Abstract
Background
MMP-7 and TIMP-1 may play a role in the pathogenesis of cancer disease. In this study we investigated plasma levels of selected metalloproteinase and its tissue inhibitor in comparison to plasma levels of the commonly accepted tumor markers (CA 125 and HE4) in selected histological types of epithelial ovarian cancer patients as compared to control groups: patients with a benign ovarian tumor and healthy subjects. Plasma levels of MMP-7 and TIMP-1 were determined using ELISA, CA 125 and HE4 – by CMIA methods.
Results
Plasma levels of all biomarkers studied were significantly higher in ovarian cancer patients as compared to both control groups. MMP-7 demonstrated comparable to HE4 or CA125 values of diagnostic sensitivity (SE: 61%; 68%; 58%, respectively), specificity (SP: 95%; 95%; 98%, respectively), positive (PPV: 93%; 96%; 98%, respectively) and negative predictive values (NPV: 61%; 66%; 60%, respectively) in the groups tested. The combined use of the aforementioned biomarkers resulted in a further increase in diagnostic criteria and AUC, especially in the early stages of the disease.
Conclusions
These findings suggest the usefulness of combining MMP-7 with CA 125 and HE4 in the diagnosis of epithelial ovarian cancer as a new tumor marker panel.
Electronic supplementary material
The online version of this article (doi:10.1186/s13048-017-0338-z) contains supplementary material, which is available to authorized users.
Keywords: MMP-7, TIMP-1, HE4, CA125, Epithelial ovarian cancer, Tumor markers
Background
Ovarian cancer (OC) is a highly lethal gynecological cancer. Approximately 23% of gynecological cancers are ovarian in origin, but 47% of all deaths from cancer of the female genital tract occur in women with cancer of this organ. Malignant tumors of the ovaries occur at all ages with variation in histological sub-type by age [1, 2]. Established risk factors for epithelial ovarian tumors include reproductive risk factors and inherited pathological mutations in the BRCA1 and BRCA2 genes [3, 4]. Initially, OC lacks clear symptoms, which prevents early diagnosis and treatment. Many potential biomarkers have been identified or used in recent years in the diagnostics of ovarian cancer patients [5, 6]. At present, CA 125 (carbohydrate antigen 125) [7] is the best-known ovarian cancer biomarker, although novel biomarkers such as HE4 (human epididymis protein 4), applicable to the diagnosis of this malignancy, have been researched recently [7, 8]. This glycoprotein belongs to a family of protease inhibitors and it is presumed to function as a is trypsin inhibitor. It is expressed in normal glandular epithelium of the reproductive tract, respiratory epithelium and distal renal tubules [9, 10]. In benign conditions, the highest HE4 concentrations have been observed in both women and men with renal failure. HE4 has been shown to be overexpressed in 93% of serous, 100% of endometrioid, and 50% of clear cell ovarian carcinomas. Similarly to other tumor markers, it is neither a strictly organ-specific nor a tumor-specific factor. Significant HE4 gene expression and strong immunoreactivity has been found in some lung, endometrial, renal, thyroid and breast carcinomas [9, 11]. When comparing the two aforementioned biomarkers it is believed that levels of HE4 are less frequently elevated in benign gynecological conditions than those of CA125 [12].
Different types of proteins, other than the commonly accepted and used tumor markers - such as cytokines (M-CSF, VEGF) [13, 14] and metalloproteinases are currently being investigated [15, 16]. Metalloproteinases have the ability to degrade extracellular matrix proteins, thereby facilitating tumor invasiveness, which also occurs through their interaction with growth factors, cytokines and proteases [17]. The tissue inhibitors of metalloproteinases (TIMPs: TIMP-1 and -2) regulate MMPs’ activation by binding as a complex [18]. MMP-7 (matrilysin) is the smallest proteinase from the entire metalloproteinases family with broad proteolytic activity against proteoglycans, elastin, laminin, collagens [19]. Overexpression and increased serum levels of this metalloproteinase have been confirmed in prostate, lung, renal, colorectal or breast malignancies [20–24]. Some clinical studies have also revealed significantly elevated TIMP-1 levels in the plasma of patients with colorectal, prostate and pancreatic cancers, which was associated with worse clinical outcomes [25–27].
The aim of this study was to determine plasma levels of metalloproteinase 7 and its tissue inhibitor 1 in comparison to HE4 and CA125 plasma levels in epithelial ovarian cancer patients and in relation to the control groups: patients with benign ovarian tumors and healthy subjects. The diagnostic criteria (sensitivity, specificity, predictive values of positive and negative test results) and the receiver-operating characteristic curve (ROC) for parameters investigated alone and in combinations were defined. Furthermore, correlations between the biomarkers tested were established.
These data may be used in the evaluation of the usefulness of MMP-7 and TIMP-1 in diagnosing ovarian cancer and in discriminating it from benign ovarian tumors.
Methods
Patients
Table 1 shows the tested groups. The study included 100 epithelial ovarian cancer patients (EOC) (sub-types serous and endometrioid) diagnosed by the Gynecology Group. Clinical stages and histological classification based on the criteria of the International Federation of Gynecology and Obstetrics (FIGO) and the World Health Organization (WHO) were established in all cases. Ovarian cancer histopathology was established in all cases by tissue biopsy of the tumor or post surgery from tumor cancer tissues. Patients with renal failure were excluded from our study due to very high HE4 concentration levels, undistinguishable from ovarian cancer. None of the patients had received chemo- or radiotherapy before blood sample collection.
Table 1.
Characteristics of ovarian cancer patients and control groups: benign ovarian tumor and healthy subjects
| Study group | Number of patients | |
|---|---|---|
| Epithelial ovarian cancer patients | 100 (100%) | |
| • median age (range) | 59 (46–87) | |
| - sub-type serous epithelial | 54 (54%) | |
| • median age (range) | 59 (46–81) | |
| - sub-type endometrioid epithelial | 46 (46%) | |
| • median age (range) | 59 (49–87) | |
| Tumor stage | IA-T1aN0M0 | 5 (5%) |
| IB-T1bN0M0 | 7 (7%) | |
| IC-T1cN0M0 | 13 (13%) | |
| IIA-T2aN0M0 | 8 (8%) | |
| IIB-T2bN0M0 | 9 (9%) | |
| IIC-T2cN0M0 | 8 (8%) | |
| IIIA-T3aN0M0 | 9 (9%) | |
| IIIB-T3bN0M0 | 9 (9%) | |
| IIIC-T3cN0M0 | 7 (7%) | |
| IV(metastases) | 25 (25%) | |
| Menopausal status: | ||
| - postmenopausal | 100 (100%) | |
| Benign ovarian tumor patients | 80 (100%) | |
| - type cystis serous | 40 (50%) | |
| - type cystis endometriosis | 40 (50%) | |
| Median age (range) | 54 (48–68) | |
| Menopausal status: | ||
| - postmenopausal | 80 (100%) | |
| Healthy subjects | 80 (100%) | |
| Median age (range) | 55 (47–64) | |
| Menopausal status: | ||
| - postmenopausal | 80 (100%) | |
Pretreatment staging procedures included physical and blood examinations, ultrasound scanning and chest X-rays. In addition, radioisotope bone scans, the examination of bone marrow aspirates, and brain and CT scans were performed where necessary.
The control groups were comprised of 80 benign ovarian tumor patients (cystis serous or cystis endometrioides) and 80 healthy volunteers. The benign ovarian tumor histopathology was established in all cases by tissue biopsy of the ovarian tumor or post surgery.
The healthy women group were also examined by a gynecologist prior to blood collection and subjects with a clinical history of endometriosis or mild gynecological conditions were excluded. Women included in the control group were volunteers without visible/perceptible changes in the adnexa and in the anamnesi. The group was examined by a gynecologist prior to blood collection and the ultrasound examination was performed in every case.
The ovarian cancer patients and the control group (benign lesions) were treated in the Department of Gynecology, University Hospital in Bialystok, Poland, in the years 2009–2014. The study was approved by the local Ethics Committee of the Medical University in Bialystok, numbers: R-I-002/314/2009 and R-I-002/262/2010 and all the patients gave their informed consent for study participation.
Biochemical analyses
Venous blood samples were collected from each patient. Blood was collected into a heparin sodium tube, centrifuged 1000 rpm for 15 min. to obtain plasma samples and stored at −850 C until assayed. Tested metalloproteinase-7 (MMP-7) and tissue inhibitor of metalloroteinase-1 (TIMP-1) were measured with the enzyme-linked immunosorbent assay (ELISA) (Quantikine Human Total MMP-7 Immunoassay, Human TIMP-1 Immunoassay, R&D systems) according to the manufacturer’s protocols. Duplicate samples were assessed for each patient.
The intra-assay coefficient of variation (CV%) of MMP-7 is reported to be 3.7% at a mean concentration of 4.58 ng/ml, SD = 0.168. The inter-assay coefficient of variation (CV%) of MMP-7 is reported to be 4.1% at a mean concentration of 4.82 ng/ml, SD = 0.198.The intra-assay coefficient of variation (CV%) of TIMP-1 – 5.0% at a mean concentration of 6.95 ng/ml, SD = 0.35. The inter-assay coefficient of variation (CV%) of TIMP-1 – 4.9% at a mean concentration of 6.90 ng/ml, SD = 0.34. The assay showed no significant cross-reactivity or interference with numerous human metalloproteinases and tissue inhibitors of metalloproteinases (TIMPs).
Plasma concentrations of CA125 and HE4 were measured by chemiluminescent microparticle immunoassay (CMIA) (Abbott, Chicago, IL, USA). The intra-assay CV for CA125 is reported to be 2.4% at a mean concentration of 43.5 U/ml, SD = 1.1. The inter-assay CV for CA125–3.9% at a mean concentration of 43.5 U/ml, SD = 1.7. The intra-assay CV for HE4–3.7% at a mean concentration of 39.0 pmol/L, SD = 1.4. The inter-assay CV for HE4–2.8% at a mean concentration of 39.0 pmol/L, SD = 1.1.
Statistical analysis
Statistical analysis was performed using the STATISTICA 8.0 PL program. A preliminary statistical analysis (Chi-square test) revealed that the distribution of MMP-7, TIMP-1 and tumor markers levels did not follow a normal distribution. Consequently, nonparametric methods were used to compare tumor marker levels between patient groups. Comparisons between two groups were performed using the Mann-Whitney test. In the case of multiple groups, Kruskal-Wallis tests were calculated with post hoc comparisons according to the Dwass-Steele-Critchlow-Fligner method. ROC analyses were utilised in the evaluation of the diagnostic power of tumor markers. Markers were compared by assessing the significance of differences between the areas under their corresponding ROC curves. In addition, markers were compared by assessing the differences in sensitivity and specificity obtained for the optimal cut-off points. The construction of the ROC curves was performed using the GraphRoc Program for Windows.
Data were presented as median and range. Statistically significant differences were defined as comparisons resulting in p < 0.05. The Spearman rank correlation was used in the correlation analyses.
The cut-off of MMP-7 (5.04 ng/ml), TIMP-1 (253.33 ng/ml), HE4 (93.81 pmol/L) and CA125 (107.09 U/ml) were calculated as the 95th percentile from the control group of healthy blood donors.
Results
In the total group of ovarian cancer (OC) patients, plasma levels of MMP-7 (5.60 ng/ml), TIMP-1 (170.79 ng/ml) and tumor markers, HE4 (207.09 pmol/L) or CA125 (139.70 U/ml) were found to be statistically higher compared to the healthy subjects (3.25 ng/ml; 128.88 ng/ml; 54.00 pmol/L; 12.70 U/ml) (p < 0.001, respectively) (Table 2). Moreover, we observed significant differences between the concentrations of all the parameters when every stage of cancer advancement (I-IV) was compared with the corresponding control group (with the exception of TIMP-1 – stage II): I -p < 0.001 (MMP-7, HE4 and CA125); II - p < 0.001 (in all cases); III - p < 0.001 (MMP-7, HE4 and CA125) and p = 0.001 (TIMP-1); IV - p < 0.001 (MMP-7, HE4 and CA125) and p = 0.011 (TIMP-1). Plasma concentrations of all aforementioned biomarkers were also significantly different in the advanced stages (III-IV) in comparison to those found in the early stages (I-II): MMP-7, TIMP-1, HE4 and CA125 in the comparison of stage III with stage I (p = 0.037; p = 0.005; p = 0.011; p = 0.002, respectively) and HE4 and CA125 in the comparison of stage III with stage II (p = 0.004; p = 0.013) or MMP-7, TIMP-1 and CA125 in the comparison of stage IV with I (p = 0.011; p = 0.033; p = 0.007), MMP-7 and CA125 in the comparison of stage IV with II (p = 0.010; p = 0.025) of tumor advancement.
Table 2.
Plasma levels of MMP-7, TIMP-1, HE4 and CA 125 in tested groups (statistically significant when p < 0.05)
| Groups | MMP-7 (ng/ml) |
TIMP-1 (ng/ml) |
HE4 (pmol/L) |
CA125 (U/ml) |
|
|---|---|---|---|---|---|
| Ovarian cancer Median Range | Stage I |
a, b4.76 1.98–17.86 |
a108.35 4.60–328.90 |
a, b118.70 34.50–1093.80 |
a66.73 10.60–557.20 |
| Stage II |
a, b4.73 2.24–18.00 |
151.70 15.20–839.00 |
a, b120.90 38.30–1205.70 |
a, b61.45 9.80–2060.78 |
|
| Stage III |
a, b, d7.92 1.98–17.80 |
a, b, d241.20 26.00–554.70 |
a, b, d650.55 48.70–1810.60 |
a, b, d766.20 10.10–2742.00 |
|
| Stage IV |
a, b, d12.27 2.00–27.40 |
a, b, d252.60 28.00–642.00 |
a, b372.95 37.80–1944.20 |
a, b, d541.13 14.30–8602.30 |
|
| Total group |
a, b5.60 1.98–27.40 |
a, b170.79 4.60–839.00 |
a, b207.09 34.50–1944.20 |
a, b139.70 9.80–8602.30 |
|
| Control groups Median Range | Benign ovarian tumor total group | 3.18 1.33–24.25 |
107.00 6.71–309.06 |
57.70 34.90–202.90 |
c22.90 5.80–748.00 |
| Healthy subjects | 3.25 1.75–8.42 |
128.88 23.38–266.09 |
54.00 15.00–408.89 |
12.70 1.49–36.60 |
|
aStatistically significant when comparing EOC patients with healthy subjects
bStatistically significant when comparing EOC patients with benign ovarian tumor total group
cStatistically significant when comparing patients with benign ovarian tumor and healthy subjects
dStatistically significant when comparing EOC patients in stage III or IV with stage I or II
Ovarian cancer patients (total group) had statistically considerably higher levels of all the researched factors (p < 0.001; in all cases) than patients with ovarian cysts (Table 2). We also observed similar, significantly higher concentrations of MMP-7 in stages I-IV (p < 0.001 in all cases), of TIMP-1 in stages III-IV (p < 0.001), of HE4 in stages I-IV (p < 0.001 in all cases) and of CA125 in stages II-IV (p = 0.002; p < 0.001; p < 0.001) of OC in comparison with the total benign lesions group.
We also noticed significant differences in the concentrations of CA125 when the ovarian cysts group was compared with the healthy subjects group (p < 0.001).
Table 3 presents the diagnostic criteria of parameters tested in OC patients. We observed higher SE ranges of MMP-7 and tumor markers in more advanced ovarian tumor stages (exception – TIMP-1). They were the highest for HE4. Interestingly, MMP-7 presented better results than CA125 in the groups with stage I-II (Table 3). Combined use of the studied biomarkers resulted in an increase in diagnostic SE to a very high range in stage I: 75% and II: 81%for the combination of MMP-7 with HE4 and CA125. The maximum ranges (96–100%) were obtained for the combinations of MMP-7 + HE4; HE4 + CA125; MMP-7 + CA125 as well as for the combination of MMP-7 or its tissue inhibitor with both markers in stages III-IV (Table 4).
Table 3.
The diagnostic criteria of MMP-7, TIMP-1, HE4 and CA 125 in epithelial ovarian cancer patients
| Epithelial ovarian cancer | Diagnostic criteria (%) | MMP-7 | TIMP-1 | HE4 | CA125 |
|---|---|---|---|---|---|
| Stage I | SE | 42 | 4 | 54 | 35 |
| SP | 95 | 95 | 95 | 98 | |
| PPV | 78 | 25 | 81 | 90 | |
| NPV | 83 | 73 | 80 | 75 | |
| Stage II | SE | 46 | 23 | 54 | 38 |
| SP | 95 | 95 | 95 | 98 | |
| PPV | 78 | 67 | 82 | 90 | |
| NPV | 81 | 76 | 71 | 82 | |
| Stage III | SE | 77 | 35 | 75 | 77 |
| SP | 95 | 95 | 95 | 98 | |
| PPV | 87 | 75 | 88 | 95 | |
| NPV | 92 | 78 | 86 | 90 | |
| Stage IV | SE | 79 | 16 | 88 | 83 |
| SP | 95 | 95 | 95 | 98 | |
| PPV | 86 | 80 | 86 | 95 | |
| NPV | 93 | 76 | 90 | 92 | |
| Total group | SE | 61 | 20 | 68 | 58 |
| SP | 95 | 95 | 95 | 98 | |
| PPV | 93 | 87 | 96 | 98 | |
| NPV | 61 | 44 | 66 | 60 |
Table 4.
The diagnostic criteria of MMP-7, TIMP-1 in combination with HE4 and CA 125 in epithelial ovarian cancer patients
| Epithelial ovarian cancer | Diagnostic criteria (%) | MMP-7 + HE4 | MMP-7 + CA125 | TIMP-1 + HE4 | TIMP-1 + CA 125 | HE4 + CA 125 | MMP-7 + HE4 + CA125 | TIMP-1 + HE4 + CA 125 |
|---|---|---|---|---|---|---|---|---|
| Stage I | SE | 71 | 63 | 54 | 42 | 63 | 75 | 63 |
| SP | 91 | 94 | 91 | 94 | 94 | 89 | 89 | |
| PPV | 74 | 79 | 68 | 71 | 79 | 72 | 68 | |
| NPV | 89 | 87 | 84 | 81 | 87 | 91 | 86 | |
| Stage II | SE | 62 | 65 | 62 | 42 | 73 | 81 | 73 |
| SP | 91 | 94 | 91 | 94 | 94 | 89 | 89 | |
| PPV | 73 | 81 | 73 | 73 | 83 | 75 | 73 | |
| NPV | 85 | 87 | 85 | 80 | 90 | 92 | 89 | |
| Stage III | SE | 96 | 92 | 88 | 85 | 96 | 100 | 100 |
| SP | 91 | 94 | 91 | 94 | 94 | 89 | 89 | |
| PPV | 81 | 86 | 79 | 85 | 86 | 79 | 79 | |
| NPV | 98 | 97 | 95 | 94 | 98 | 100 | 100 | |
| Stage IV | SE | 92 | 96 | 79 | 83 | 96 | 96 | 96 |
| SP | 91 | 94 | 91 | 94 | 94 | 89 | 89 | |
| PPV | 79 | 85 | 76 | 83 | 85 | 77 | 77 | |
| NPV | 97 | 98 | 92 | 94 | 98 | 98 | 98 | |
| Total group | SE | 80 | 81 | 71 | 63 | 82 | 88 | 83 |
| SP | 91 | 94 | 91 | 94 | 94 | 89 | 89 | |
| PPV | 93 | 95 | 92 | 94 | 95 | 93 | 92 | |
| NPV | 75 | 74 | 67 | 62 | 77 | 83 | 77 |
The diagnostic specificities of the biomarkers tested (SP) presented high, comparable values: 95%–98% (Table 3).
PPV in the total group of OC patients had very high values (87%–98%) for all the parameters tested, NPV was the highest for HE4 (66%) (Table 3). Combined use of the biomarkers studied for the remaining group resulted in an increase in NPV (83%) values and a decrease in PPV (95%) (Table 4). A maximum range of NPV (98–100%) was obtained for the combination of MMP-7 with HE4 and/or CA125 in stages III-IV of ovarian cancer.
To evaluate the dependence between the investigated parameters we used the Spearman’s rank correlation (Table 5). There were only positive significant correlations in the ovarian cancer total group: between the HE4 and CA125 concentrations (R = 0.39; p < 0.001), between the CA125 and MMP-7 concentrations (R = 0.27; p = 0.007,) between the CA125 and TIMP-1 concentrations (R = 0.30; p = 0.002), between the HE4 and MMP-7 concentrations (R = 0.35; p < 0.001), and between the HE4 and TIMP-1 or the MMP-7 and TIMP-1 concentrations (R = 0.24; p = 0.014). Furthermore, significant positive correlations were noticed between the HE4 and MMP-7 (R = 0.24; p = 0.008) or TIMP-1 (R = 0.27; p = 0.002) concentrations as well as between the MMP-7 and TIMP-1 concentrations (R = 0.25; p = 0.006) in the ovarian cysts group.
Table 5.
The Spearman rank correlation for MMP-7, TIMP-1, HE4 and CA125 in tested groups
| MMP-7 | TIMP-1 | HE4 | CA125 | |||
|---|---|---|---|---|---|---|
| EOC | MMP-7 | R | 1.00 | 0.24 | 0.35 | 0.27 |
| p | 0.014 | <0.001 | 0.007 | |||
| TIMP-1 | R | 0.24 | 1.00 | 0.24 | 0.30 | |
| p | 0.014 | 0.014 | 0.002 | |||
| HE4 | R | 0.35 | 0.24 | 1.00 | 0.39 | |
| p | <0.001 | 0.014 | <0.001 | |||
| CA125 | R | 0.27 | 0.30 | 0.39 | 1.00 | |
| p | 0.007 | 0.002 | <0.001 | |||
| Benign Ovarian Tumor | MMP-7 | R | 1.00 | 0.25 | 0.24 | 0.06 |
| p | 0.006 | 0.008 | 0.526 | |||
| TIMP-1 | R | 0.25 | 1.00 | 0.27 | 0.001 | |
| p | 0.006 | 0.002 | 0.966 | |||
| HE4 | R | 0.24 | 0.27 | 1.00 | 0.17 | |
| p | 0.008 | 0.002 | 0.070 | |||
| CA125 | R | 0.06 | 0.001 | 0.17 | 1.00 | |
| p | 0.526 | 0.966 | 0.070 | |||
| Healthy Subjects | MMP-7 | R | 1.00 | 0.12 | −0.12 | −0.05 |
| p | 0.336 | 0.331 | 0.682 | |||
| TIMP-1 | R | 0.12 | 1.00 | 0.13 | −0.03 | |
| p | 0.336 | 0.286 | 0.794 | |||
| HE4 | R | −0.12 | 0.13 | 1.00 | 0.14 | |
| p | 0.331 | 0.286 | 0.268 | |||
| CA125 | R | −0.05 | −0.03 | 0.14 | 1.00 | |
| p | 0.682 | 0.794 | 0.268 | |||
Bold data are statistically significant when p < 0.05
The relationship between diagnostic SE and SP was illustrated by the ROC (receiver-operating characteristics) curve. The AUCs of all compared biomarkers (with the exception of TIMP-1) were significantly higher compared to AUC = 0.5 in every studied OC group (Tables 6, 7). We demonstrated that the CA125 (0.8988) and HE4 (0.8836) areas under the ROC curve were the largest in the total group of OC (Table 6; Fig. 1). The AUCs of CA125 and HE4 were also the largest in the groups of patients with stages I-IV of the disease. Combining the studied parameters resulted in a further increase in the area under the ROC curve in every case (especially for the combination of MMP-7 + HE4 + CA125) to the value: 0.8635 in stage I; 0.9385 in stage II; 0.9935 in stage III, 0.9788 in stage IV and 0.9382 in the total OC group (Table 7). It should be emphasised that the areas under the ROC curve in various stages of cancer for MMP-7 in combination with HE4 or CA125 were as large as those for the combination of CA125 and HE4.
Table 6.
The diagnostic criteria of the ROC curve for MMP-7, TIMP-1, HE4 and CA125 in epithelial ovarian cancer patients
| Epithelial ovarian cancer | The ROC criteria | MMP-7 | TIMP-1 | HE4 | CA 125 |
|---|---|---|---|---|---|
| Stage I | AUC | 0.7801a | 0.5769 | 0.8343a | 0.8324a |
| SE | 0.0693 | 0.0718 | 0.0582 | 0.0471 | |
| 95% C.I. | 0.644–0.916 | 0.436–0.718 | 0.720–0.948 | 0.740–0.925 | |
| pAUC = 0.5 | 0.0001 | 0.2840 | <0.001 | <0.001 | |
| Stage II | AUC | 0.7938a | 0.6109 | 0.8462a | 0.8825a |
| SE | 0.0639 | 0.0772 | 0.0479 | 0.0397 | |
| 95% C.I. | 0.669–0.919 | 0.460–0.762 | 0.752–0.940 | 0.805–0.960 | |
| pAUC = 0.5 | <0.001 | 0.1506 | <0.001 | <0.001 | |
| Stage III | AUC | 0.8905a | 0.7740a | 0.9521a | 0.9331a |
| SE | 0.0485 | 0.0643 | 0.0288 | 0.0327 | |
| 95% C.I. | 0.796–0.986 | 0.648–0.900 | 0.896–1.008 | 0.869–0.997 | |
| pAUC = 0.5 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage IV | AUC | 0.8679a | 0.7314a | 0.8994a | 0.9458a |
| SE | 0.0624 | 0.0778 | 0.0511 | 0.0252 | |
| 95% C.I. | 0.746–0.990 | 0.579–0.884 | 0.799–1.000 | 0.896–0.995 | |
| pAUC = 0.5 | <0.001 | 0.0029 | <0.001 | <0.001 | |
| Total group | AUC | 0.8335a | 0.6372a | 0.8836a | 0.8988a |
| SE | 0.330 | 0.0425 | 0.0263 | 0.0235 | |
| 95% C.I. | 0.769–0.898 | 0.554–0.721 | 0.832–0.935 | 0.853–0.945 | |
| pAUC = 0.5 | <0.001 | 0.0013 | <0.001 | <0.001 |
C.I. – confidence intervals of AUC
aStatistically significant when comparing tested parameters AUC’s with 0.5 AUC
Bold data are statistically significant when p < 0.05
Table 7.
The diagnostic criteria of the ROC curve for MMP-7, TIMP-1in combination with HE4 and CA125 in epithelial ovarian cancer patients
| EOC | The ROC criteria | MMP-7 + HE4 | MMP-7 + CA 125 | TIMP-1 + HE4 | TIMP-1 + CA 125 | HE4 + CA 125 | MMP-7 + HE4 + CA 125 | TIMP-1 + HE4 + CA 125 |
|---|---|---|---|---|---|---|---|---|
| Stage I | AUC | 0.8596a | 0.7981a | 0.7795a | 0.6532a | 0.8474a | 0.8635a | 0.8071a |
| SE | 0.0571 | 0.0689 | 0.0668 | 0.0730 | 0.0573 | 0.0566 | 0.0646 | |
| 95% C.I. | 0.748–0.972 | 0.663–0.933 | 0.648–0.910 | 0.510–0.796 | 0.735–0.960 | 0.752–0.974 | 0.681–0.934 | |
| pAUC = 0.5 | <0.001 | <0.001 | <0.001 | 0.0359 | <0.001 | <0.001 | <0.001 | |
| Stage II | AUC | 0.8456a | 0.9284a | 0.8290a | 0.7479a | 0.9331a | 0.9385a | 0.8982a |
| SE | 0.0521 | 0.0262 | 0.0556 | 0.0722 | 0.0255 | 0.0234 | 0.0401 | |
| 95% C.I. | 0.744–0.948 | 0.877–0.980 | 0.720–0.938 | 0.607–0.889 | 0.883–0.983 | 0.893–0.984 | 0.820–0.977 | |
| pAUC = 0.5 | <0.001 | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 | |
| Stage III | AUC | 0.9864a | 0.9704a | 0.9657a | 0.9349a | 0.9893a | 0.9935a | 0.9923a |
| SE | 0.0085 | 0.0233 | 0.0225 | 0.0412 | 0.0070 | 0.0052 | 0.0060 | |
| 95% C.I. | 0.970–1.003 | 0.925–1.016 | 0.922–1.010 | 0.854–1.016 | 0.976–1.003 | 0.983–1.004 | 0.981–1.004 | |
| pAUC = 0.5 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Stage IV | AUC | 0.9513a | 0.9519a | 0.9673a | 0.9423a | 0.9487a | 0.9788a | 0.9526a |
| SE | 0.0406 | 0.0399 | 0.0155 | 0.0329 | 0.0370 | 0.0119 | 0.0405 | |
| 95% C.I. | 0.872–1.031 | 0.874–1.030 | 0.937–0.998 | 0.878–1.007 | 0.876–1.021 | 0.955–1.002 | 0.873–1.032 | |
| pAUC = 0.5 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Total group | AUC | 0.9109a | 0.9137a | 0.8858a | 0.8205a | 0.9309a | 0.9382a | 0.9202a |
| SE | 0.0237 | 0.0239 | 0.0263 | 0.0332 | 0.0205 | 0.0198 | 0.0222 | |
| 95% C.I. | 0.864–0.957 | 0.867–0.961 | 0.834–0.937 | 0.755–0.885 | 0.891–0.971 | 0.899–0.977 | 0.877–0.964 | |
| pAUC = 0.5 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
C.I. – confidence intervals of AUC
aStatistically significant when comparing tested parameters AUC’s with 0.5 AUC
Bold data are statistically significant when p < 0.05
Fig. 1.
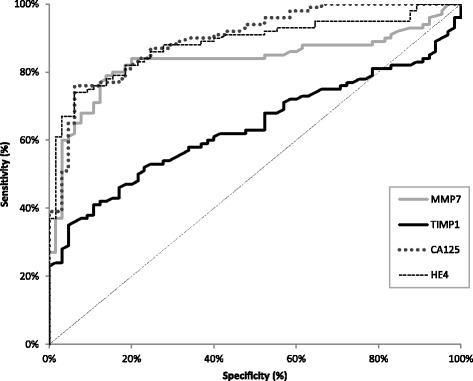
Diagnostic criteria of ROC curve for tested parameters in total EOC group
Discussion
Enhanced activity of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) has been proven to be closely associated with tumor aggressiveness, metastasis and poor prognosis [15, 26]. In this study we investigated the diagnostic usefulness of MMP-7 and TIMP-1 separately, and in combination with HE4 and CA125, which may improve the effectiveness of non-invasive diagnostics in patients with epithelial ovarian malignancies. Furthermore, we performed a comparison of the received results with the control group results (benign ovarian lesions patients and healthy subjects). Additionally, we estimated the diagnostic utility of the aforementioned parameters in correlation to the stage of cancer disease.
Our results showed significantly higher plasma concentrations of the commonly used tumor markers in every stage of advancement as well as in the total OC group in comparison to the healthy subjects group and these results are in line with our previous papers [13, 28] and with research results published by other authors [29, 30]. We found comparable results regarding MMP-7 in the ovarian cancer [31]. Moreover, the overexpression of this metalloproteinase was associated with poor survival and/or correlated with the tumor stage of various malignancies [32–35]. Our results are consistent with the results of Määttä et al. [36] who observed increased levels of TIMP-1 in the course of ovarian cancer, although the tested group was considerably smaller (22 cases) and composed of serous, mucinous and others malignant ovarian tumors. These data are also very similar to the studies of researchers who compared patients with breast cancer [37] with healthy volunteers.
In opposition to our findings, Acar et al. [31] found no significant differences in serum MMP-7 levels in patients with benign ovarian disease (only 10 cases were included) when compared to patients with malignant disease. The results reported in the available literature regarding TIMP-1 [36–38] correspond to the results of the current study and to our previous publications regarding breast cancer [39, 40]. Regardless of the menopausal status and composition of the groups compared, statistically higher concentrations of comparative tumor markers (p = 0.001 up to p < 0.0001) were observed in ovarian cancer groups in comparison with benign diseases control groups [12, 29, 30, 41]. These results correspond to our previous publications [13, 28]. Other researchers, in line with the present study, have reported a lack of statistically significant differences in serum MMP-7 concentrations between benign ovarian lesions and healthy women groups [31]. By contrast, Beeghly-Faidel et al. [42] found a significantly higher MMP-7 expression in endometrial hyperplasia in comparison with normal endometrium. We were unable to confirm our findings regarding TIMP-1 in the published literature since no reports on the subject are available. Our present observations confirm the results of our previous study, which found significantly higher concentrations of CA125 in a group of 70 postmenopausal women with benign lesions of the ovary (cysts) [43].
The Spearman’s rank correlation test revealed that the degree of correlation between the concentrations of MMP-7, TIMP-1, HE4 and CA125 was not particularly strong (R:0.24–0.39).
This may indicate that each of the markers was elevated independently of the remaining ones and supports the proposition of a combined analysis. Unfortunately, we could not compare our data regarding MMP-7 and tissue inhibitor of metalloproteinase −1 with other publications. A positive correlation between TIMP-1 and CA15–3 concentrations in a group including 100 breast cancer patients (stages I-IV) (R = 0.28) has also been previously revealed [39]. Some authors have demonstrated significant positive correlations between CA125 and HE4 levels in patients with ovarian malignancies (R = 0.54) [44, 45].
The present study demonstrated that diagnostic sensitivity was the highest for HE4, although SE of MMP-7 reached equal or even higher values than CA125, especially in stages I-II. Our results are in agreement with the published literature [29, 46]. It is worth emphasizing that we found a maximum increase in diagnostic sensitivity for the combination of MMP-7 with both tumor markers to 75% in stage I, even to 81%–100% in stages II-IV as compared with the use of either marker alone or of both comparative tumor markers together. Several studies have confirmed this observation - they found sensitivity to be greater than in either marker used alone: MMP-7, CCL18 (CC chemokine 18), CCL11 (CC chemokine 11) and CA125 in ovarian cancer (SE in the early stages 94.4%) [46]. This conclusion is also in accordance with our previous papers in which the diagnostic criteria of selected cytokines and aforementioned tumor markers were evaluated in various gynecological malignancies [47, 48]. Diagnostic specificity (SP) reached very high and equal values for all biomarkers studied and this was in accordance [40, 43] with the available literature in the course of various malignant and benign diseases.
Notably, MMP-7 revealed high and comparable values of PPV and NPV to the values presented by HE4 and CA125 in every stage of advancement and in the total OC group. In the current study, the combination of both comparative tumor markers with MMP-7 had unquestionably higher NPV value ~100%. Unfortunately, we were unable to compare the findings concerning our diagnostic panel with the papers published since no reports on the subject are available. Interestingly, the presented results of the classic tumor markers diagnostic criteria in OC are partially in accordance with a publication by Hamed et al. [29] who observed higher values of PPV and NPV for HE4 or CA125 separately (93.1%/80.7% and 92.7%/87.2%, respectively) in 30 patients with epithelial ovarian cancer versus 20 healthy women of varying menopausal status.
The area under the ROC curve (AUC) of 1 indicates a desirable, high diagnostic power of a test. Following our analysis, HE4 (0.8836) and CA125 (0.8988) showed the largest areas under the ROC curve in the total group of ovarian cancer as well as in the groups divided according to tumor stage. Moreover, we demonstrated that the utilisation of a combined panel of MMP-7 with both known tumor markers undoubtedly improved cancer detection in every stage, but especially in the early stages of the disease (0.8343 and 0.8324 vs 0.8635; respectively – I stage). In line with the present data, preoperative serum TIMP-1 concentration showed insufficient diagnostic power (AUC = 0.730) in differentiating between low malignant potential and malignant ovarian tumors [36]. In a few previous publications the AUC values for differentiating ovarian cancer were significantly higher for the combination of various biomarkers [36, 49], which is in line with our findings [13, 28, 43]. Differences in study results might be due to differences in the histological types or disease stages of ovarian cancer and in the number of patients enrolled in each study.
Conclusions
In summary, to the authors’ knowledge, our report is the first to evaluate the diagnostic usefulness of MMP-7 and TIMP-1 independently and, especially, in combination with both established ovarian tumor markers. The results of this study suggest that combining MMP-7, HE4 and CA125 measurements might enable the improved, early detection of selected histological types of EOC when compared with the use of either marker alone. Moreover, the investigated metalloproteinase presented similar to HE4 and CA125 diagnostic usefulness in opposition to TIMP-1 whose presented diagnostic usefulness was undoubtedly insufficient.
Acknowledgements
This study was financed by a Statutory Grant for Scientific Research from the Polish Ministry of Science and Higher Education in the years 2015-2016.
Funding
This study was financed by a Statutory Grant for Scientific Research from the Polish Ministry of Science and Higher Education (N N407 530,738 and N/ST/ZB/15/003/2208 and N/ST/ZB/16/002/2208). The founding body did not participate in the preparation of this manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Additional file 1].
Authors’ contributions
GEB conducted the immunoassays, performed the statistical analysis and drafted the manuscript. EG conducted data acquisition and participated in sequence alignment. MZ conducted the immunoassays. EKG conducted the immunoassays. JO helped to draft the manuscript. MS helped to draft the manuscript. LC helped to draft the manuscript. MD participated in the design and coordination of the study and data interpretation. SŁ conceived of the study, conducted the immunoassays and helped to draft the manuscript. All authors have read and approved of the final manuscript.
Authors’ information
This study is a continuation of our research programme concerning cancers of the breast and the reproductive organs, of which several previous manuscripts have been published in this journal in the last few years.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the local Ethics Committee of the Medical University in Bialystok, numbers: R-I-002/314/2009 and R-I-002/262/2010 and all the patients gave their informed consent for study participation.
Abbreviations
- AFP
α-fetoprotein
- AUC
Area under ROC curve
- CA 125
Carbohydrate antigen 125
- CA19–9
Carbohydrate antigen 19–9
- CCL
CC chemokine
- CEA
Carcinoembryonic antigen
- CMIA
Chemiluminescent microparticle immunoassay
- CRP
C Reactive Protein
- CT
Computed tomography
- CV
Coefficient of variation
- ELISA
Enzyme-linked immunosorbent assay
- EOC
Epithelial ovarian cancer
- FIGO
International Federation of Gynecology and Obstetrics
- HE4
Human epididymis protein 4
- HGFs
Hematopoietic growth factors
- M-CSF
Macrophage-colony stimulating factor
- MMP-7
Metalloproteinase 7
- NPV
Negative predictive value
- OC
Ovarian cancer
- PPV
Positive predictive value
- ROC
Receiver-operating characteristics
- SAA
Serum amyloid A
- SD
Standard deviation
- SE
Diagnostic sensitivity
- SP
Diagnostic specificity
- TIMP-1
Tissue inhibitor of metalloproteinase-1
- TNM
Tumor-nodulus-metastasis classification
- VEGF
Vascular endothelial growth factor
- WHO
World Health Organization
- βFGF
Basic fibroblast growth factor
Additional file
Spearman rank correlation. (XLSX 10 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13048-017-0338-z) contains supplementary material, which is available to authorized users.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;6:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–216. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Vergote I, Banerjee S, Gerdes AM, van Asperen C, Marth C, Vaz F. Current perspectives on recommendations for BRCA genetic testing in ovarian cancer patients. Eur J Cancer. 2016;69:127–134. doi: 10.1016/j.ejca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Xu K, Yang S, Zhao Y. Prognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysis. Oncotarget. 2017;8:285–302. doi: 10.18632/oncotarget.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina R, Escudero JM, Auge JM, Filella X, Foj L, Torne A, et al. HE4 a novel tumour marker for ovarian cancer: comparison with CA 125 and ROMA algorithm in patients with gynaecological diseases. Tumor Biol. 2011;32:1087–1095. doi: 10.1007/s13277-011-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban A, Miszczyk L. Ovarian cancer – diagnostical and therapeutical dilema in oncological gynecology. Wspol Onkol. 2003;7:294–300. [Google Scholar]
- 7.Schummer M, Drescher C, Forrest R, Gough S, Thorpe J, Hellstrom I, et al. Evaluation of ovarian cancer remission markers HE4, MMP7 and mesothelin by comparison to the established marker CA125. Gynecol Oncol. 2012;125:65–69. doi: 10.1016/j.ygyno.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KKL, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol. 2013;128:239–244. doi: 10.1016/j.ygyno.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 10.Galgano MT, Hampton GM, Frierson HF., Jr Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–853. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 11.Jiang SW, Chen H, Dowdy S, Fu A, Attewell J, Kalogera E, et al. HE4 transcription and splice variants-specific expression in endometrial cancer and correlation with patient survival. Int J Mol Sci. 2013;14:22655–22677. doi: 10.3390/ijms141122655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore RG, Miller MC, Steinhoff MM, Skates SJ, Lu KH, Lambert-Messerlian G, et al. Serum HE4 levels are less frequently elevated than CA 125 in women with benign gynecologic disorders. Am J Obstet Gynecol. 2012;206:351.e1–8. [DOI] [PMC free article] [PubMed]
- 13.Ławicki S, Gacuta-Szumarska E, Będkowska GE, Szmitkowski M. Hematopoietic cytokines as tumor markers in gynecological malignancies. A multivariate analysis in epithelial ovarian cancer patients. Growth Factors. 2012;30:357–366. doi: 10.3109/08977194.2012.724407. [DOI] [PubMed] [Google Scholar]
- 14.Candido dos Reis FJ, Moreira de Andrade J, Bighetti S. CA 125 and vascular endothelial growth factor in the differential diagnosis of epithelial ovarian tumors. Gynecol Obstet Invest. 2002;54:132–136. doi: 10.1159/000067877. [DOI] [PubMed] [Google Scholar]
- 15.Behrens P, Rothe M, Florin A, Wellman A, Wernert N. Invasive properties of serous human epithelial ovarian tumors are related to Ets-1, MMP-1 and MMP-9 expression. Int J Mol Med. 2001;8:149–154. doi: 10.3892/ijmm.8.2.149. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cells invasion. Arch Gynecol&Obstet. 2012;286:1537–1543. doi: 10.1007/s00404-012-2456-6. [DOI] [PubMed] [Google Scholar]
- 17.Stamenkovic I. Extracellular matrix remodeling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 18.Worley JR, Thompkins PB, Lee MH, Hutton M, Soloway P, Edwards DR, et al. Sequence motifs of tissue inhibitor of metalloproteinases 2 (TIMP-2) determining progelatinase a (proMMP-2) binding and activation by membrane-type metalloproteinase 1 (MT1-MMP) Biochem J. 2003;372:799–809. doi: 10.1042/bj20021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22:145–152. doi: 10.1023/A:1023039230052. [DOI] [PubMed] [Google Scholar]
- 20.Yin H, Sheng Z, Zhang X, Du Y, Qin C, Liu H, et al. Overexpression of SOX18 promotes prostate cancer progression via the regulation of TCF1, c-Myc, cyclin D1 and MMP-7. Oncol Rep. 2017;37:1045–1051. doi: 10.3892/or.2016.5288. [DOI] [PubMed] [Google Scholar]
- 21.Xiao XY, Lang XP. Correlation between MMP-7 and bFGF expressions in non-small cell lung cancer tissue and Clinicopathologic features. Cell Biochem Biophys. 2015;73:427–432. doi: 10.1007/s12013-015-0656-y. [DOI] [PubMed] [Google Scholar]
- 22.Niedworok C, vom Dorp F, Tschirdewahn S, Rübben H, Reis H, Szucs M, et al. Validation of the diagnostic and prognostic relevance of serum MMP-7 levels in renal cell cancer by using a novel automated fluorescent immunoassay method. Int Urol Nephrol. 2016;48:355–361. doi: 10.1007/s11255-015-1185-8. [DOI] [PubMed] [Google Scholar]
- 23.Xing XJ, Gu XH, Ma TF. Relationship of serum MMP-7 levels for colorectal cancer: a meta analysis. Tumour Biol. 2014;35:10515–10522. doi: 10.1007/s13277-014-2349-3. [DOI] [PubMed] [Google Scholar]
- 24.Yari K, Rahimi Z, Payandeh M, Rahimi Z. MMP-7 A-181G polymorphism in breast cancer patients from western Iran. Breast Care (Basel) 2015;10:398–402. doi: 10.1159/000442231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen IJ, Brünner N, Dowell B, Davis G, Nielsen HJ, Newstead G, et al. Plasma TIMP-1 and CEA as markers for detection of primary colorectal cancer: a prospective validation study including symptomatic and non-symptomatic individuals. Anticancer Res. 2015;35:4935–4941. [PubMed] [Google Scholar]
- 26.Gong Y, Chippada-Venkata UD, Galsky MD, Huang J, Oh WK. Elevated circulating tissue inhibitor of metalloproteinase 1 (TIMP-1) levels are associated with neuroendocrine differentiation in castration resistant prostate cancer. Prostate. 2015;75:616–627. doi: 10.1002/pros.22945. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson C, Elliott V, Menon U, Apostolidou S, Fourkala OE, Gentry-Maharaj A. Evaluation in pre-diagnosis samples discounts ICAM-1 and TIMP-1 as biomarkers for earlier diagnosis of pancreatic cancer. J Proteome. 2015;113:400–402. doi: 10.1016/j.jprot.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Ławicki S, Będkowska GE, Gacuta-Szumarska E, Szmitkowski M. The plasma concentration of VEGF, HE4 and CA125 as a new biomarkers panel in different stages and sub-types of epithelial ovarian tumors. J Ovarian Res. 2013;6:45–55. doi: 10.1186/1757-2215-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamed EO, Ahmed H, Sedeek OB, Mohammed AM, Abd-Alla AA, Abdel Ghaffar HM. Significance of HE4 estimation in comparison with CA 125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn Pathol. 2013;8:s11. doi: 10.1186/1746-1596-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggeri G, Bandiera E, Zanotti L, Belloni S, Ravaggi A, Romani C, et al. HE4 and epithelial ovarian cancer: comparison and clinical evaluation of two immunoassays and a combination algorithm. Clin Chim Acta. 2011;412:1447–1453. doi: 10.1016/j.cca.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Acar A, Onan A, Coskun U, Uner A, Bagriacik U, Atalay F, et al. Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med Oncol. 2008;25:279–283. doi: 10.1007/s12032-007-9031-1. [DOI] [PubMed] [Google Scholar]
- 32.Kren L, Goncharuk VN, Krenova Z, Stratil D, Hermanova M, Skrickova J, et al. Expression of matrix metalloproteinases 3, 10 and 11 (stromelysins 1,2 and 3) and matrix metalloproteinase 7 (matrilysin) by cancer cells in non-small cell lung neoplasms. Clinicopathologic studies Cesk Patol. 2006;42:16–19. [PubMed] [Google Scholar]
- 33.Sier CF, Hawinkels LJ, Zijlmans HJ, Zuidwijk K, de Jonge-Muller ES, Ferreira V. Endothelium specific matrilysin (MMP-7) expression in human cancers. Matrix Biol. 2008;27:267–271. doi: 10.1016/j.matbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Sica GS, Fiorani C, Stolfi C, Monteleone G, Candi E, Amelio I, et al. Peritoneal expression of matrilysin helps identify early post-operative recurrence of colorectal cancer. Oncotarget. 2015;6:13402–13415. doi: 10.18632/oncotarget.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koskensalo S, Mrena J, Wiksten JP, Nordling S, Kokkola A, Hagstrom J, et al. MMP-7 overexpression is an independent prognostic marker in gastric cancer. Tumor Biol. 2010;31:149–155. doi: 10.1007/s13277-010-0020-1. [DOI] [PubMed] [Google Scholar]
- 36.Määttä M, Talvensaari-Mattila A, Turpeenniemi-Hujanen T, Santala M. Matrix metalloproteinase-2 (MMP-2) and −9 (MMP-9) and their tissue inhibitors (TIMP-1 and TIMP-2) in differential diagnosis between low malignant potential (LMP) and malignant ovarian tumours. Anticancer Res. 2007;27:2753–2758. [PubMed] [Google Scholar]
- 37.Wu Z-S, Wu Q, Yang J-H, Wang H-Q, Ding X-D, Yang F, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008;122:2050–2056. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 38.Holten-Andersen L, Christensen IJ, Jensen SB, Reibel J, Laurberg S. Nauntofte bet al. Saliva and plasma TIMP-1 in patients with colorectal cancer: a prospective study. Scand J Gastroenterol. 2012;47:1234–1241. doi: 10.3109/00365521.2012.711855. [DOI] [PubMed] [Google Scholar]
- 39.Ławicki S, Zajkowska M, Głażewska EK, Będkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of VEGF, MMP-9, and TIMP-1 in the diagnosis of patients with breast cancer. Onco Targets Ther. 2016;9:911–919. doi: 10.2147/OTT.S99959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ławicki S, Głażewska EK, Sobolewska M, Będkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of macrophage colony-stimulating factor, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 as a new biomarkers of breast cancer. Ann Lab Med. 2016;36:223–229. doi: 10.3343/alm.2016.36.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng H, Gao Y. Serum HE4 as a useful biomarker in discriminating ovarian cancer from benign pelvic disease. Int J Gynecol Cancer. 2012;22:1000–1005. doi: 10.1097/IGC.0b013e318249bee7. [DOI] [PubMed] [Google Scholar]
- 42.Beeghly-Faidel A, Xiang Y-B, Deming SL, Long JR, Xu W-H, Cai Q, et al. No association between matrix metalloproteinase (MMP)-1, −3, and −7 SNPs and endometrial cancer risk. Cancer Epidemiol Biomark Prev. 2009;18:1925–1928. doi: 10.1158/1055-9965.EPI-09-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Będkowska GE, Ławicki S, Gacuta E, Pawłowski P, Szmitkowski M. M-CSF in a new biomarker panel with HE4 and CA125 in the diagnostics of epithelial ovarian cancer patients. J Ovarian Res. 2015;8:27–38. doi: 10.1186/s13048-015-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park Y, Lee J-H, Hong DJ, Lee EY, Kim H-S. Diagnostic performances of HE4 and CA 125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem. 2011;44:884–888. doi: 10.1016/j.clinbiochem.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Azzam AZ, Hashad DI, Kamel NA. Evaluation of HE4 as an extrabiomarker to CA 125 to improve detection of ovarian carcinoma: is it time for a step forward? Arch Gynecol Obstet. 2013;288:167–172. doi: 10.1007/s00404-013-2722-2. [DOI] [PubMed] [Google Scholar]
- 46.Zohny SF, Fayed ST. Clinical utility of circulating matrix metalloproteinase-7 (MMP-7), CC chemokine ligand 18 (CCL 18) and CC chemokine ligand 11 (CCL 11) as a markers for diagnosis of epithelial ovarian cancer. Med Oncol. 2010;27:1246–1253. doi: 10.1007/s12032-009-9366-x. [DOI] [PubMed] [Google Scholar]
- 47.Ławicki S, Będkowska GE, Gacuta-Szumarska E, Knapp P, Szmitkowski M. Pretreatment plasma levels and diagnostic utility of hematopoietic cytokines in cervical cancer or cervical intraepithelial neoplasia patients. Folia Histochem Cytobiol. 2012;50:213–219. doi: 10.5603/FHC.2012.0030. [DOI] [PubMed] [Google Scholar]
- 48.Ławicki S, Będkowska GE, Gacuta-Szumarska E, Szmitkowski M. Hematopoietic cytokines as tumor markers in gynecological malignancies: a multivariate analysis with ROC curve in endometrial cancer patients. Growth Factors. 2012;30:29–36. doi: 10.3109/08977194.2011.627332. [DOI] [PubMed] [Google Scholar]
- 49.Fawzy A, Mohamed MR, Ali MA, Abd El-Magied MH, Helal AM. Tissues CA125 and HE4 gene expression levels offer superior accuracy in discriminating benign from malignant pelvic masses. Asian Pac J Cancer Prev. 2016;17:323–333. doi: 10.7314/APJCP.2016.17.1.323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its Additional file 1].


