Abstract
The use of non-human primates (NHPs) in studies of volitional, oral self-administration of alcohol can help address the complex interplay between stress and excessive alcohol consumption. There are aspects to brain, endocrine and behavior of NHPs, particularly macaques, that provide a critical translational link towards understanding the risks and consequences of alcohol use disorders (AUDs) in humans. These include wide individual differences in escalating daily alcohol intake, accurate measures of hypothalamic-pituitary-adrenal (HPA) axis hormonal interactions, neuroanatomical specificity of synaptic adaptations to chronic alcohol, genetic similarities to humans, and the ability to conduct in vivo brain imaging. When placed in a framework that alcohol addiction is a sequence of dysregulations in motivational circuitry associated with severity of AUD, the NHP can provide within-subject information on both risks for and consequences of repeatedly drinking to intoxication. Notably, long-term adaptations in neurocircuitry that mediate behavioral reinforcement, stress responses and executive functions are possible with NHPs. We review here the substantial progress made using NHPs to address the complex relationship between alcohol and stress as risk factors and consequences of daily drinking to intoxication. This review also highlights areas where future studies of brain and HPA axis adaptations are needed to better understand the mechanisms involved in stress leading to excessive alcohol consumption.
Keywords: Alcohol, ethanol, self-administration, monkey, allostasis, stress
I: Introduction
As this special issue on the neuropharmacology of alcoholism highlights, a useful approach to conceptualizing alcohol use disorders (AUDs) is based in understanding the cascading effects of repeatedly drinking to intoxication on neurocircuitry that mediate behavioral reinforcement, stress responses and executive functions (Koob and Volkow, 2016). Notably, this framework provides specific, testable hypotheses regarding the temporal sequence and specific mediators of dysregulation in motivational circuitry associated with severity of AUD. In this review, we highlight the non-human primate (NHP) studies of volitional, oral intake of alcohol and focus on the changes in the stress system as well as the proportion of monkeys that escalate and maintain drinking excessive levels. We emphasize the importance of NHPs as a translational link to human AUD both in terms of neuroendocrine physiology and the capacity to longitudinally assess neurocircuitry changes amenable to addressing the paradigm put forward by Koob and colleagues.
There is now strong evidence that the mammalian response to stress is an orchestration of endocrine, neural and behavioral processes that, in the face of chronic alcohol, can become maladaptive and propagate further escalations in alcohol intake (Becker, 2012; Blaine et al., 2015; Sinha et al., 2011). This underscores the phenomenon that the relationship between stress and alcohol is bidirectional. On one hand, stress is an etiological factor in the development of alcohol use disorders (Keyes et al., 2012) while on the other hand, pathological (i.e., allostatic) adaptations in the stress response occur due to continued alcohol consumption (Richardson et al., 2008; Sinha, 2012). Hypothalamic-pituitary-adrenal (HPA) axis activation is integral to the definition of stress, which is defined as any physical or psychological stimulus that challenges homeostasis and activates the HPA axis (Smith and Vale, 2006). However, regulation of the HPA axis is complex, flexible and reactive to changes in both the internal and external environments. Under chronic stress, the hypothalamic and extra-hypothalamic response loses flexibility and undergoes a compromised response to additional stressors resulting in a shift from homeostasis to allostasis (McEwen et al., 1993; McEwen, 1998).
The development of AUDs and resultant drinking severity are associated with altered HPA axis dysregulation, with most data primarily centered on glucocorticoid regulation (Wand, 2008). Beyond the HPA axis, the glucocorticoid receptor (GR) is abundantly expressed in mesolimbic reward circuitry (Harfstrand et al., 1986; Morimoto et al., 1996) and the extended amygdala (Patel et al., 2000; Pryce, 2008). Thus, the extra-hypothalamic corticotrophin-releasing factor (CRF) system can influence responses to stress via the amygdala and other limbic regions (Schulkin et al., 1998). Counter to the suppression of hypothalamic CRF, glucocorticoids increase CRF expression in the amygdala (Schulkin et al., 1998) and up-regulation of CRF by corticosterone contributes to anxiety-like and fearful behaviors to perceived stress. Indeed, in a recent study that overexpressed CRF in the central amygdala region of young rhesus macaques, anxious temperament was increased compared to cage-mate controls (Kalin et al., 2016). In turn, anxiety and negative emotional states facilitate the initiation, maintenance and relapse to alcohol use (Sinha, 2013). Notably, in animal studies, the GR antagonist mifepristone (MIFI) blocks alcohol-induced place preference (Rotter et al., 2012), reduces alcohol intake (Koenig and Olive, 2004; Vendruscolo et al., 2012; Vendruscolo et al., 2015), reduces alcohol withdrawal symptoms (Jacquot et al., 2008) and protects hippocampal neurons from injury due to binge-like alcohol consumption (Cippitelli et al., 2014). The translation of the efficacy of MIFI was recently shown to reduce cue-induced alcohol craving and alcohol intake in human subjects with AUD (Vendruscolo et al., 2015). Perhaps understudied in terms of adrenal hormonal response in chronic alcohol effects is the mineralocorticoid system, which is key to fluid regulation and motivated behavior at the level of the amygdala (Sakai et al., 2000).
In order to dissect the interactions of stress and excessive drinking, animal models play a key role. Monkey models of alcohol self-administration, as with other animal models, are able to reduce the impact of several key factors (e.g., nutritional status, housing environment, age at first intoxication, exposure to stressors, etc.) and isolate critical variables related to excessive alcohol consumption (Grant and Bennett, 2003). Monkeys have a prolonged adolescence and young adulthood phase, close genetic similarities, similar expansion of the cerebral cortex, and in the case of old world monkeys, neuroendocrine similarities to humans. These similarities are especially advantageous when studying endocrine physiology, where there are notable differences between primates (human and NHP) and rodents. For example, the relative distribution of opioid receptors in the frontal cortex differs substantially between rodents and humans (Mansour et al., 1988), corticotropin releasing factor binding protein (CRF-BP) is found both centrally and peripherally in primates while only in the brain and pituitary of rodents (Bowman et al., 2001; Seasholtz et al., 2002) and important morphological differences in the pituitary gland exist between rodents and humans (Kelberman et al., 2009). At the level of the adrenal gland, the primary glucocorticoid, providing negative feedback to the brain and pituitary to restore homeostasis following HPA axis activation, is corticosterone in rodents and cortisol in primates. The zona reticularis adrenocortical layer, where the primary adrenal androgen dehydroepiandrosterone (DHEA) is synthesized, is absent in rodents (Conley et al., 2004). And finally, rats have low levels of 5ß-reduced neuroactive steroids indicating species differences in neuroactive metabolites of adrenal steroids that may contribute to the subjective effects of alcohol (Helms et al., 2012c; Morrow et al., 2006; Porcu et al., 2009).
Given the translation potential of old world macaques and baboons, these NHPs have been particularly useful for addressing stress as an etiological factor in AUDs (Barr and Goldman, 2006; Cederbaum et al., 2009; Grant and Bennett, 2003; Grant et al., 2014). In comparisons to humans, macaque monkeys have similar alcohol absorption and metabolism rates and can self-administer large quantities of alcohol over months and years (Baker et al., 2014; Green et al., 1999; Vivian et al., 2001). As discussed above, the adaptive response to chronic stress and chronic alcohol self-administration can result in a pathological state that involves the physiological integration of multiple organs impacting the primate HPA axis and brain. Thus, studies that address allostatic mechanisms involving longitudinal adaptations in brain circuitry are uniquely possible in NHPs (Grayson et al., 2014; Miranda-Dominquez et al., 2014). Importantly, like humans, NHPs show wide individual differences in their chronic intake of alcohol over years, with a large proportion voluntarily drinking amounts similar to human alcoholic chronic drinking levels (Baker et al., 2014; Baker et al., 2017; Mendelson and Mello, 1972;). More broadly, macaques and humans share similar genetic and epigenetic composition (Barr, 2013; Cervera-Juanes et al., 2016). For example, a recent study found CRH and serotonin gene polymorphisms in male rhesus macaques predicted a blunted ACTH response to dexamethasone (Ferguson et al., 2012). Thus gene-endocrine interactions can confer greater risk for HPA axis dysfunction, and can be utilized as a valuable tool in evaluating risk factors for a variety of behavioral disorders, including alcohol use disorders.
This review will focus on advances in our understanding of the interactions between stress and alcohol self-administration stemming primarily from a NHP model of alcohol self-administration designed to address these interactions. In these studies, each animal lives with an operant panel in the home-cage environment (see Vivian et al., 2001; Grant et al., 2008 for detailed descriptions of operant panels and procedures) where they are trained to obtain all food and fluids. Rather than using food or fluid deprivation or flavoring the alcohol solution to induce alcohol drinking, fluid is made available while small quantities of food are available on a fixed-time schedules (i.e., no response is required for the time-scheduled delivery of a food pellet). This schedule heightens HPA activity and results in adjunctive drinking in non-deprived monkeys (Mello and Mendelson, 1971; Grant and Johanson, 1988; Grant et al., 2008). This schedule-induced polydipsia procedure has been applied to induce daily consumption of alcohol (4% w/v ethanol) in a protocol that increases the daily dose from 0.5 g/kg/day, to 1.0 g/kg/day to 1.5 g/kg/day, in 30-day epochs of each dose. The blood ethanol concentrations (BEC) obtained during this procedure ensures animals experience an intoxicating dose (80 mg/dl) (Grant et al., 2008). After being induced to drink 1.5 g/kg/day for at least 30 consecutive days, the induction schedule is terminated and monkeys have concurrent “open-access” to water and alcohol 22-h/day with food available in three equal meals at 2-hr intervals.
Applying a standard protocol to assess the population distribution of daily alcohol drinking, given nearly unlimited access to 4% (w/v diluted in water) (Baker et al., 2014; Grant et al., 2008), has provided longitudinal self-administration and endocrine data from dozens of macaques that are conducive for studying adaptations in neurocircuitry that integrate genetic (Cervera-Juanes et al., 2017; Iancu et al., 2017) and neuroimaging techniques (Kroenke et al., 2013; 2014). Using this animal model, novel approaches to both genetic (Cervera-Juanes et al., 2016) and MRI connectivity studies can help address highly translational endocrine, neuroanatomical and behavioral measures of the temporal sequence and specific mediators of dysregulation in motivational circuitry associated with severity of AUDs.
II: Assessment of Risks for Heavy Drinking and Stress Interactions
Major risk factors for heavy drinking identified in macaque monkeys include age at the onset of drinking, social setting, endocrine factors and epigenetic processes. Across cultures, alcohol use most often begins during late adolescence (Hall et al., 2016) and individuals who begin heavy drinking during adolescence are more likely to to acquire AUDs (Dawson et al., 2008; Grant et al., 2001; Grant et al., 2006). The adolescent and young adult stages of life are known to be particularly stressful, both socially and biologically, as there is rapid growth in secondary sex characteristics and new social pressures placed on individuals. For male macaques, the late adolescent to young adult stage is also stressful and a time when individuals sever formed social relationships and leave the natal troop to find a place in a new troop, perhaps to ensure genetic diversity (Soumi, 2011). Female monkeys also experience profound social changes in late adolescent/young adult stage of life with sexual maturity and also show heightened HPA activity (Gust et al., 2000).
In the first study to explicitly study age as a risk factor for heavy drinking, male rhesus monkeys with a range of ages were enrolled into an alcohol self-administration protocol described previously (Helms et al., 2014a). Similar to humans, male rhesus who first experienced alcohol intoxication as late adolescents continued to increase their intake as they matured into young adults; while those monkeys who first experienced intoxication as young adults maintained greater alcohol intake on a daily basis compared to late adolescents. First experiencing alcohol intoxication in middle-age resulted in the lowest risk for heavy drinking. We have also observed the same relationship between age and heavy alcohol intake in female rhesus macaques (unpublished observation). Of the HPA-related hormones studied in the late adolescent/young adult male monkeys (ACTH, cortisol, deoxycorticosterone (DOC), DHEA-S, and aldosterone) the neurosteroid DOC at baseline was uniquely predictive of attaining high BECs during open-access self-administration (Helms et al., 2014b). Thus, both the young adult stage and circulating DOC appear related to risk for heavy drinking, although these factors cannot be disentangled with the current datasets.
Risk for heavy alcohol consumption due to stress can also be framed in terms of social setting and social rank. Standard housing conditions for NHP self-administration studies are individual cages with visual, olfactory, auditory contact and limited physical contact (animals can touch each others hands, if two neighboring monkeys are participating). To evaluate dominance rank, dividers between four individual cages were removed for two hours each day and social rank was determined by the outcomes of aggressive and affiliative interactions. Under a longitudinal design, late adolescent (4.2–5.3 years) cynomolgus male monkeys alternated housing conditions between individually (limited physical contact as described above) or social housing (group of 3–4 monkeys) every 3 months and blood samples were acquired in the early morning, mid-day and evening to capture the diurnal peak and trough. Independent of social rank, having direct contact with conspecifics temporarily elevated cortisol across all dominance ranks, demonstrating that social housing was a mild stressor. During social housing, ACTH was elevated across all social ranks, with the greatest increase in the dominant monkeys, particularly soon after social housing was implemented (Helms et al., 2012a). Diurnal cortisol was generally stable across housing conditions, but greater in the dominant monkeys during the fist 2 weeks of social housing. It should be noted that a study using a similar design of changing housing conditions from individual to social groups also did not document a change in cortisol as a function of social rank, but in these studies ACTH was not measured (Czoty et al., 2009; Morgan et al., 2000). Following the housing manipulation, under the alcohol self-administration protocol of 22hr/day alcohol access for over 12 months the dominant monkeys were the least likely to become heavy drinkers (Helms et al., 2012a). Thus, it appears that dominance confers a slight protection from future heavy drinking. In a recent analysis of acquiring the blood samples from the home cage (low stress condition) as opposed to removal from the cage and restrained in a chair (mild stress), dominant monkeys under stable housing conditions had the least reactive HPA response relative to animals with intermediate and subordinate status (Jimenez et al., 2017). Specifically, dominant animals had higher cortisol under low stress conditions, but subordinates reacted more strongly during a mild stress condition. One can hypothesize that having greater fluctuations in HPA axis response to mild stress (reflective of social subordination) may contribute to future alcohol-seeking behavior. Indeed, in a previous study with peer-reared monkeys, individuals that had a large cortisol response to social isolation stress were found to consume more alcohol in 1-hr daily sessions, independent of early-life stress (Fahlke et al., 2000). Although there are hypotheses that heightened HPA glucocorticoid response may be additive to the reinforcing effects of drugs of abuse, supportive data appears strongest within rodent studies. For example, Piazza and LeMoal (1998) reported that rodents will self-administer glucocorticoids and that this behavior was related to dopaminergic tone in the mesolimbic dopaminergic system. However, a majority of both male and female rhesus macaques trained to self-administer on a fixed-ratio schedule would not self-administer dexamethasone, a synthetic glucocorticoid (Broadbear et al., 1999). These studies illustrate a critical difference in the reinforcing properties of glucocorticoids between these species and highlight the translational value of the NHP model. Overall, social setting (group or individual) has a greater impact on HPA axis activity than social rank, but both variables are important to consider when investigating the HPA axis response to stress and evaluating HPA axis activity as a risk factor for chronic alcohol self-administration in NHPs. Future studies with NHPs having access to alcohol within a troop setting would improve this translational approach to understand socially derived stress and its impact on the development of excessive alcohol intakes.
A history of stress, particularly early childhood stress, can result in anxious as well as antisocial temperaments in mature humans and NHPs (Higley et al., 1991; Kagan, 1994; Kay et al., 2010). These temperaments can be viewed as endophenotypes with genetic basis towards development of alcohol use disorders (McClintick and Grant, 2016) and temperament can predict adolescent alcohol use in humans (Dick et al., 2013). Monkeys also exhibit stable temperaments that can be quantified when confronted with strangers or novel objects, by the degree of behavioral inhibition (anxious animals) or aggressive responses (aggressive animals) (Kalin and Shelton, 1989). In a recent study, a group of 21 male and 11 female rhesus monkeys in the late adolescent/early adult stage of life were typed for temperament prior to being induced to drink alcohol (McClintick and Grant, 2016). The monkeys with aggressive temperaments self-administered more alcohol and attained higher BECs than non-aggressive monkeys. No differences were found between anxious and non-anxious monkeys. The relationship between aggression and alcohol drinking was observed across sex and is not sex-specific (McClintick and Grant, 2016). The lack of influence of an anxious temperament is in contrast to previous studies of peer-reared monkeys where stress and anxiety predicted increased alcohol intake (Higley et al., 1991; Schwandt et al., 2010), however both the early rearing environments and the alcohol access conditions were very different between these sets of studies. Human studies show a strong relationship between early adverse childhood stress and risk for alcohol and substance abuse, with evidence that the threshold for risk of alcohol dependence is experiencing 2 or more adverse events (Pilowsky et al., 2009). Future NHPs can help address if the developmental stage, the nature of the stressor, or the chronicity of stress produces changes in neurocircuitry that produces a trajectory in which anxious temperament is established and translates into risk for excess alcohol drinking later in life.
A final risk factor to consider is the genetic and genomic influences on stress alcohol interactions. Macaques have an advantage of sharing similar genetic and epigenetic composition with humans (Barr, 2013). There are examples of common polymorphisms, such as the monoamine oxidase promoter (MAOA-LPR), that are associated with heavy alcohol consumption, strengthening the rationale NHPs provide a unique opportunity to investigate the genetic basis of risk for AUDs (Barr, 2013; Schwandt et al., 2010). A further opportunity exists in determining if epigenetic processes, such as DNA methylation and histone modification, can be used longitudinally to characterize the relationship with acquiring excessive drinking. Finally, an opportunity exists to determine if epigenetic changes in peripheral tissue, such as blood, reflect the same modifications as the brain. Using this approach the rhesus monoamine oxidase A (MAO-A) expression in the blood of alcohol-naïve subjects was found to be negatively correlated with subsequent alcohol self-administration (Cervera-Juanes et al., 2015). Further, the methylation of the promoter region of the MAO-A gene was associated with MAO-A expression in blood and in the nucleus accumbens (Cervera-Juanes et al., 2016). Because MAO-A is involved in monoaminergic metabolism (serotonin, dopamine and norepinephrine), discrete epigenetic changes altering the expression of this enzyme has direct implications for homeostatic physiological and behavioral processes involved in the risk for heavy drinking.
Interestingly, rodent studies have revealed epigenetic changes that persist across generations. Specifically, the risk for future alcohol consumption may be measurable in DNA methylation profiles that have been found to link parental alcohol exposure and the HPA axis activity of alcohol-naïve offspring (Asimes et al., 2016; Todkar et al., 2016). These studies have opened a door to understanding environmental impacts, such as early life stress and paternal/maternal stressors including alcohol intake, on the epigenetic changes that may confer a risk for the development of AUDs and may provide new avenues for the development of pharmacological interventions. Although epigenetic studies of the hypothalamic PVN and HPA axis activation have yet to be explored as a risk factor in monkeys that self-administer alcohol, future studies should address this possibility.
III: Consequences of Heavy Drinking on Stress Systems
As mentioned previously, repeated activation of the HPA axis results in a shift in the physiological processes that support homeostasis towards a new set point, known as an allostatic state or allostasis. Evidence for an allostatic state in the HPA axis of macaques following long-term alcohol consumption is robust. In cynomolgus males, the diurnal rhythms of both ACTH and cortisol were flattened after six months of daily self-administration (Helms et al., 2012a; 2013), documenting a profound change in homeostatic mechanisms. Furthermore, the effect of introducing social housing, which had previously activated the HPA axis, was dampened with chronic drinking (Helms et al., 2012a). The range of average daily alcohol intake in this cohort was 1.1 – 4.3 g/kg/day (averaged over 12-months of open-access spanning both individual and group housing), suggesting that this moderate dose (1.0 g/kg is an approximate dose equivalent of 4 drinks/day) is sufficient to blunt the diurnal rhythm. We regularly observe a dissociation between ACTH and cortisol during alcohol self-administration, with cortisol levels being less sensitive than ACTH to environmental stressors (i.e., social housing or restraint) as well as pharmacological manipulation of the HPA axis (response to naloxone or o-CRF). In this context, there is not a general lack of ACTH sensitivity at the adrenal gland, as hormones from each layer of the adrenal cortex were not uniformly desensitized. Specifically, male rhesus macaques have significantly lower basal ACTH following long-term daily alcohol self-administration while the adrenal steroids are differentially affected (Helms et al., 2014b). Cortisol (secreted from the zona fasciculata) remained constant across the experimental phases, similar to sulfated dehydroepiandrosterone (DHEA-S, an androgen secreted from the zona reticularis). Mineralocorticoids, however, were significantly increased during open-access conditions. Specifically, aldosterone and deoxycorticosterone (DOC; mineralocorticoids secreted from the zona glomerulosa) increased significantly during open-access compared to pre-alcohol (Aoun et al., 2017; Helms et al., 2014b). Additionally, DOC measured at baseline and after 12-months of open-access was negatively correlated with average BEC during open access conditions, while aldosterone was related to the volume of water and alcohol consumed (Helms et al., 2014b).
To further investigate this relationship, HPA axis activation of DOC secretion was measured longitudinally in cynomolgus males (Jimenez et al., 2017). Similar to the rhesus males, basal DOC measured under low stress (home-cage) conditions was significantly elevated during open-access. To better understand how the HPA axis stimulates DOC secretion, pharmacological challenges were used to target each level of the HPA axis. These data demonstrated that basal DOC is preserved despite aberrant responding to pharmacological challenges targeting the hypothalamus and pituitary. Specifically, alcohol induction resulted in a dampened response to exogenous ACTH and ovine-CRF (targeting the adrenal cortex and pituitary gland, respectively) while the response to naloxone (a non-selective opioid receptor antagonist targeting the central nervous system and hypothalamic PVN) was greatly potentiated. After six-months of open-access conditions, basal DOC was significantly elevated and while the response to the pharmacological challenges had normalized, they remained significantly different than baseline, and provide evidence of an allostatic state (Jimenez et al., 2017). These results have implications for neurosteroid activity in the brain, specifically the neuroactive metabolite of DOC, (3α,5α)-3,21-dihydroxypregnan-20-one (THDOC), a positive modulator at the GABAA receptor. Furthermore, these results highlight the need for a greater understanding of the mineralocorticoid system in long-term alcohol use and chronic stress.
Across species, central mechanisms of allostasis following alcohol self-administration have been demonstrated in brain regions that contribute to the stress response. At the apex of the HPA axis are parvocellular neurons in the hypothalamic PVN where integration from a complex hierarchy of afferent signals converge to generate an appropriate HPA axis response (Herman et al., 2003). A unique relationship between distinct GABAergic and glutamatergic terminals in the PVN, as measured by ultrastructural immunogold presynaptic density, was found with water and alcohol consumption in female rhesus macaques (Jimenez et al., 2015). In this analysis, presynaptic glutamate immunogold density in recurrent (i.e. axon terminals from parvocellular neurons that remain within the PVN) was negatively correlated with average daily alcohol consumption. These data complement rodent models of both acute (Lee et al., 2004) and repeated (Lee and Rivier, 1997; Richardson et al., 2008) alcohol exposure influencing parvocellular PVN neurons and suggest that there is a direct interaction between alcohol and parvocellular neurons.
Projecting to the PVN, the bed nucleus of the stria terminalis (BNST), part of the extended amygdala that is involved in neuroendocrine activity (Crestani et al., 2013) is influenced by alcohol in humans (O’Daly et al., 2012) and rodents (Kash et al., 2009; Kash, 2012; Olive et al., 2002). In male rhesus macaques, Pleil and colleagues (2015) report an increase in the frequency of spontaneous inhibitory postsynaptic currents (sIPSC) in the BNST. Using cluster analysis, 40% of the variance in sIPSC frequency was accounted for by DOC (negative association), lifetime alcohol and neuronal capacitance (positive associations). The frequency, but not amplitude, of postsynaptic events are representative of presynaptic release probability, rather than postsynaptic receptor expression or subunit composition (Siggins et al., 2005; Weiner and Valenzuela, 2005). Because the BNST has a heterogeneous neuronal population, this data suggests that inhibitory signaling to a particular neuronal phenotype (indicated by capacitance) is altered by chronic alcohol self-administration, albeit possibly through alchol’s effects on circulating adrenal steroids.
Further evidence for alcohol-dependent changes in presynaptic release probability were recently reported in the nucleus accumbens core (NAcc). As noted in the previous section, gene regulation and expression by epigenetic alterations in chromatin structure, such as DNA methylation or acetylation may provide a cumulative record of genomic adaptations to chronic alcohol exposure. Using this approach, Cervera-Juanes and colleagues (2017) report that male rhesus macaques who met criteria for heavy or very heavy drinking status (Baker et al., 2014) had a greater proportion of CpG hypermethylation of synaptic genes. Importantly, the differentially regulated regions (DMRs) between heavy/very heavy and light/binge drinking monkeys were largely hypermethylated and correlated with average daily intake. Of the 17 DMRs that distinguished these two drinking phenotypes, eight were mapped to genes that regulate neurotransmitter release and receptor trafficking in a coordinated fashion (Cervera-Juanes et al., 2017).
The nucleus accumbens also shows region specific adaptations to chronic drinking at the level of synaptic activity as measured by fast scan cyclic voltammetry (FSCV) (Siciliano et al., 2015, 2016). Specifically, kappa-opioid receptor (KOR) regulation of dopaminergic release is altered in macaques following chronic alcohol self-administration, resulting in increased accumbens dopamine release and uptake, but a decreased release and uptake in the caudate (Siciliano et al., 2015). Further, the relative changes in terminal function between the accumbens and the caudate were highly correlated with alcohol intake, suggesting these adaptations are involved in elevated alcohol consumption. The interplay between the accumbens and the caudate provides important support for a neurocircuitry-based approach in understanding possible adaptations in motivational transitions towards heavy drinking. It should also be noted that the supersensitivity of the KOR to alcohol treatment was first found in rodents (Walker and Koob, 2008), showing strong translational implications for the kappa/dynorphin system in the regulation of alcohol consumption. From an investigation of dopamine autoreceptors following 6 months of chronic alcohol self-administration also using FSCV, we had documented a shift from nearly equal D2/D3 regulation towards D2 dominant autoreceptor regulation of presynaptic dopamine release in the NAcc (Siciliano et al., 2016). Autoregulation of dopamine neurotransmission is implicated in most motivational assays involving food and drug reinforcement. Further research is required to understand the link between the differentially methylated regions of multiple genes involved in synaptic regulation, autoreceptor control of dopamine release, and the regional specificity of dopamine release in the neuroadaptation to alcohol.
Regional specificity in response to chronic alcohol self-administration in NHPs has also been documented in the adaptation of medium spiny neurons (MSNs) in the caudate and putamen using patch clamp techniques and ex vivo slice recordings (Cuzon Carlson et al., 2011). Unlike the studies conducted in the accumbens where brain slices were prepared from monkeys that were drinking daily, these studies investigated the striatum of monkeys that were in prolonged abstinence form chronically drinking alcohol. The data showed a selective increase in dendritic spine density and enhanced glutamatergic transmission in the putamen but not in the caudate nucleus. In addition, the intrinsic excitability of the MSNs and GABAergic transmission was selectively decreased in the putamen of abstinent drinkers. Both the morphological and the neurophysiological changes indicate a shift in the inhibitory/excitatory transmission towards stronger putamen synaptic activation (Cuzon Carlson et al., 2011). These cellular adaptations have implications for an increase in habitual behaviors, particularly in the abstinent state and susceptibility to relapse.
Other studies of synaptic recordings from the monkey model of chronic alcohol self-administration show a general dampening effect on oscillatory regions in the brain. Welsh and colleagues (2010) found hyperexcitability related to t-type calcium channel activation in neurons within the inferior olive in cynomolgus monkeys following chronic alcohol self-administration in the non-abstinent state. Interestingly, slices from monkeys in extended abstinence (35 days) had below-normal function, suggesting overcompensation, or an allostatic state in movement related circuits. Alcohol’s effect on another oscillatory region, the lateral geniculate nucleus (LGN) of the thalamus has also been studied in this monkey model (Breckinridge Carden et al., 2006). The LGN regulates sleep/wake cycles, arguably one of the most critical daily rhythms that is disrupted in subjects with AUDs (see Chakravorty et al., 2016 for review). Burst firing within the LGN was significantly dampened following long-term alcohol consumption in cynomolgus males (Breckinridge Carden et al., 2006), providing a possible cellular basis for understanding, and treating, alcohol induced sleep disruption. Although currently unexplored, the mechanism behind the flattened diurnal glucocorticoid rhythm described above may also be a centrally mediated oscillatory consequence of long-term alcohol consumption.
The final consideration of chronic adaptations to alcohol stress interactions is at the level of the immune system. The interplay between alcohol, stress and the immune system is complex (Barr et al., 2016). Generally, long-term heavy alcohol consumption is associated with an increased risk of infection and chronic disease. However, long-term moderate alcohol intake (BEC below 80 mg/dl) in rhesus males was found to improve B-and T-cell response following a vaccine booster compared to “heavy drinkers” (BEC averaging over 80 mg/dl) and alcohol-naïve controls (Messaoudi et al, 2013), a consequence of alcohol-modulated gene expression in leukocytes (Barr et al., 2015). In a cohort of cynomolgus males, sixty immune signaling molecules (ISMs) were assayed from the same plasma samples as ACTH and cortisol across the experimental phases. During induction and self-administration, nearly half (28/60) of the ISMs were found to decrease (Helms et al., 2012b). These data suggest that long-term alcohol consumption negatively impacts NF-kB and STAT/JAK immune pathways in coordination with the development of an allostatic state in HPA axis signaling (Helms et al., 2012b). Although there is a rich body of literature between stress and immunity as well as alcohol and immunity, there is considerable work that remains in understanding the relationship between these factors. Further, neuroimmune signaling molecules in the limbic regions of the brain provide an additional aspect of alcohol allostasis that is under active investigation (see Reviews by Crews and deTimary in this special issue).
IV: Summary
The monkey model of alcohol self-administration provides the opportunity to study risks for and consequences of long-term alcohol self-administration related to stress at multiple levels. From social hierarchy, temperament, and age to presynaptic neurotransmitter release and adrenal adaptation, the monkey model has strengthened our understanding of the complex interplay between stress and excessive alcohol consumption. Future studies aimed at expanding our understanding of the relationship between alcohol self-administration and stress will focus on how the three primary components of the HPA axis interact with and are controlled by interactions with extra-HPA systems (Figure 1). An important step is to investigate functional adaptations of parvocellular neurons in the PVN either with morphological, physiological or in vivo imaging techniques. Further, longitudinal analysis of CRF-binding protein (CRF-BP) and transcriptome sequencing of each layer of the adrenal cortex will provide insight into functional changes at each level of the HPA axis during the course of chronic alcohol self-administration. These analyses can be complemented with longitudinal hormone measures, including circulating concentrations under basal conditions and in response to specific pharmacological challenges to better understand alcohol allostasis. Next, understanding how these components interact as a system can be enriched by a thorough investigation of mineralocorticoids including longitudinal analysis of circulating hormones (including oxytocin (OXY), DOC and aldosterone), their neuroactive metabolites and their respective receptors. Finally, understanding the HPA axis in a broader context by integrating the relationship with the autonomic nervous system, as well as limbic regions such as the BNST, amygdala and striatum is necessary. The NHP’s translational potential through longitudinal resting state functional connectivity MRI (Grayson et al., 2014; Miranda-Dominguez 2014) and advanced genetic sequencing (Inacu et al., 2017) will help enable these goals.
Figure 1. Alcohol self-administration and HPA axis interactions in nonhuman primates (NHPs).
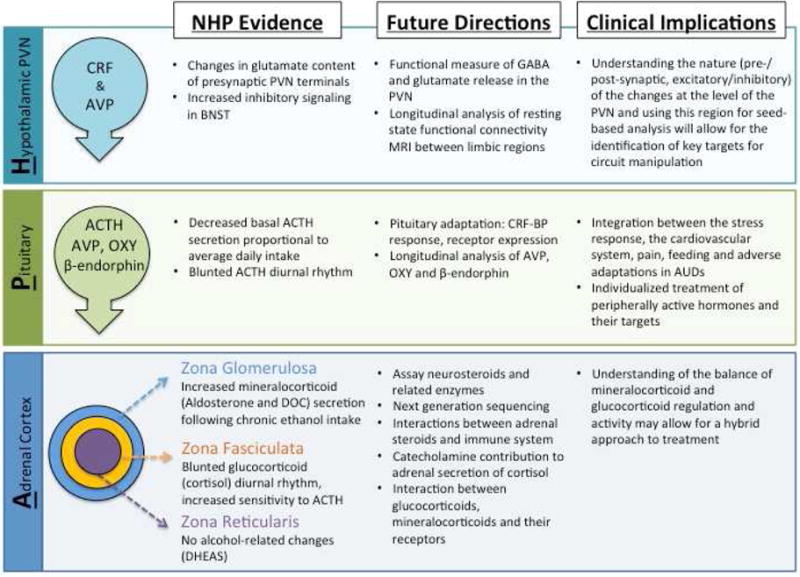
At the apex of the HPA axis is the hypothalamic paraventricular nucleus (PVN) where corticotropin-releasing factor (CRF) and arginine-vasopressin (AVP) are released in response to real or perceived threats to homeostasis. GABA and glutamate are the principle neurotransmitters in relaying information to the PVN, and targets for alcohol pharmacology. Activation of parvocellular neurons stimulates adrenocorticotropin hormone (ACTH) release from the anterior pituitary which increases steroidogenesis from each layer of the adrenal cortex. The left-hand column highlights some of the evidence from the NHP model showing that each level of the hypothalamic-pituitary-adrenal axis is effected by long-term alcohol self-administration. The future directions (center column) are aimed at better understanding the physiological impact of alcohol at each level and the interactions among the three principal components. While this list is not exhaustive, it highlights some of the projects that have clinical implications (right-hand column).
AUD: alcohol use disorder; RNST bed nucleus of the stria terminalis; CRF-BP: corticotropin-releasing factor binding protein; DOC: deoxycorticosterone; DHEAS: sulfated dihydroepiandrosterone; OXY: oxytocin
The future of alcohol research should continue towards understanding risks for and consequences of harmful alcohol intake at an organismal level. While the NHP model of alcohol self-administration has unique strengths, so too do many other preclinical and clinical models. The NHP model described here is built firmly on a foundation of rodent and human research, and will continue to bridge these two. Importantly, the NHP model will to extend our knowledge where it is uniquely situated to do so. Ultimately, understanding the mechanisms and interactions of central and peripheral processes of allostasis in an individualized context will aid in developing pharmacological intervention and treatment strategies.
This review covers the use of non-human primates (NHPs) in volitional, oral alcohol self-administration studies with the interaction of stress response to exacerbate alcohol drinking.
When placed in a framework that alcohol addiction is a sequence of dysregulations in motivational circuitry associated with severity of AUD, the NHP can provide within-subject information on both risks for and consequences of repeatedly drinking to intoxication.
This review also highlights areas where future studies of brain and HPA axis adaptations are needed to better understand the mechanisms involved in stress leading to excessive alcohol consumption.
Acknowledgments
This research was supported by AA 13641, AA 13510, AA 10760 and AA 019431 from NIH to Kathleen Grant.
Abbreviations
- ACTH
adrenocorticotropin hormone
- AVP
arginine-vasopressin
- AUD
alcohol use disorder
- BEC
blood ethanol concentration
- BNST
bed nucleus of the stria terminalis
- CRF-BP
corticotropin-releasing factor binding protein
- CRF
corticotropin-releasing factor
- DHEA
dehydroepiandrosterone
- DMR
differentially methylated region
- DOC
deoxycorticosterone
- FSCV
fast-scan cyclic voltammetry
- GABA
gamma-aminobutyric acid
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- ISM
immune-signaling molecule
- KOR
kappa opioid receptor
- LGN
lateral geniculate nucleus
- MAO-A
monoamine oxidase A
- MAOA-LPR
monoamine oxidase promoter
- MRI
magnetic resonance imaging
- MSN
medium spiny neuron
- NAcc
nucleus accumbens core
- NHP
nonhuman primate
- o-CRF
ovine-corticotropin-releasing factor
- OXY
oxytocin
- PVN
hypothalamic paraventricular nucleus
- THDOC
(3α,5α)-3,21-dihydroxypregnan-20-one
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Aoun EG, Jimenez VA, Vendruscolo LF, Walter NAR, Barbier E, Ferrulli A, Haass-Koffler CL, Darakajian P, Lee MR, Addolorato G, Heilig M, Hitzemann R, Koob GF, Grant KA, Leggio L. Relationship between the aldosterone – mineralocorticoid receptor pathway and alcohol drinking: preliminary translational findings across monkeys, rats and humans. Molecular Psychiatry. 2017 doi: 10.1038/mp.2017.97. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimes A, Torcaso A, Pinceti E, Kim CK, Zeleznik-Le NJ, Pak TR. Adolescent binge-pattern alcohol exposure alters genome-wide DNA methylation patterns in the hypothalamus of alcohol-naïve male offspring. Alcohol. 2016 doi: 10.1016/j.alcohol.2016.10.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Farro J, Gonzales S, Helms C, Grant KA. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin Exp Res. 2014;38(11):2835–2843. doi: 10.1111/acer.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Walter NA, Salo A, Rivas P, Moore S, Gonzales S, Grant KA. Identifying future drinkers: behavioral analysis of monkeys initiating drinking to intoxication is predictive of future drinking classification. Alcohol Clin Exp Res. 2017 doi: 10.1111/acer.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS. Non-human primate models of alcohol-related phenotypes: the influence of genetic and environmental factors. Curr Top Behav Neurosci. 2013;13:223–249. doi: 10.1007/7854_2011_142. [DOI] [PubMed] [Google Scholar]
- Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addiction Bio. 2006;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Barr T, Girke T, Sureshchandra S, Nguyen C, Grant K, Messaoudi I. Alcohol consumption modulates host defense in rhesus macaques by altering gene expression in circulating leukocytes. Journal of Immunology. 2015;196(1):182–195. doi: 10.4049/jimmunol.1501527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr T, Helms C, Grant K, Messaoudi I. Opposing effects of alcohol on the immune system. Progress in neuropsychopharmacology and biological psychiatry. 2016:242–251. doi: 10.1016/j.pnpbp.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–458. doi: 10.35946/arcr.v34.4.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Seo D, Sinha R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol doi. 2015 doi: 10.1111/adb.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman ME, Lopata A, Jaffe RB, Golos TG, Wickings J, Smith R. Corticotropin-releasing hormone-binding protein in primates. Am J Primatol. 2001;53:123–130. doi: 10.1002/1098-2345(200103)53:3<123::AID-AJP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Breckinridge Carden W, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, Godwin DW. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Research. 2006;1089(1):1–9. doi: 10.1016/j.brainres.2006.02.135. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Glucocorticoid-reinforced responding in the rhesus monkey. Psychopharmacology (Berl) 1999;147(1):46–55. doi: 10.1007/s002130051141. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Cervera-Juanes R, Wilhem LJ, Park B, Lee R, Locke J, Helms C, Gonzales S, Wand G, Jones SR, Grant KA, Ferguson B. MAOA expression predicts vulnerability for alcohol use. Mol Psychiatry. 2015;21:472–479. doi: 10.1038/mp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera-Juanes R, Wilhelm LJ, Park B, Grant KA, Ferguson B. Genome-wide analysis of the nucleus accumbens identifies DNA methylation signals differentiating low/binge from heavy alcohol drinking. Alcohol. 2016 doi: 10.1016/j.alcohol.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera-Juanes R, Wilhelm LJ, Park B, Grant KA, Ferguson B. Alcohol-dose-dependent DNA methylation and expression in the nucleus accumbens identifies coordinated regulation of synaptic genes. Transl Psychiatry. 2017;7:e994. doi: 10.1038/tp.2016.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40(11):2271–2282. doi: 10.1111/acer.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, Eskay RL. Binge-like ethanol consumption increases corticosterone levels and neurodegeneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addict Biol. 2014;19:27–36. doi: 10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird MI. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Crestani CC, Alves FHF, Gomes FV, Resstel LBM, Correa FMA, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Current Neuropharmacology. 2013;11:141–159. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36(12):2513–28. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21(1):68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse SJ, Hickman M, Heron J, Macleod J, Joinson C, Maughan B, Lewis G, Kendler KS. Adolescent alcohol use is predicted by childhood temperament factors before age 5, with mediation through personality and peers. Clin Exp Res. 2013;37(12):2108–2117. doi: 10.1111/acer.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. ACER. 2000;24(5):644–650. [PubMed] [Google Scholar]
- Ferguson B, Hunter JE, Luty J, Street ST, Woodall A, Grant KA. Genetic load is associated with hypothalamic-pituitary-adrenal axis dysregulation in macaques. Genes, Brain and Behavior. 2012;11:949–957. doi: 10.1111/j.1601-1B3X.2012.00B56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at the onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100(3):235–55. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW. Drinking typography established by schedule-induced polydipsia predicts chronic heavy drinking in a monkey model of ethanol self- administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Ferguson B, Helms C, McClintick M. Drinking to Dependence Risk Factors in Nonhuman Primates. In: Noronha A, Cui C, Harris A, Crabbe JC, editors. Neurobiology of Alcohol Dependence. San Diego: Academic Press; 2014. pp. 408–420. 2014, Chapter 20. [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuanf MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2006;36:109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large scale systems organization in the rhesus macaque brain. J Neurosci. 2014;34:2065–2074. doi: 10.1523/JNEUROSCI.3038-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (macaca fascicularis) Alcohol Clin Exp Res. 1999;23(4):611–6. [PubMed] [Google Scholar]
- Gust DA, Wilson ME, Stocker T, Conrad S, Plotsky PM, Gordon TP. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys 1. The Journal of Clinical Endocrinology & Metabolism. 2000;85(7):2556–63. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson J-A. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WD, Patton G, Stockings E, Weiner M, Lynskey M, Morley KI, Degenhardt L. Why young people’s substance use matters for global health. Lancet Psychiatry. 2016;3:265–279. doi: 10.1016/S2215-0366(16)00013-4. [DOI] [PubMed] [Google Scholar]
- Helms CM, McClintick MN, Grant KA. Social rank, chronic ethanol self-administration, and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharmacology (Berl) 2012a;224(1):133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Jeng S, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol Clin Exp Res. 2012b;36(6):955–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C, Rossi DJ, Grant KA. Neurosteroid influences on sensitivity to ethanol. Frontiers in endocrinology. 2012c;3(10):1–19. doi: 10.3389/fendo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gonzales SW, Green HL, Szeliga KT, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fasicularis) Psychopharmacology. 2013;228(4):541–549. doi: 10.1007/s00213-013-3052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rau A, Shaw J, Stull C, Gonzales SW, Grant KA. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology. 2014a;231:1853–1861. doi: 10.1007/s00213-013-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Park B, Grant KA. Adrenal steroid hormones and ethanol self-administration in male rhesus macaques. Psychopharmacology. 2014b;231:3425–3436. doi: 10.1007/s00213-014-3590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Nueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Lnnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Scie USA. 1991;88(16):7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu OD, C A, Walter NAR, Darakjian P, Oberbeck DL, Daunais JB, et al. On the Relationships in Rhesus Macaques between Chronic Ethanol Consumption and the Brain Transcriptome. Addict Biol. doi: 10.1111/adb.12501. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the glucocorticoid antagonist, mifepristone, on the consequence of withdrawal from long term alcohol consumption. Alcohol Clin Exp Res. 2008;32:2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Jimenez VA, Helms CM, Cornea A, Meshul CK, Grant KA. An ultrastructural analysis of the effects of ethanol self-administration on the hypothalamic paraventricular nucleus in rhesus macaques. Front Cell Neurosci. 2015;9:260. doi: 10.3389/fncel.2015.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez VA, Porcu P, Morrow L, Grant KA. Adaptations in Basal and Hypothalamic-Pituitary-Adrenal-Mediated Deoxycorticosterone Responses Following Ethanol Self-Administration in Cynomolgus Monkeys. Frontiers in experimental endocrinology. 2017;8:19. doi: 10.3389/fendo.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez VA, Allen DC, McClintick MN, Grant KA. Social setting, social rank and HPA axis response in Cynomolgus monkeys. Psychopharmacology. doi: 10.1007/s00213-017-4596-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. On the nature of emotion. Monogr Soc Res Child Dev. 1994;59(2–3):7–24. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviours in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Krovner R, Riedel MK, Fekete EM, Roseboom PH, Tromp DPM, Grabow BP, Olsen ME, Brodsky EK, McFarlin DR, Alexander AL, Emborg ME, Block WF, Fudge JL, Oler JA. Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Bio Psych. 2016;80:345–355. doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012;46:303–308. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DB, Marsiske M, Suomi SJ, Higley JD. Exploratory factor analysis of human infant temperament in the rhesus monkey. Infant Behav Dev. 2010;33:111–114. doi: 10.1016/j.infbeh.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Lovell-Badge R, Robinson ICA, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30(7):790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. Stress and alcohol: epidemiologic evidence. Alcohol Res. 2012;34:391–400. doi: 10.35946/arcr.v34.4.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psyychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psych. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Flory GS, Park B, Shaw J, Rau AR, Grant KA. Chronic ethanol (EtOH) consumption differentially alters gray and white matter EtOH methyl ÅH magnetic resonance intensity in the primate brain. Alcohol Clin Exp Res. 2013;37(8):1325–32. doi: 10.1111/acer.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA. Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI. Neuropsychopharmacology. 2014;39(4):823–830. doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J neurosci. 1997;17(22):8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145(10):4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends in Neurosciences. 1988;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McClintick MN, Grant KA. Aggressive temperament predicts ethanol self-administration in late adolescent male and female rhesus macaques. Psychopharmacology (Berl) 2016;233(23–24):3965–3976. doi: 10.1007/s00213-016-4427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sakai RR, Spencer RL. Adrenal steroid effects on the brain: versatile hormones with good and bad effects. In: Schulkin J, editor. Hormonally-induced Changes in Mind and Brain. Academic Press; San Diego: 1993. pp. 157–189. 1993. [Google Scholar]
- Mello NK, Mendelson JH. Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom Med. 1972;34(2):139–164. doi: 10.1097/00006842-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Asquith M, Engelmann F, Park B, Brown M, Rau A, Shaw J, Grant KA. Moderate alcohol consumption enhances vaccine-induced responses in the rhesus macaques. Vaccine. 2013;32:43–61. doi: 10.1016/j.vaccine.2013.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. J Neurosci. 2014;34:5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52(3):115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshal J, Ball D, Phillips ML, Williams SS, Stphens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in he bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Gluccorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pilowsky DJ, Keyes KM, Hansin D. Adverse childhood events and lifetime alcohol dependence. Am J Public Health. 2009;99:258–263. doi: 10.2105/AJPH.2008.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in human and rat serum. Steroids. 2009;74(4–5):463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Helms CM, Sobus JR, Daunais JB, Grant KA, Kash TL. Effects of chronic alcohol consumption on neuronal function in the non-human primate BNST. Addiction biology. 2015;21(6):1151–1167. doi: 10.1111/adb.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary- adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter A, Biermann T, Amato D, Schumann G, Desrivieres S, Kornhuber J, Müller CP. Glucocorticoid receptor antagonism blocks ethanol-induced place preference learning in mice and attenuates dopamine D2 receptor adaptation in the frontal cortex. Brain Res Bull. 2012;88:519–524. doi: 10.1016/j.brainresbull.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Sakai RR, McEwen BS, Fluharty SJ, MA LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000;57:1337–1345. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Chen S, Higley JD, Suomi SJ, Heilig M, Barr CS. alcohol response and consumption in adolescent rhesus macaques: life history and genetic influences. 2010 doi: 10.1016/j.alcohol.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammels. Journal of Endocrinology. 2002;175:89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR. Voluntary ethanol intake predicts k-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci. 2015;35(15):5959–5968. doi: 10.1523/JNEUROSCI.4820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR. Voluntary ethanol intake predicts K-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci. 2015;35(15):5959–5968. doi: 10.1523/JNEUROSCI.4820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Mateo Y, Helms CM, Lovinger DM, Grant KA, Jones SR. Chronic ethanol self-administration in macaques shifts dopamine feedback inhibition to predominantly D2 receptors in nucleus accumbens core. Drugs and Alcohol Dependence. 2016;158:159–163. doi: 10.1016/j.drugalcdep.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacology and Therapeutics. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress lead to risk of alcohol relapse? Alcohol Res. 2012;34:432–440. doi: 10.35946/arcr.v34.4.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. 2013;23:649–654. doi: 10.1016/j.conb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary- adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Risk, Resilience and Gene-Environment Interplay in Primates. J Can Acad Child Adolesc Psychiatry. 2011;20:289–297. [PMC free article] [PubMed] [Google Scholar]
- Todkar A, Granholm L, Ajumah M, Nisson KW, Comasco E, Nylander I. HPA axis gene expression and DNA methylation profiles in rats exposed to early life stress, adult voluntary ethanol drinking and single housing. Front Mol Neurosci. 2016;26(8):90. doi: 10.3389/fnmol.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. doi: 10.1111/j.1530-0277.2001.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a moticational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G. The influence of stress on the transition from drug use to addiction. Alcohol Res Health. 2008;31:119–136. [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacology & Therapeutics. 2005;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odigiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proceedings of the national academy of sciences. 2010;108(25):10314–10319. doi: 10.1073/pnas.1017079108/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]


