Abstract
Objective
Animal models and studies in adults have demonstrated that copper restriction increases severity of liver injury in non-alcoholic fatty liver disease (NAFLD). This has not been studied in children. We aimed to determine if lower tissue copper is associated with increased NAFLD severity in children.
Methods
This was a retrospective study of pediatric patients who had a liver biopsy including a hepatic copper quantitation. The primary outcome compared hepatic copper concentration in NAFLD versus non-NAFLD. Secondary outcomes compared hepatic copper levels against steatosis, fibrosis, lobular inflammation, balloon degeneration, and NAFLD activity score (NAS).
Results
The study analysis included 150 pediatric subjects (102 with NAFLD and 48 non-NAFLD). After adjusting for age, BMI z-score, GGT, ALT, and total bilirubin, NAFLD subjects had lower levels of hepatic copper than non-NAFLD (p=0.005). Additionally, tissue copper concentration decreased as steatosis severity increased (p<0.001). Copper levels were not associated with degree of fibrosis, lobular inflammation, portal inflammation, or balloon degeneration.
Conclusions
In this cohort of pediatric subjects with NAFLD, we observed decreased tissue copper levels in subjects with NAFLD when compared to non-NAFLD subjects. Additionally, tissue copper levels were lower in subjects with non-alcoholic steatohepatitis, a more severe form of the disease, when compared to steatosis alone. Further studies are needed to explore the relationship between copper levels and NAFLD progression.
Keywords: hepatic copper, steatosis, NAFLD, fibrosis, children
Introduction
While the increasing clinical and economic burden of non-alcoholic fatty liver disease (NAFLD) has been well-defined1, the etiopathogenesis of disease needs further characterization. The severity of NAFLD ranges widely in children, from steatosis alone (NAFL) to steatosis plus severe inflammation (NASH) and/or progressive fibrosis. The genetic and environmental contributors to the severity of NAFLD remain unclear.
Copper is an essential microelement and is utilized by enzymes controlling a wide range of intracellular processes including oxidative phosphorylation, scavenging of reactive oxygen species, mitochondrial respiration, post-translation processing of collagen, elastin and neuropeptides, synthesis of neurotransmitters as well as those important in bidirectional transfer of iron ions across plasma membranes2, 3. The balance of copper in biologic systems is vital because excess free copper ions can cause damage to cellular components; yet deficiency will interrupt the function of cellular enzymes that require this cofactor3. Copper deficits result in impaired energy production, abnormal cholesterol and glucose metabolism and increased oxidative damage1, 4.
Studies in NAFLD animal models have demonstrated that copper deficiency (levels <1.6ppm) contributes to NASH severity6 and when combined with high fructose feeding, liver injury worsens through impairment of mitochondrial beta-oxidation, decreased levels of glutathione (GSH) and superoxide dismutase -1 (SOD-1) in hepatocytes7. Additionally, copper has been demonstrated to mediate lipid metabolism via increased size of circulating VLDL and expression of fatty-acid synthase in animal models9, 10 Human studies in adults also report a correlation between low serum and hepatic tissue copper levels and NAFLD11, 12. Although this has been reported in adults, hepatic tissue copper content in children with NAFLD has yet to be characterized. The objective of this study was to explore the association between tissue copper levels measured at the time of a clinically indicated liver biopsy and NAFLD, using a pediatric population. We hypothesized that, similarly to adult studies, lower hepatic copper levels would be associated with presence of disease, and that the concentrations would decrease with increased severity of NAFLD.
Methods
The study design was a retrospective chart review and was approved by the Children’s Healthcare of Atlanta Institutional Review Board, IRB Study Number 15-028. The Children’s Healthcare of Atlanta Egleston Department of Pathology database was searched for patient records with the keywords “liver biopsy” and “copper” from January 2001 to November 2015. Patients ≤ 25 years old at the time of biopsy were eligible for inclusion. 226 patient charts were retrospectively reviewed and data collected included anthropometrics, laboratory results including tissue copper level, final clinical diagnosis (as assigned by the pediatric hepatologist or gastroenterologist), and final pathologic diagnosis. Of the 226 patients, 19 were excluded because they did not have hepatic copper measurements and an additional 34 were excluded for conditions known to cause copper accumulation in the liver including Wilson’s disease, cholestatic liver disease, and cirrhosis. 173 subjects remained for analysis, from which 107 were characterized as NAFLD and 66 as non-NAFLD. We further limited our analysis to subjects that had tissue copper levels within normal range (10– ≤ 35 μg/g), since higher than usual levels can not only occur in cholestatic liver diseases and Wilson’s, but also in conditions of advanced fibrosis and other conditions which ultimately may obscure the relationship between NAFLD and copper, and distort the results. The cohort included 102 NAFLD- characterized subjects and 48 non-NAFLD. Results of the 173 subjects are provided in supplementary tables and figures.
Pathology Review
For this study, all biopsy specimens were re-evaluated by a pediatric pathologist blinded to the clinical information. Each biopsy was systematically assessed and scored using the NAFLD Activity Score (NAS) and fibrosis staging as outlined by the Central Pathology Committee of the NASH Clinical Research Network (CRN) 13. Diagnosis, stage of fibrosis, lobular inflammation, ballooning, and severity of steatosis were evaluated using the scoring system proposed by the NASH CRN.
Tissue Copper Levels and Clinical Labs
Tissue copper levels were measured at the time of liver biopsy when clinically indicated. Tissue is collected at the time of a liver biopsy. At least 0.3 mg of tissue is placed in a metal free container and fresh-frozen at −80°C, and sent for measurement to Mayo Medical Laboratories. Normal tissue copper levels are 10 – ≤ 35 μg/g tissue.
Statistical Analyses
Statistical analyses were carried out using SAS v9.4 (Carey, NC). Data are presented as means with corresponding standard deviations, unless indicated otherwise. Comparisons of copper concentrations across NAFLD versus non-NAFLD, fibrosis stage, severity of steatosis, lobular inflammation, portal inflammation, balloon degeneration, and NAS were evaluated using linear regression. For each exposure category examined, models were adjusted for age, BMI z-score, gamma glutamyl transferase (GGT), alanine aminotransferase (ALT), and total bilirubin. Type I error was set at alpha equals 0.05.
Results
Study demographics
This study analyzed data from 150 pediatric patients, 102 of which were characterized as NAFLD and 48 as non-NAFLD that had tissue copper levels within the normal specified range of 10–≤35ug/g. The average age was 13.7 with an age range of 2–20 years old. The NAFLD group was primarily male (76%) while the non-NAFLD was primarily female (66%). Differences were observed between the two groups in BMI, ALT, and tissue copper levels (Table 1). Demographics and laboratory results that included subjects with copper levels above 35 μg/g (n=173) are shown in Supplementary Table 1.
Table 1.
Descriptive statistics of study population.
| Clinical Manifestation | NAFLD n=102 |
non-NAFLD n=48 |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Sex, Male | 75.50% | 33.30% | ||
| Age | 13.9 | 3 | 13.4 | 5 |
| BMI z-score | 2.23 | 0.4 | 0.5 | 1.2 |
| ALT (5–35 U/L) | 114.2 | 70.4 | 247.8 | 619.1 |
| Ceruloplasmin (20–43 mg/dL) | 25.4 | 5.9 | 27.9 | 8.2 |
| Tissue copper (10 – ≤35 μg/g) | 16 | 6.2 | 21.8 | 7.3 |
Analysis included subjects with tissue copper concentrations within normal range, 10– ≤ 35 μg/g.
BMI, Body Mass Index; ALT, alanine aminotransferase.
Tissue copper in Pediatric NAFLD vs. non-NAFLD
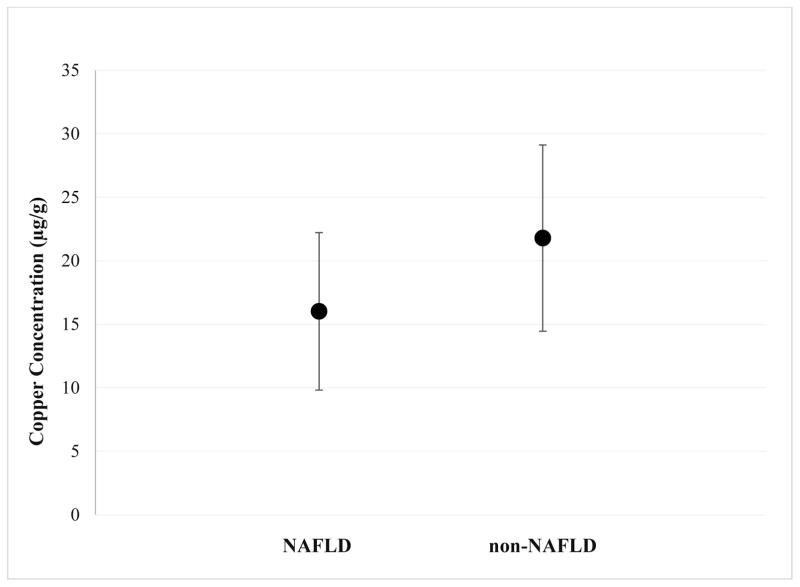
Tissue copper was measured at time of liver biopsy as part of the clinical assessment by sending tissue samples in metal free containers to the Mayo Clinic for analysis. Statistically significant differences between subjects with NAFLD and non-NAFLD subjects were observed, 16.03± 6.2 μg/g versus 21.79 ± 7.3 μg/g, respectively (p=0.005) (Table 1, Figure 1). After adjusting for age, BMI z-score, liver enzymes, and total bilirubin, the differences in tissue copper levels remained significant between NAFLD and non-NAFLD (p<0.005) (Table 2). Results including all subjects are displayed in Supplementary Table 1.
Figure 1.
Comparison of mean hepatic copper (μg/g) in NAFLD (n=102) versus non-NAFLD (n=48). NAFLD subjects had lower mean tissue copper than non-NAFLD subjects (16.03 vs. 21.79, p=0.005).
Table 2.
Adjusted estimates for mean hepatic copper tissue concentrations.
| Comparison | Parameter Estimate | 95%lower | 95%upper | p-value |
|---|---|---|---|---|
| NAFLD vs. non-NAFLD | −5.61 | −9.45 | −1.77 | 0.005 |
| NAFL vs. NASH | 2.67 | −0.01 | 5.34 | 0.054 |
| Steatosis Grade | −4.10 | −5.65 | −2.54 | <0.001 |
| Lobular Inflammation | 0.17 | −1.86 | 2.20 | 0.870 |
| NAS | −1.38 | −2.47 | −0.29 | 0.015 |
| Portal Inflammation | 1.59 | −0.26 | 3.43 | 0.100 |
| Fibrosis | −1.41 | −4.32 | 1.49 | 0.468 |
| Ballooning | 0.04 | −1.91 | 1.99 | 0.968 |
Analysis included subjects with tissue copper concentrations within normal range, 10– ≤ 35 μg/g. Adjusted for age, BMI z-score, GGT, ALT, total bilirubin
NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NAS, NAFLD Activity Score.
Comparison of copper within subjects with NAFLD
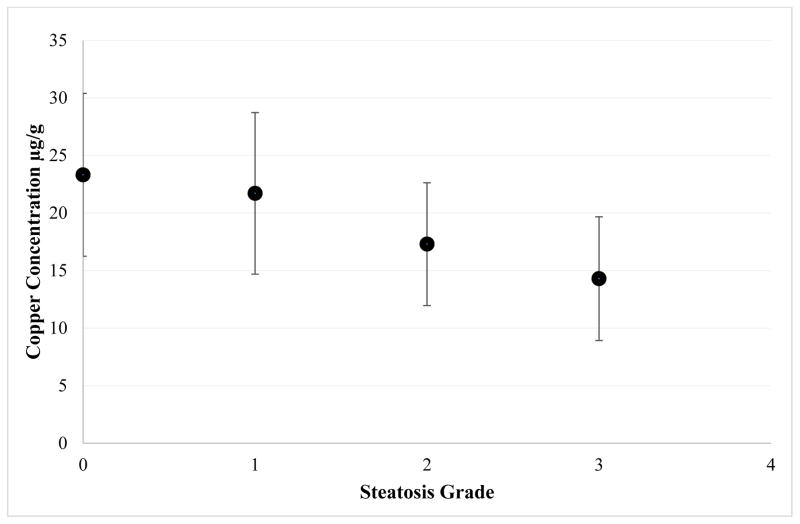
When analyzing data by disease severity, after adjusting for age, BMI z-score, liver enzymes and total bilirubin levels, differences were observed when categorizing NAFLD as simple steatosis (NAFL) vs. steatohepatitis (NASH), with lower levels of copper in the latter, that trended towards significance (p=0.054) (Table 2). When analyzing subjects by steatosis grade, statistically significant differences were observed between groups, where tissue copper levels declined as steatosis grade increased (p <0.001, Table 2 and Figure 2). The comparison of tissue copper and NAS score also demonstrated a statistically significant, inverse relationship with lower levels of tissue copper seen in the patients with higher NAS scores (p=0.015). Lastly, no statistically significant differences were observed between groups when looking at degree of fibrosis (p=0.47), lobular inflammation (p=0.87), portal inflammation (p=0.10), or balloon degeneration (p=0.97) (Table 2).
Figure 2.
Relationship between mean hepatic copper (μg/g) against severity of steatosis (n=150). Steatosis severity increases as hepatic copper decreases, p<0.001. Steatosis grade is based on criteria set by the NASH CRN, Grade 0: <5%, Grade 1: 5–33%, Grade 2: 34–66%, Grade 3: >66% steatosis.
Discussion
NAFLD is a complex disease, with both environmental and genetic factors contributing to its pathogenesis. In this study, we compared hepatic tissue copper levels in a large cohort of children who had undergone liver biopsy for clinical diagnosis. This is the first study to demonstrate differences in the tissue copper levels in children with NAFLD compared to non-NAFLD, and lower levels in pediatric NASH.
Copper deficiency can pose increased risks in NAFLD where copper depletion is associated with exacerbation of existing factors. For example, in NAFLD animal studies, a high fructose diet in the setting of copper depletion results in further impairment of anti-oxidant defenses, with decreased expression of hepatic glutathione peroxidases (GPx) and superoxide dismutase (SOD-1), and increased 4-hydroxynoenol (4-HNE) and glutathione disulfide/glutathione (GSSG/GSH) ratio, both markers of oxidative stress6, when compared to NAFLD alone. It is important to investigate the correlation between high fructose intake and copper deficiency, as currently in the United States, fructose intake in adolescents is higher than any other age group14, 15. The mechanisms by which copper deficiency and excess fructose lead to liver injury and fat accumulation begin with decreasing host expression of the small intestinal copper transporter (Ctr-1)7, thereby negating the ability to compensate for copper deficiency. This results in the worsening of copper deficiency, which has downstream effects on mitochondrial fatty acid synthesis. Song et al demonstrated a pattern of microvesicular steatosis in copper-deficient, fructose-fed mice, suggesting derangements in mitochondrial oxidative phosphorylation16. Moreover, the combination of copper deficiency and high fructose intake upregulates fatty acid synthase and downregulates carnitine palmitoyl transferase (CPT-1), the rate limiting step in mitochondrial fatty acid beta oxidation, which may further promote de novo lipogenesis7. The effects of excess fructose and low copper on hepatic fat accumulation and impaired lipid handling is further supported by an increase in expression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA reductase, the rate limiting enzyme in cholesterol biosynthesis)17, 18 and pyruvate dehydrogenase, the enzyme which generates the acetyl-CoA backbone of fatty acids19, 20. This is further supported by Song et al who showed an increase in known metabolites of lipogenesis fecal samples of copper-deficient mice fed a high fructose diet16.
In this study of children, we observed an inverse association between hepatic tissue copper levels and severity of steatosis. Interestingly, no associations were observed between copper concentrations and specific histologic features such as degree of fibrosis, lobular inflammation, portal inflammation, and balloon degeneration. A negative association was seen between tissue copper and NAFLD severity by NAS score. Subjects with NASH, which is a diagnosis combining features of inflammation, hepatocyte ballooning and steatosis displayed lower tissue copper levels compared to NAFL subjects supporting the hypothesis generated in animal studies that copper deficiency is associated with increased severity of injury in NAFLD.
A strength of this study includes its ability to describe tissue copper levels in children and also to show that copper levels are lower in NASH compared to NAFL. A limitation to this study is its retrospective nature of analysis, which limits the exploration of causality. Additionally, current measurements of tissue copper do not include defatting the tissue first, which is postulated to factitiously lower copper concentrations. Animal studies suggest that fat may interfere with copper measurement, giving falsely low copper values that are lower as the fat content increases (less tissue)21, however even when tissues were defatted prior to copper measurement, the difference between NAFLD and non-NAFLD remained, where lower copper concentrations were found in obese mice when compared to normal weight mice. In our study, the comparison of copper and steatosis could have been affected by this methodology problem, and it is important to interpret findings with caution and to not infer causality. Larger, prospective studies are warranted that measure tissue copper concentrations in defatted tissue samples and may more accurately examine the relationship between copper levels and NAFLD and its role in the progression of the disease. In conclusion, in our study we observed that tissue copper levels were inversely correlated to degree of steatosis, where subjects with non-alcoholic steatohepatitis displayed lower tissue copper levels than subjects with steatohepatitis alone. If causality can be established, modest copper supplementation would be a low-risk, potential therapy for NAFLD, especially in the pediatric population.
Supplementary Material
What is known
Copper restriction increases severity of liver injury in NAFLD.
This has been shown in animal models and adult studies.
What is new
Subjects with NAFLD had low hepatic tissue copper concentrations.
Lower copper levels were found in NASH compared to NAFL suggesting that severity of liver injury might be affected by copper concentrations.
Acknowledgments
Grant Support: The study was funded, in part, from grants from the National Institutes of NIH R03 DK096157 (MBV) and NIH K23 DK080953-05 (MBV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest/Financial Disclosure: Dr. Vos reports paid and unpaid consulting for pediatric NAFLD: Aegerion, Allegan, Intercept, Shire, Immuron and Target Pharmasolutions; and money or inkind research services: AMRA, Resonance Health and Target Pharmasolutions. All other authors have no conflicts of interest to declare.
Author Contributions: MM – collected data, interpreted data results, wrote first draft of manuscript, SC – interpreted histology and results, reviewed manuscript, MS – study design, contributed to manuscript, LS – data analysis and interpretation, contributed to manuscript, JK – data analysis and interpretation, contributed to manuscript, CM – study design, contributed to manuscript, MV – study design, interpretation of results, contributed to manuscript.
References
- 1.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Peds. 2013;162:496–500. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zatulovskaia YA, Ilyechova EY, Puchkova LV. The Features of Copper Metabolism in the Rat Liver during Development. PLoS One. 2015;10:e0140797. doi: 10.1371/journal.pone.0140797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper Biomed Pharmacother. 2003;57:386–98. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hordyjewska A, Popiolek L, Kocot J. The many “faces” of copper in medicine and treatment. Biometals. 2014;27:611–21. doi: 10.1007/s10534-014-9736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubino JT, Franz KJ. Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J Inorg Biochem. 2012;107:129–43. doi: 10.1016/j.jinorgbio.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Song M, Schuschke DA, Zhou Z, et al. Modest fructose beverage intake causes liver injury and fat accumulation in marginal copper deficient rats. Obesity (Silver Spring) 2013;21:1669–75. doi: 10.1002/oby.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song M, Schuschke DA, Zhou Z, et al. High fructose feeding induces copper deficiency in Sprague-Dawley rats: a novel mechanism for obesity related fatty liver. J Hepatol. 2012;56:433–40. doi: 10.1016/j.jhep.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman A, Aigner E, Weghuber D, et al. The Potential Role of Iron and Copper in Pediatric Obesity and Nonalcoholic Fatty Liver Disease. Biomed Res Int. 2015;2015:287401. doi: 10.1155/2015/287401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.al-Othman AA, Rosenstein F, Lei KY. Copper deficiency alters plasma pool size, percent composition and concentration of lipoprotein components in rats. J Nutr. 1992;122:1199–204. doi: 10.1093/jn/122.6.1199. [DOI] [PubMed] [Google Scholar]
- 10.al-Othman AA, Rosenstein F, Lei KY. Copper deficiency increases in vivo hepatic synthesis of fatty acids, triacylglycerols, and phospholipids in rats. Proc Soc Exp Biol Med. 1993;204:97–103. doi: 10.3181/00379727-204-43640. [DOI] [PubMed] [Google Scholar]
- 11.Aigner E, Theurl I, Haufe H, et al. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008;135:680–8. doi: 10.1053/j.gastro.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Stattermayer AF, Traussnigg S, Aigner E, et al. Low hepatic copper content and PNPLA3 polymorphism in non-alcoholic fatty liver disease in patients without metabolic syndrome. doi: 10.1016/j.jtemb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Vos MB, Kimmons JE, Gillespie C, et al. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh JA, Sharma AJ, Grellinger L, et al. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr. 2011;94:726–34. doi: 10.3945/ajcn.111.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei X, Song M, Yin X, et al. Effects of Dietary Different Doses of Copper and High Fructose Feeding on Rat Fecal Metabolome. J Proteome Res. 2015;14:4050–8. doi: 10.1021/acs.jproteome.5b00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunce GE. Hypercholesterolemia of copper deficiency is linked to glutathione metabolism and regulation of hepatic HMG-CoA reductase. Nutr Rev. 1993;51:305–7. doi: 10.1111/j.1753-4887.1993.tb03061.x. [DOI] [PubMed] [Google Scholar]
- 18.Yount NY, McNamara DJ, Al-Othman AA, et al. The effect of copper deficiency on rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. J Nutr Biochem. 1990;1:21–7. doi: 10.1016/0955-2863(90)90094-2. [DOI] [PubMed] [Google Scholar]
- 19.Millo H, Werman MJ. Hepatic fructose-metabolizing enzymes and related metabolites: role of dietary copper and gender. J Nutr Biochem. 2000;11:374–81. doi: 10.1016/s0955-2863(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 20.Sheline CT, Choi DW. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann Neurol. 2004;55:645–53. doi: 10.1002/ana.20047. [DOI] [PubMed] [Google Scholar]
- 21.Church SJ, Begley P, Kureishy N, et al. Deficient copper concentrations in dried-defatted hepatic tissue from ob/ob mice: A potential model for study of defective copper regulation in metabolic liver disease. Biochem Biophys Res Commun. 2015;460:549–54. doi: 10.1016/j.bbrc.2015.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.