J. (1998) Proc. Natl. Acad. Sci. USA 95, 13260-13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli, A., Petersen, C. I., deBlaquiere, M., Blakely, R. D. & DeFelice, L. J. (1997) J. Neurosci. 17, 3401-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, F., Lester, H. A. & Mager, S. (1996) Biophys. J. 71, 3126-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cammack, J. N. & Schwartz, E. A. (1996) Proc. Natl. Acad. Sci. USA 93, 723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvelli, L., McDonald, P. W., Blakely, R. D. & Defelice, L. J. (2004) Proc. Natl. Acad. Sci. USA 101, 16046-16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahlig, K. M., Javitch, J. A. & Galli, A. (2004) J. Biol. Chem. 279, 8966-8975. [DOI] [PubMed] [Google Scholar]
- 20.Robbins, A. K. & Horlick, R. A. (1998) BioTechniques 25, 240-244. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer, J. V. & Javitch, J. A. (1998) Proc. Natl. Acad. Sci. USA 95, 9238-9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoshbouei, H., Wang, H., Lechleiter, J. D., Javitch, J. A. & Galli, A. (2003) J. Biol. Chem. 278, 12070-12077. [DOI] [PubMed] [Google Scholar]
- 23.Mosharov, E. V., Gong, L. W., Khanna, B., Sulzer, D. & Lindau, M. (2003) J. Neurosci. 23, 5835-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram, S. L., Prasad, B. M. & Amara, S. G. (2002) Nat. Neurosci. 5, 971-978. [DOI] [PubMed] [Google Scholar]
- 25.Wightman, R. M., Jankowski, J. A., Kennedy, R. T., Kawagoe, K. T., Schroeder, T. J., Leszczyszyn, D. J., Near, J. A., Diliberto, E. J., Jr., & Viveros, O. H. (1991) Proc. Natl. Acad. Sci. USA 88, 10754-10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciolkowski, E. L., Maness, K. M., Cahill, P. S. & Wightman, R. M. (1994) Anal. Chem. 66, 3611-3617. [Google Scholar]
- 27.Zhang, D. Q., Stone, J. F., Zhou, T., Ohta, H. & McMahon, D. G. (2004) NeuroReport 15, 1761-1765. [DOI] [PubMed] [Google Scholar]
- 28.Sonders, M. S., Zhu, S. J., Zahniser, N. R., Kavanaugh, M. P. & Amara, S. G. (1997) J. Neurosci. 17, 960-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garris, P. A., Ciolkowski, E. L., Pastore, P. & Wightman, R. M. (1994) J. Neurosci. 14, 6084-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulzer, D. & Pothos, E. N. (2000) Rev. Neurosci. 11, 159-212. [DOI] [PubMed] [Google Scholar]
- 31.Cragg, S. J. & Rice, M. E. (2004) Trends Neurosci. 27, 270-277. [DOI] [PubMed] [Google Scholar]
- 32.Sitte, H. H., Huck, S., Reither, H., Boehm, S., Singer, E. A. & Pifl, C. (1998) J. Neurochem. 71, 1289-1297. [DOI] [PubMed] [Google Scholar]
- 33.Torres, G. E., Gainetdinov, R. R. & Caron, M. G. (2003) Nat. Rev. Neurosci. 4, 13-25. [DOI] [PubMed] [Google Scholar]
- 34.Sung, U., Apparsundaram, S., Galli, A., Kahlig, K. M., Savchenko, V., Schroeter, S., Quick, M. W. & Blakely, R. D. (2003) J. Neurosci. 23, 1697-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilgemann, D. W. (2003) Proc. Natl. Acad. Sci. USA 100, 386-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artigas, P. & Gadsby, D. C. (2003) Ann. N.Y. Acad. Sci. 986, 116-126. [DOI] [PubMed] [Google Scholar]
- 37.Gnegy, M. E., Khoshbouei, H., Berg, K. A., Javitch, J. A., Clarke, W. P., Zhang, M. & Galli, A. (2004) Mol. Pharmacol. 66, 137-143. [DOI] [PubMed] [Google Scholar]
- 38.Kantor, L. & Gnegy, M. E. (1998) J. Pharmacol. Exp. Ther. 284, 592-598. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.