Abstract
Individuals exposed to adverse childhood experiences (ACEs) are vulnerable to various health problems later in life. This study was designed to determine whether participation in an efficacious program to enhance supportive parenting would ameliorate the association between ACEs and prediabetes status at age 25. Rural African American parents and their 11-year-old children (N = 390) participated in the Strong African American Families (SAAF) program or a control condition. Each youth at age 25 provided a total ACEs score and a blood sample from which overnight fasting glucose was assayed. Logistic regression equations were used to test the hypotheses. The logistic regression analyses revealed a significant interaction between total ACEs and random assignment to SAAF or control, OR = 0.56, 95% CI [0.36, 0.88]. Follow-up analyses indicated that, for participants in the control condition, a 1-point increase in ACEs was associated with a 37.3% increase in risk of having prediabetes. ACEs were not associated with the likelihood of having prediabetes among participants in the SAAF condition. Control participants with high total ACEs scores were 3.54 times more likely to have prediabetes than were SAAF participants with similar scores. This study indicated that participation at age 11 in a randomized controlled trial designed to enhance supportive parenting ameliorated the association of ACEs with prediabetes at age 25. If substantiated, these findings may provide a strategy for preventing negative health consequences of ACEs.
1. Introduction
Interest is mounting among pediatrics scientists and practitioners regarding the hypothesis that adverse childhood experiences (ACEs) influence physical health across the lifespan (Miller et al., 2011a; Shonkoff et al., 2009). This interest has been fueled by recent studies showing that people exposed to major psychological stresses in childhood experience elevated morbidity and mortality from the chronic diseases of aging. Adverse childhood experiences include maltreatment, neglect, and household dysfunction before the age of 18 years (Anda et al., 2006; Felitti and Anda, 2010). ACEs are associated with an elevated risk of heart, lung, and liver disease; cancer; and health-related quality of life (Anda et al., 2009; Caspi et al., 2006; Chartier et al., 2010; Dong et al., 2004; Dube et al., 2009; Felitti et al., 1998; Middlebrooks and Audage, 2008; Springer et al., 2007). Individuals who report ACEs also demonstrate greater use of health care services and higher health care costs (Anda et al., 2008; Chartier et al., 2010). This research reflects a dose-response relationship between numbers of ACEs and health outcomes, with the risk of health problems later in life increasing as exposure to ACEs increases (Felitti, 1993; Felitti et al., 1998).
This study was designed to advance understanding of the association between ACEs and subsequent health status by testing hypotheses involving prediabetes among African American young adults living in the rural southern United States. Prediabetes is characterized by impaired glucose control; it is a precursor to diabetes and a contributor to numerous chronic diseases of aging, including heart disease and stroke (Middlebrooks and Audage, 2008). Although more than 80 million people in the United States are prediabetic, with the prevalence for African Americans being alarmingly high at 39% (Centers for Disease Control and Prevention, 2014), little is known about the link between ACEs and prediabetes. The study hypotheses were tested with a sample of rural African American youths who took part in a randomized prevention trial and were followed from preadolescence (age 11) to young adulthood (age 25). The preventive intervention, the Strong African American Families (SAAF) program, was designed to enhance supportive parenting and reduce harsh parenting (Brody et al., 2004); it will be described in more detail later. To measure prediabetes, an overnight fasting blood sample was assayed. The following sections present the study hypotheses and the rationale for them.
Accumulating evidence suggests that children exposed to ACEs display lasting alterations in the activity of the sympathetic-adrenal-medullary system and the hypothalamic-pituitary-adrenal axis (Garner et al., 2012; Middlebrooks and Audage, 2008; Miller et al., 2011a). Dysregulation of these systems has downstream implications for glucose metabolism, insulin sensitivity, fat accumulation, and inflammatory signaling, all of which are implicated in the pathogenesis of prediabetes and type 2 diabetes (Black, 2006; Hotamisligil, 2006). Thus, exposure to ACEs was hypothesized to predict prediabetes status during young adulthood.
The primary purpose of this study was to determine whether participation in a prevention program that enhances supportive parenting will ameliorate the association between ACEs and prediabetes status. To address this question, secondary analyses were conducted on data from the SAAF randomized, controlled trial. SAAF was designed to mitigate the negative impact of life stress on rural African American youths by increasing supportive parenting processes. Recent observational studies show that a history of receiving supportive parenting has health benefits that help to mitigate some of the hormonal, metabolic, and cardiovascular changes that follow childhood adversity. Supportive parenting buffers the effects of low childhood socioeconomic status (SES) on proinflammatory signaling profiles (Chen et al., 2011), allostatic load (Brody et al., 2014a), and metabolic profiles (Miller et al., 2011b) in adulthood. SAAF has demonstrated stress-buffering capacities for a wide range of psychosocial outcomes. Its results include increases in self-control and decreases in both drug use and conduct problems. These outcomes remained detectable at least 2 years after participation in SAAF (Brody, 2016). SAAF also has favorable effects on several health-relevant biological processes, including inflammation (Miller et al., 2014), catecholamine levels (Brody et al., 2014b), and epigenetic aging (Brody et al., 2015), 8 years after participation in the prevention program. Accordingly, this study tested the hypothesis that exposure to ACEs would be associated with prediabetes status among young adults who had been assigned to the control condition but not among those who had been assigned to the SAAF condition.
2. Methods
2.1. Participants
Participants in the SAAF trial included 667 African American families who resided in nine rural counties in Georgia. Details of the original SAAF prevention trial are provided elsewhere (Brody et al., 2004). Briefly, a targeted youth from each family (mean age at pretest = 11.2, SD = 0.34) and the parent who had primary responsibility for the youth’s care took part in data collections. At pretest, although the primary caregivers in the sample worked an average of 39.4 hours per week, 46.3% lived below federal poverty standards. From the original sample of 667 youths, 500 were randomly selected, due to funding constraints, to participate in a collection of biological data at age 19; these data focused on hormonal assessments. At age 25, 408 of the subsample of 500 agreed to participate in a follow-up assessment by completing questionnaires. Of the 408 participants who completed questionnaires, 391 agreed to a blood draw for assaying of fasting blood glucose. One participant was excluded due to missing data on self-reported adverse childhood experiences. The remaining 390 participants constituted the sample in the present study. At age 11, 227 (62% of the original sample) of these participants had been assigned randomly to the SAAF condition and 163 (55% of the original sample) had been assigned randomly to the control condition. The attrition rates in the control and the SAAF conditions did not differ significantly. The original random assignment oversampled participants into the SAAF condition to ensure that the analyses involving the intervention condition would be adequately powered (see Brody et al, 2004); this accounts for the greater number of 25-year-olds who had been assigned to the SAAF group. At age 11, parents gave written informed consent to their own and their minor youths’ participation, and minor youths gave written assent to their own participation. Each family was paid $100 after the assessment at pretest. At age 25, young adults gave written informed consent to their own participation, and each participant was paid $160 after the assessment and blood draw. The University of Georgia’s Institutional Review Board reviewed and approved all study procedures.
2.2. Intervention implementation
The SAAF prevention program consisted of seven consecutive, 2-hour weekly meetings held at community facilities, with separate parent and youth skill-building curricula and a family curriculum (see Brody et al.(Brody et al., 2012) for a complete description, including a summary of efficacy findings). Parents in the prevention condition were taught the consistent provision of instrumental and emotional support, high levels of monitoring and control, adaptive racial socialization strategies, and methods for communicating about sex and alcohol use. Youths learned adaptive behaviors to use when encountering racism, the importance of forming goals for the future and making plans to attain them, and resistance efficacy skills. Approximately 70% of the families attended four or more sessions. During the weeks when the intervention families participated in the prevention sessions, the control families received three leaflets via postal mail that described adolescent development and provided tips for stress management and exercise promotion. Past intervention assessments showed no evidence of control participants’ having received information provided to the SAAF participants. To preserve the random nature of the group assignments, the analyses reported here included all families who completed the pretest regardless of the number of prevention sessions that they actually attended (an intent-to-treat analysis). These families were retained in the analysis to preclude the introduction of self-selection bias into the findings.
2.3. Data collection procedures
All data were collected in participants’ homes using a standardized protocol. A field researcher who was also a certified phlebotomist went to each participant’s home to collect self-report data and to draw a blood sample to be used to assess prediabetes status.
2.4. Measures
2.4.1. Intervention status and gender
Intervention status and gender were dummy coded. SAAF participants were coded 1 and control participants were coded 0; male participants were coded 1 and female participants were coded 0.
2.4.2. Family socioeconomic disadvantage
Six dichotomous variables formed an index of socioeconomic disadvantage at age 11 that was used as a control in the data analyses. A score of 1 was assigned to each of the following: family poverty based on federal guidelines, primary caregiver unemployment, receipt of Temporary Assistance for Needy Families, primary caregiver single parenthood, primary caregiver education level less than high school graduation, and caregiver-reported inadequacy of family income. The scores were summed to form the index.
2.4.3. Adverse childhood experiences
Young adults reported adverse childhood experiences on the Adverse Childhood Experiences (ACEs) questionnaire (Felitti et al., 1998). An ACEs score was calculated by summing dichotomized yes/no responses across 10 ACEs categories indicating the presence or absence of particular adversities that participants may have experienced before the age of 18 years: living with someone who was mentally ill or depressed, was a problem drinker or alcoholic or used street drugs, or went to prison; having parents who were separated or divorced; witnessing domestic violence; experiencing physical neglect (did not have enough to eat, wore dirty clothes, or no one protected them) or emotional neglect (no one in the family loved them or thought they were important); and experiencing physical abuse (parents or other adults hit, beat, kicked, or physically injured them), verbal abuse (parents or other adults swore at, insulted, or humiliated them), or sexual abuse (by adults or persons 5 or more years older than they were). The study sample’s ACEs scores ranged from 0 to 7 (M = 1.25, SD = 1.44). The scores represented the following percentages: 0 = 35.6% (36.2% in the control group, 35.2% in the SAAF group); 1 = 35.1% (36.2% in the control group, 34.4% in the SAAF group), 2 = 13.3% (12.9% in the control group, 13.7% in the SAAF group), 3 = 7.7% (8.0% in the control group, 7.5% in the SAAF group), 4 = 3.6% (3.1% in the control group, 4.0% in the SAAF group), 5 = 2.6% (2.5% in the control group, 2.6% in the SAAF group), 6 = 1.3% (1.2% in the control group, 1.3% in the SAAF group), 7 = 0.8% (0 in the control group, 1.3% in the SAAF group).
2.4.4. Young adult type 2 prediabetes status
A field researcher who was also a certified phlebotomist went to each participant’s home to draw a fasting blood sample. Participants were requested not to eat or drink after midnight prior to the blood draw. Blood was drawn into Serum Separator Tubes (Becton-Dickinson, Franklin Lakes, NJ), and centrifuged on site according to the manufacturer’s instructions. Serum was harvested and frozen immediately on dry ice. At the end of the study, glucose was measured photometrically using a UV test on a Roche/Hitachi cobas c502 analyzer. The average intra- and inter-assay coefficients of variation were 0.7% and 1.8%, respectively. This assay has a dynamic range of 2–750 mg/dL. The operationalization of the prediabetes outcome conformed to the American Diabetes Association’s (2015) prescription for determining clinical prediabetes status. This cutoff has both scientific and clinical validity. Young adults were classified as having prediabetes if they had a fasting glucose level between 100 mg/dL and 125 mg/dL, and as having diabetes if they had a fasting glucose level ≥ 126 mg/dL. Of 390 participants, 50 (12.8%) were classified as having prediabetes and 5 (1.3%) were classified as having diabetes. Because the size of the subsample with diabetes was deemed inadequate for meaningful analyses, the outcome of interest in this study was the presence of either prediabetes or diabetes (total n = 55, 14.1%: control group n = 25, 15.3%; SAAF group n = 30, 13.2%).
2.4.5. Potential confounds
The analyses controlled for adiposity, operationalized as BMI assessed at the home visit. The field researcher recorded the participant’s weight and height and used them to calculate the BMI (weight in kilograms divided by the square of height in meters).
2.5. Plan of analysis
The data were analyzed in the following sequence. First, a two-factor multivariate analysis of variance was executed to evaluate the equivalence of the demographic and study variables for attrition status × prevention status. The results of the equivalence analysis were explained by the presentation of F-tests, along with means of continuous variables or frequencies of categorical variables. Second, the descriptive statistics and Pearson product-moment correlations among study variables for the control group and the SAAF group were computed. Third, a logistic regression model was executed to test the study hypothesis that participation in SAAF would ameliorate the association between ACEs and a heightened risk of prediabetes. The glucose concentrations were not normally distributed (mean = 91.84, SD = 20.23; skewness = 7.68, kurtosis = 77.60; Shapiro-Wilk test = 0.45, df = 391, p < .001) and remained so even after a log transformation was applied (mean = 1.96, SD = 0.07; skewness = 3.71, kurtosis = 27.91; Shapiro-Wilk test = 0.73, df = 391, p < .001). Therefore, a logistic regression was used to test the study hypothesis with prediabetes status coded as a binary outcome variable according to criteria from the American Diabetes Association (2015). The regression model included main effects of ACEs and assignment to SAAF or to the control condition, and a product term representing the interaction of the two variables. The odds ratios (ORs) and 95% confidence intervals (CIs) were also reported. All the analyses were conducted using SPSS version 22.0.
3. Results
To determine group equivalence, a two-factor multivariate analysis of variance was executed on the demographic and study variables for participants who did or did not provide blood samples at age 25 by prevention group assignment at age 11. Neither significant main effects of prevention group assignment nor significant interaction effects emerged (see Table 1). The hypothesis was tested that participation in SAAF would ameliorate the association between ACEs and a heightened risk of having prediabetes. Table 2 presents descriptive statistics and correlations among study variables for the control group and the SAAF group. A logistic regression equation, adjusted for gender, including main effects of ACEs and intervention assignment, as well as a multiplicative interaction term involving ACEs and assignment to SAAF or the control condition, was executed. The interaction analysis was executed based on the conventions that Aiken and West (1991) prescribed, whereby the ACEs are first mean centered and interactions are calculated as the product of the centered ACEs and prevention status. The analysis revealed a main effect for ACEs and a significant interaction between ACEs and SAAF participation (Table 3, Model 1, OR = 0.57, 95% CI [0.37, 0.88]). This interaction retained its significance when the control variables were included in the analyses (Table 3, Model 2, OR = 0.56, 95% CI [0.36, 0.88]). The estimated prevalence of prediabetes was plotted by ACEs and prevention status. These results, depicted in Figure 1, show that, among youths from the control condition, a 1-point increase in ACEs was associated with a 37.3% increase in risk of having prediabetes at age 25 (OR = 1.37, 95% CI [1.02, 1.84]). ACEs, however, were not associated with the likelihood of having prediabetes among participants who had been assigned to the SAAF condition (OR = 0.77, 95% CI [0.55, 1.07]). Among participants who experienced high childhood adversity (e.g., scored 3 or greater on the ACEs questionnaire), those who had been assigned randomly to the control condition had an estimated prevalence of prediabetes of 22.3%, whereas those who had been assigned randomly to the SAAF condition had an estimated prevalence of prediabetes of 7.5%. Furthermore, youths who experienced high childhood adversity and had been assigned randomly to the control condition were 3.54 times more likely to have prediabetes than were youths who experienced similar levels of childhood adversity and had been assigned randomly to the SAAF condition (odds of having prediabetes for control group = 0.223/(1 − 0.223) = 0.2870; odds of having prediabetes for SAAF group = 0.075/(1 − 0.075) = 0.0811; OR = 0.2870/0.0811 = 3.54).
Table 1.
Characteristics of Subjects With and Without Fasting Glucose Data.
| With Fasting Glucose Data
|
Without Fasting Glucose Data
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SAAF
|
Control
|
SAAF
|
Control
|
||||||
| % or M | SD | % or M | SD | %or M | SD | % or M | SD | F | |
|
|
|
|
|
|
|||||
| Age 11 | (n = 227) | (n = 163) | (n = 142) | (n = 135) | F (1, 663) | ||||
| Gender, male | 39.2% | 41.7% | 57.0% | 57.0% | 0.104 | ||||
| Parent age | 37.74 | 7.89 | 37.63 | 7.12 | 37.65 | 7.60 | 37.94 | 7.86 | 0.110 |
| Family SES disadvantage | 2.52 | 1.43 | 2.24 | 1.47 | 2.27 | 1.47 | 2.04 | 1.58 | 0.055 |
| Family poverty | 39.2% | 36.8% | 43.0% | 32.6% | 1.076 | ||||
| (n = 226) | (n = 163) | (n = 140) | (n = 135) | F (1, 660) | |||||
| Parent =< high school education | 59.7% | 47.9% | 50.7% | 45.9% | 0.809 | ||||
| (n = 227) | (n = 163) | (n = 140) | (n = 135) | F (1, 661) | |||||
| Parent unemployment | 22.0% | 19.0% | 27.1% | 20.0% | 0.397 | ||||
| (n = 226) | (n = 163) | (n = 137) | (n = 135) | F (1, 657) | |||||
| Single-parent family | 61.1% | 57.1% | 52.6% | 51.1% | 0.106 | ||||
|
| |||||||||
| Age 25 | (n = 227) | (n = 163) | (n = 10) | (n = 7) | F (1, 403) | ||||
| ACEs score | 1.30 | 1.51 | 1.18 | 1.33 | 0.60 | 0.70 | 0.43 | 0.79 | 0.004 |
ACEs, adverse childhood experiences; SAAF, Strong African American Families program; SES, socioeconomic status.
Table 2.
Descriptive Statistics and Correlations among Study Variables for Control and SAAF Groups.
| Variables | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| % or M | 13.2% | 39.2% | 2.52 | 30.48 | 1.30 | |
| SD | – | – | 1.43 | 8.76 | 1.51 | |
|
| ||||||
| 1. Prediabetes/ diabetes status (age 25) | — | −.047 | .066 | .149* | −.104 | |
| 2. Gender, male | .020 | — | −.074 | −.237*** | −.066 | |
| 3. Family SES disadvantage (age 11) | .093 | .065 | — | .088 | .084 | |
| 4. BMI (age 25) | .204** | −.256** | .068 | — | −.068 | |
| 5. ACEs score (age 25) | .161* | .102 | .107 | −.076 | — | |
|
| ||||||
| % or M | 15.3% | 41.7% | 2.24 | 30.16 | 1.18 | |
| SD | – | – | 1.47 | 8.72 | 1.33 | |
Upper diagonal: descriptive statistics and Pearson correlations for SAAF group (n = 227); lower diagonal: descriptive statistics and Pearson correlations for control group (n = 163). ACEs, adverse childhood experiences; BMI, body mass index; SES, socioeconomic status.
p < .05.
p < .01.
p < .001.
Table 3.
Adverse Childhood Experiences and Intervention Status as Predictors of Prediabetes Status (N = 390).
| Prediabetes Statusa (age 25) | ||||
|---|---|---|---|---|
|
|
||||
| Model 1
|
Model 2
|
|||
| OR | 95% CI | OR | 95% CI | |
| 1. Gender, male | 0.84 | 0.47, 1.53 | 1.10 | 0.58, 2.06 |
| 2. ACEs score (age 25) | 1.35 | 1.01, 1.79 | 1.37 | 1.02, 1.84 |
| 3. Intervention (SAAF) | 0.83 | 0.46, 1.51 | 0.79 | 0.43, 1.44 |
| 4. ACEs × SAAF | 0.57 | 0.37, 0.88 | 0.56 | 0.36, 0.88 |
| 5. Family SES disadvantage (age 11) | — | — | 1.13 | 0.92, 1.38 |
| 6. BMI (age 25) | — | — | 1.06 | 1.02, 1.09 |
Prediabetes at age 25 was defined as fasting blood glucose between 100 mg/dL and 125 mg/dL; n = 50. The prediabetes group included 5 participants who had diabetes, glucose level ≥ 126. Total prediabetes group n = 55. ACEs, adverse childhood experiences; BMI, body mass index; OR, odds ratio; CI, confidence interval; SAAF, Strong African American Families program; SES, socioeconomic status.
Figure 1.
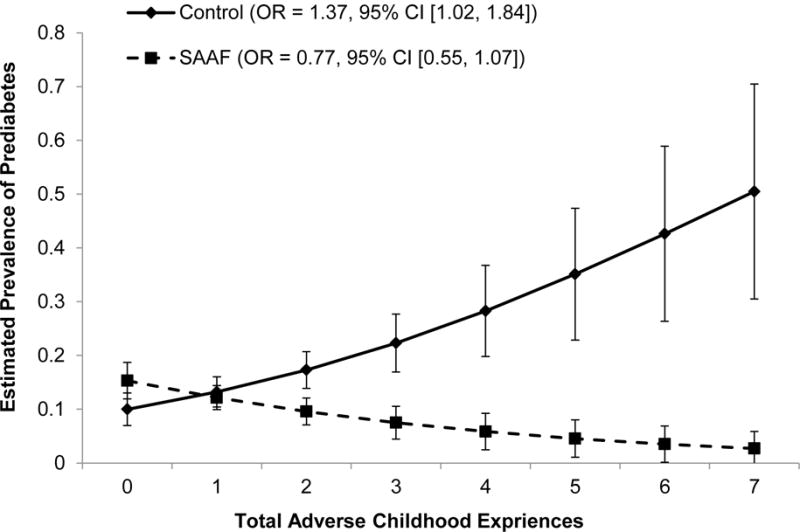
The effect of adverse childhood experiences on youths’ prediabetes status at age 25 by intervention status, controlling for gender, family SES disadvantage at age 11, and BMI at age 25. Numbers in parentheses refer to simple slopes for the control group and the Strong African American Families intervention group.
4. Discussion
This study extends knowledge by documenting an association between ACEs and prediabetes status and demonstrating that participation in a parenting-based prevention program ameliorated this association. These findings are also noteworthy because the study was conducted with a sample of African American participants from low-SES backgrounds in the rural South, a region with some of the highest rates of prediabetes in the United States (Menke et al., 2015). This is the first demonstration that a prevention program designed to enhance protective parenting processes can ameliorate the association between ACEs exposure and risk of developing prediabetes in young adulthood. From a public health perspective, uncoupling the link between ACEs and prediabetes is important because progression to type 2 diabetes reduces patients’ quality of life and is a significant public health problem in the United States, with estimated financial costs of nearly $250 billion annually (American Diabetes Association, 2013). Hence, the 3.54-fold higher risk among participants with high ACEs in the control condition compared with similar participants in the SAAF condition is likely to have individual, public health, and economic implications.
The protective properties of SAAF converge with “parenting effects” commonly observed in animal models, wherein caregiving tendencies exert lasting influences on offspring physiology, particularly in the brain and in the endocrine and immune systems (Coe et al., 2007; Hofer, 1987; Zhang et al., 2006). In human studies, mounting evidence reveals that supportive parenting can favorably mold stress-response tendencies among vulnerable children (Gunnar and Quevedo, 2007). As mentioned in the Introduction, parenting has been shown to buffer the influence of exposure to adversity on children’s neuroendocrine, immune, and neurocognitive systems (Shonkoff et al., 2009). The results in this report reinforce the importance of parenting during childhood and adolescence for health outcomes across the lifespan (Repetti et al., 2002; Troxel and Matthews, 2004).
From a primary prevention standpoint, efficacious family-centered programs designed to enhance supportive parenting are available for African American preadolescents, adolescents, and young adults (Brody, 2016). Participation in these programs has demonstrated stress-buffering effects on psychosocial outcomes such as adolescent drug use, and on biomarkers such as adolescents’ catecholamine levels, inflammation levels, epigenetic aging (Brody et al., 2016), and, as this report demonstrates, prediabetes status. These stress buffering associations are strongest for youths growing up in the most challenging socioeconomic circumstances (Miller et al., 2014). Taken together, accumulating research suggests that education and support for parents, even those living in challenging circumstances, that enables them to learn and practice important caregiving skills has the potential to promote supportive parenting, parents’ confidence, and children’s health and well-being.
Several limitations of this study must be noted. First, the SAAF trial was not designed to examine changes in prediabetes status. Prediabetes was not assessed when participants were 11 years of age; therefore, it could not be determined whether fasting glucose levels changed differentially over time for members of the intervention and control groups. At study entry, the SAAF and control groups were similar in terms of family poverty, parental education, family structure, parental age, and youth gender, suggesting that randomization worked to minimize pretrial group differences. Although the two groups were equivalent, unmeasured selection bias may have been operating. Second, because of the relatively small subsample of young adults who had prediabetes, the precise parenting practices, family strengths, or biological processes that could be responsible for the SAAF buffering effects could not be identified. We considered the possibility that SAAF rendered youths less vulnerable to substance use, the development of depressive symptoms or obesity, or the adoption of an unhealthful lifestyle, all of which would serve as potential mediators. These indirect/mediating pathways, however, did not reach statistical significance. Future research involving a larger sample of participants in which ACEs are assessed prior to the identification of prediabetes status is needed to identify the operative mediators. Finally, the findings’ generalizability must be documented with other groups living in rural and in urban settings.
5. Conclusion
The findings underscore the vulnerability of children with high levels of ACEs to prediabetes in young adulthood. Other research also suggests that supportive parenting has the potential to offset these health risks. In this study, high levels of ACEs were associated with prediabetes status, but only among young adults from the control condition of the SAAF randomized trial; those who had participated in SAAF 14 years previously were more than three times less likely to have developed prediabetes. If substantiated, these findings may provide a strategy for preventing the health consequences of ACEs.
Highlights.
Exposure to adverse childhood experiences (ACEs) predicted later health problems.
At age 11, youth were assigned to a family-centered intervention or control group.
At age 25, youth provided an ACEs score, and fasting blood glucose was assayed.
ACEs were associated with prediabetes status for control group youth.
ACEs were not associated with prediabetes status for intervention group youth.
Acknowledgments
This work was supported by the National Institutes of Health grant numbers R01 HD030588 and P30 DA027827.
Abbreviations
- ACEs
adverse childhood experiences
- BMI
body mass index
- CI
confidence interval
- OR
odds ratio
- SAAF
Strong African American Families program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- American Diabetes Association. The cost of diabetes. American Diabetes Association; Alexandria, VA: 2013. [Google Scholar]
- American Diabetes Association. Diagnosing diabetes and learning about prediabetes. American Diabetes Association; Alexandria, VA: 2015. [Google Scholar]
- Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34:396–403. doi: 10.1016/j.amepre.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Dong M, Brown DW, et al. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health. 2009;9:106. doi: 10.1186/1471-2458-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Brody GH. Family-centered prevention for rural African Americans: The Strong African American Families Program (SAAF), the Strong African American Families–Teen Program (SAAF–T), and the Adults in the Making Program (AIM) In: Van Ryzin MJ, Kumpfer KL, Fosco GM, Greenberg MT, editors. Family-centered prevention programs for children and adolescents: Theory, research, and large-scale dissemination. Psychology Press; New York, NY: 2016. pp. 282–307. [Google Scholar]
- Brody GH, Kogan SM, Grange CM. Translating longitudinal, developmental research with rural African American families into prevention programs for rural African American youth. In: King RB, Maholmes V, editors. The Oxford handbook of poverty and child development. Oxford University Press-USA; New York, NY: 2012. pp. 553–570. [Google Scholar]
- Brody GH, Lei M-K, Chen E, Miller GE. Neighborhood poverty and allostatic load in African American youth. Pediatrics. 2014a;134:e1362–e1368. doi: 10.1542/peds.2014.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, et al. The Strong African American Families program: Translating research into prevention programming. Child Dev. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH. Resilience to adversity and the early origins of disease. Dev Psychopathol. 2016;28:1347–1365. doi: 10.1017/S0954579416000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH, Philibert RA. Prevention effects ameliorate the prospective association between nonsupportive parenting and diminished telomere length. Prev Sci. 2015;16:171–180. doi: 10.1007/s11121-014-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE. Prevention moderates associations between family risks and youth catecholamine levels. Health Psychol. 2014b;33:1435–1439. doi: 10.1037/hea0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: Risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States 2014. U.S. Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- Chartier MJ, Walker JR, Naimark B. Separate and cumulative effects of adverse childhood experiences in predicting adult health and health care utilization. Child Abuse Negl. 2010;34:454–464. doi: 10.1016/j.chiabu.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, et al. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ. Childhood sexual abuse, depression, and family dysfunction in adult obese patients: a case control study. South Med J. 1993;86:732–736. doi: 10.1097/00007611-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult health, well-being, social function, and healthcare. In: Lanius RA, Vermetten E, Pain C, editors. The hidden epidemic: The impact of early life trauma on health and disease. Cambridge University Press; Cambridge, UK: 2010. pp. 77–87. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Garner AS, Shonkoff JP, Siegel BS, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into lifelong health. Pediatrics. 2012;129:e224–e231. doi: 10.1542/peds.2011-2662. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early social relationships: A psychobiologist’s view. Child Dev. 1987;58:633–647. doi: 10.1111/1467-8624.ep7264468. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006 Dec 14;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Menke A, Casagrande S, Geiss LS, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015 Sep 8;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JS, Audage NC. The effects of stress on health across the lifespan. Centers for Disease Control and Prevention; Atlanta, GA: 2008. [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci U S A. 2014;111:11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011a;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011b;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–336. doi: 10.1037/0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009 Jun 3;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: Results from a large population-based sample of men and women. Child Abuse Negl. 2007;31:517–530. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA. What are the costs of marital conflict and dissolution to children’s physical health? Clin Child Fam Psychol Rev. 2004;7:29–57. doi: 10.1023/B:CCFP.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, et al. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]


