Abstract
Background
Previous studies have shown that ibuprofen is detrimental to tissue healing following acute injury; however, the effects of ibuprofen when combined with non-injurious exercise are debated.
Hypothesis
We hypothesized that administration of ibuprofen to rats undergoing a non-injurious treadmill exercise protocol would abolish the beneficial adaptations found with exercise but have no effect on sedentary muscle and tendon properties.
Study Design
Controlled laboratory study
Methods
Rats were divided into exercise or cage activity (sedentary) groups and acute (a single bout of exercise followed by 24 hours of rest) and chronic (2 or 8 weeks of repeated exercise) time points. Half of the rats received ibuprofen to investigate the effects of this drug over time when combined with different activity levels (exercise and sedentary). Supraspinatus tendons were used for mechanical testing and histology (organization, cell shape, cellularity), and supraspinatus muscles were used for morphological (fiber CSA, centrally nucleated fibers) and fiber type analysis.
Results
Chronic intake of ibuprofen did not impair supraspinatus tendon organization or mechanical adaptations (stiffness, modulus, max load, max stress, dynamic modulus, or viscoelastic properties) to exercise. Tendon mechanical properties were not diminished and in some instances increased with ibuprofen. In contrast, total supraspinatus muscle fiber cross-sectional area decreased with ibuprofen at chronic time points, and some fiber type-specific changes were detected.
Conclusions
Chronic administration of ibuprofen does not impair supraspinatus tendon mechanical properties in a rat model of exercise but does decrease supraspinatus muscle fiber cross-sectional area. Clinically, these findings suggest that ibuprofen does not detrimentally affect regulation of supraspinatus tendon adaptions to exercise but does decrease muscle growth. Individuals should be advised on the risk of decreased muscle hypertrophy when consuming ibuprofen. This fundamental study adds to the growing literature on the effects of ibuprofen on musculoskeletal tissues and provides a solid foundation on which future work can build.
Clinical Relevance
Ibuprofen is a commonly used drug by sedentary individuals and athletes. This study suggests that ibuprofen has tissue-dependent effects that should be considered when prescribing the drug.
Key Terms: Shoulder, rotator cuff, NSAIDS, Biomechanics of tendon, Muscle physiology
Introduction
In the United States, acetaminophen, ibuprofen, and aspirin are the top 3 consumed drugs by adults.12 Athletes are a sub-population among which the use of nonsteroidal anti-inflammatory drugs (NSAIDs) has become commonplace. Specifically, many athletes self-diagnose and “treat” themselves with these over-the-counter products or use them prophylactically to promote recovery and alleviate pain: 75% of high school football players had used NSAIDs in the previous 3 months, while NSAID use among Olympic athletes was second only to vitamins.7 Furthermore, 95.7% of college football players have used NSAIDs such as ibuprofen.11 Previous studies have shown that following acute tendon injury, administration of ibuprofen, which inhibits cyclooxygenases-1 and -2 (COX-1 and COX-2) of the arachidonic acid inflammatory cascade, is detrimental,e.g.,8 suggesting that early inflammation is a required component of successful tissue healing.
A mild inflammatory response may also be important in the adaptation of musculoskeletal tissues to chronic, non-injurious exercise; however current literature on the effects of ibuprofen on tissue adaptations is equivocal. For example, in a plantaris overload model in rats, one study found 50% decreased muscle hypertrophy with administration of ibuprofen.28 Although the plantaris overload model is frequently used, its rapid rate of muscle hypertrophy does not mimic the normal rate of human hypertrophy with exercise,4 which supports the use of a more clinically relevant model. In another study, ibuprofen administered to mice undergoing 4 weeks of wheel running eliminated the correlation between run distance and skeletal muscle adaptations.14 In contrast, following chronic resistance training in older adults, ibuprofen administration increased muscle hypertrophy and strength but did not affect tendon cross-sectional area or strain.5,29 Furthermore, previous studies have not been performed specifically on the supraspinatus, a commonly injured tissue of the rotator cuff. Since it has been well established that muscles and tendons are uniquely adapted to their function, it is critical to examine tissue-specific responses. Overall, little is known about the interaction between ibuprofen, a commonly consumed drug, and the response of supraspinatus muscle and tendon to exercise. Therefore, the objective of this study was to determine the effects of ibuprofen on the adaptations of supraspinatus tendon and muscle in a rat model of exercise. We hypothesized that administration of ibuprofen would abolish the beneficial adaptations found with exercise, which include enhanced tendon mechanical properties, muscle fiber hypertrophy, and increased oxidative muscle fiber type, but have no effect on sedentary muscle fiber cross-sectional area or fiber type distribution and tendon mechanical properties, organization, cell shape, or cellularity.
Methods
Study Design and Treadmill Protocols
Following approval by the University’s Institutional Animal Care and Use Committee, 167 male, Sprague-Dawley rats (400–450g at start of study) were divided into cage activity (CA) or exercise (EX) groups. These groups were further divided into an acute time point or two chronic time points with half of these animals receiving ibuprofen (IBU). Acute groups represent a short-term response (24 hours) following a single bout of exercise whereas chronic groups represent a long-term, adaptive response (after 2 or 8 weeks). Additional details describing these groups are below. The non-drug comparison groups are a subset of another study performed in the same time frame by the same personnel. Animals in the IBU groups were trained to drink from a needleless syringe2 and were administered children’s, berry-flavored, liquid ibuprofen (Major Pharmaceuticals, Livonia, MI, USA) at 20mg/kg orally every 12 hours as previously described.8
To investigate the acute effects of ibuprofen on muscle and tendon, animals in the acute EX group underwent 2 weeks of progressive, downhill treadmill training to acclimate to the treadmill, as previously described.23 Following 72 hours of rest, acute EX animals underwent a single treadmill exercise session at a constant speed of 10 meters/minute for 1 hour on a flat treadmill. These animals were then euthanized by controlled flow-rate carbon dioxide 24 hours after completion of their single bout of exercise (EX24). Animals in the IBU groups began receiving ibuprofen 24 hours prior to the start of their single bout of exercise and continued receiving ibuprofen every 12 hours until euthanasia (5 doses total, EX24IBU). A separate group of animals maintained normal cage activity for the 2 week duration and received 5 doses of ibuprofen (every 12 hours) until euthanasia 1.5 hours after their last dose of ibuprofen (CA24IBU). These animals were compared to a cage activity group that did not receive ibuprofen (CA24, Figure 1A).
Figure 1. Acute (A) and Chronic (B) Study Designs.
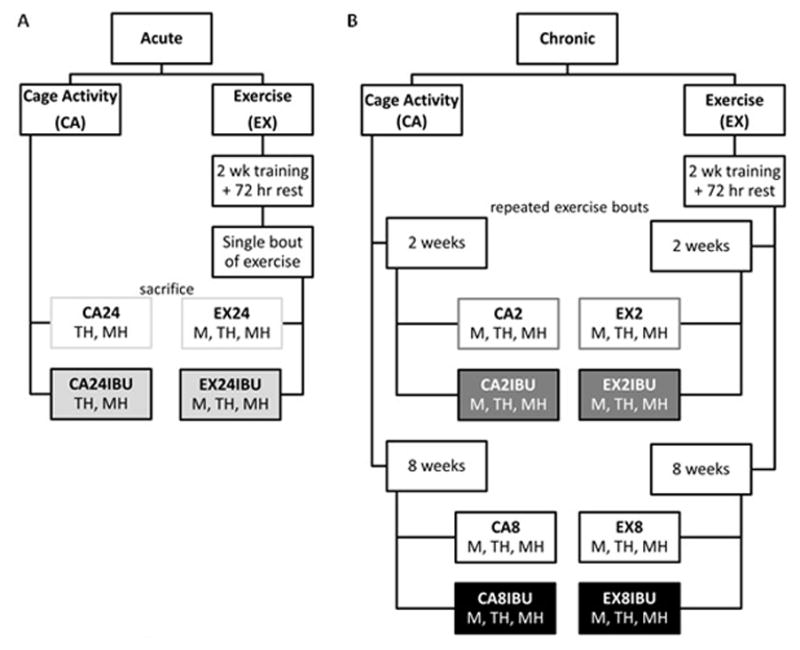
A) An acute cage activity group maintained normal cage activity, received 5 doses of ibuprofen, was euthanized 1.5 hours after their last dose of the drug (CA24IBU) and was compared to a group that did not receive ibuprofen (CA24). Rats in acute exercise groups underwent 2 weeks of treadmill training followed by 72 hours of rest. These rats were euthanized 24 hours after completion of a single bout of exercise (EX24). These animals were compared to a group that received ibuprofen beginning 1 day before their exercise session and continuing until euthanasia (EX24IBU). B) For analysis of chronic effects, control cage activity groups maintained normal cage activity for an early time point (CA2, CA2IBU) or a later time point (CA8, CA8IBU). Rats in chronic exercise groups underwent 2 weeks of treadmill training followed by 2 or 8 weeks of the exercise protocol (EX2, EX2IBU, EX8, EX8IBU). IBU groups received ibuprofen during this 2 or 8 week period. Assays were performed on supraspinatus: M- tendon mechanics, TH- tendon histology, MH- muscle histology
To investigate the chronic effects of ibuprofen on muscle and tendon adaptations to exercise, animals in the chronic EX groups underwent 2 weeks of flat treadmill acclimation and then walked on a flat treadmill at a constant speed of 10 meters/minute, 1 hour per day, 5 days per week22 for 2 or 8 weeks (EX2, EX8). Control animals maintained normal cage activity for this duration (CA2, CA8). Animals in the IBU groups received ibuprofen every 12 hours, 7 days per week for the 2 or 8 week duration (EX2IBU, EX8IBU, CA2IBU, CA8IBU, Figure 1B).
For both acute and chronic exercise groups, inclusion was set to >75% completion of the total treadmill protocol. Animals that completed <10% of the treadmill protocol (they did not even complete the 2 weeks of treadmill training and were removed before beginning the exercise protocol) were included in the CA groups.
This study was designed to test hypotheses specific to the effect of ibuprofen, separately for cage activity and exercise groups; therefore, comparisons were not made between activity levels. The study was designed to answer the following, specific, clinical questions: 1) Can sedentary individuals take ibuprofen without damaging supraspinatus tendon and muscle properties? 2) Can active individuals or athletes take ibuprofen without negating the beneficial adaptations of the supraspinatus in response to exercise? This study does not address supraspinatus tendon and muscle responses to exercise per se, but rather, the study measures tissue responses to ibuprofen at different activity levels. Furthermore, since rats continue to grow throughout their lifetime, making comparisons between time points inappropriate, the inclusion of time-matched control groups allows for rigorous comparisons specific to the effect of ibuprofen separately at each time point.
From every animal, each shoulder was designated to muscle/tendon histology or tendon mechanics. Tendon mechanical testing was not performed on the CA24 or CA24IBU groups in an effort to reduce the number of required animals. Tendon histologic changes typically precede mechanical changes, and two days is very little time for a tendon to respond with detectable changes in mechanical properties without direct injury or perturbation. At time of euthanasia, animals were weighed, shoulders allotted to histology were dissected, and the supraspinatus muscle was cut transversely through the mid-belly. This muscle piece was pinned to Styrofoam to maintain muscle architecture, covered in OCT, and immediately flash-frozen in liquid nitrogen-cooled isopentane. The specimens were stored at −80ºC until frozen sectioning. The remaining humeral head-supraspinatus tendon-muscle half was immediately placed in formalin for fixing. For mechanics, the animals were stored at −20ºC, and the shoulders were dissected and prepared at the time of testing.
Tendon Mechanical Testing
For tendon mechanical testing, animals were thawed, and the left shoulder was dissected, keeping the supraspinatus tendon attached to its bony insertion on the humerus. Verhoeff’s stain lines for optical strain measurements were placed at the bony insertion site and 2, 4, and 8 mm proximally. Tendon cross-sectional area was measured with a custom laser device.19 Cyanoacrylate was used to fix the tendon between two pieces of sandpaper at the 8 mm stain line and gripped with a custom screw clamp. Humeri were potted in PMMA, and a custom fixture secured the potted humerus for testing with an Instron ElectroPuls E3000 affixed with a 250 N load cell and a LVDT for increased displacement resolution. Tendons were submerged in a 37°C PBS bath and underwent the following testing protocol: 1) preconditioning (10 triangle cycles from 1–1.5% strain at 0.25 Hz then held at gauge length for 300s), 2) stress-relaxation at 4% strain held for 300s, 3) frequency sweep (0.1, 1, 2, 10 Hz) of 10 sine cycles with amplitude of 0.125% strain, 4) return to gauge length and held for 60s, 5) stress-relaxation at 8% strain held for 300s, 6) recovery to 4% strain held for 300s, 7) return to 8% strain and held for 300s, 8) frequency sweep (0.1, 1, 2, 10 Hz) of 10 sine cycles with amplitude of 0.125% strain, 9) return to gauge length and held for 180s, 10) ramp to failure at 0.3%/s. Due to slip in the fixture that occurred near 4% strain, only the 8% data were analyzed (steps 5, 8, 10).
Local 2D Lagrangian strain of the tendon relative to the bone during the ramp to failure was measured by tracking the stain lines with a custom MATLAB program. Stiffness was calculated as the slope of the linear region of the load-displacement curve, and elastic modulus was calculated as the slope of the linear region of the stress-strain curve. Percent relaxation was calculated as the percent change in the load after 300 seconds of relaxation. The dynamic modulus (|E*|, ratio of the stress-to-strain amplitude) and tangent of the phase angle between the stress and strain (tan(δ), ratio of the loss-to-storage modulus, a measure of viscoelasticity) were calculated using the middle 5 sine cycles at each frequency.
Tendon Histology
After fixing in 10% buffered formalin, bone-tendon-muscle units were decalcified in formic acid decalcifier (Immunocal, American MasterTech, Lodi, CA, USA) and then processed, embedded in paraffin, and sectioned at 7μm. Sections were stained with hematoxylin and eosin and the tendon midsubstance was imaged at 200x magnification and used for traditional (acute and chronic groups) and polarized light microscopy (chronic groups only). Cell density (cells/mm2) and cell shape (aspect ratio 0–1 with 1 being a circle) were quantified using commercial software (Bioquant Osteo II, Nashville, TN, USA). Custom MATLAB software was used to analyze the polarized light images and calculate the circular standard deviation, a measure of the spread of collagen fiber alignment.9
Muscle Histology
Muscle specimens were cryosectioned at 10μm, transverse to the muscle fibers. For morphological analysis, sections were stained with an anti-laminin polyclonal antibody produced in rabbit (Sigma-Aldrich, Saint Louis, MO, USA), incubated with Alexa Fluor 488 goat anti-rabbit secondary antibody (Life Technologies, Grand Island, NY, USA), mounted and cover-slipped with ProLong Gold antifade reagent with DAPI (Life Technologies, Grand Island, NY, USA), and visualized with epifluorescence on an Eclipse 90i microscope. Six images were acquired at 100x magnification per specimen (resulting in analysis of ≥ 450 fibers per specimen). The number of centrally nucleated fibers (CNF) were counted and divided by the total number of fibers to determine percent. The total average fiber cross-sectional area was analyzed with a MATLAB application.27 For fiber type analysis on chronic groups, sections were simultaneously stained with anti-myosin heavy chain-1 (MyHC-I) BA-DF, MyHC-IIa SC-71, and MyHC-IIb BF-F3 antibodies25 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), and anti-laminin antibody (Sigma-Aldrich). MyHC-IIx fibers were left unstained and were not analyzed for cross-sectional area. Sections were incubated with secondary antibodies: Alexa Fluor 546 goat anti-mouse IgM (Life Technologies), DyLight 405 goat anti-mouse IgG 2b (Jackson Immuno, West Grove, PA, USA), Alexa Fluor 488 goat anti-mouse IgG 1 (Jackson), and Alexa Fluor 647 goat anti-rabbit IgG (Jackson). Stained sections were mounted and cover-slipped with ProLong Gold antifade reagent without DAPI (Life Technologies). As previously described, the rat supraspinatus muscle fiber type distribution differs in the superficial and deep layers of the muscle;3 therefore, fiber types of these two regions were analyzed separately, and 3 images at 100x magnification were captured for each region. These images were analyzed with a MATLAB application for muscle fiber type distribution and cross-sectional area.27
Statistics
Statistical analysis was performed based on our hypotheses. To determine the effects of ibuprofen on muscle and tendon adaptations, t-tests were used to compare IBU-treated and non-drug treated groups separately for CA and EX at each time point. Comparisons between time points are inappropriate due to the significant animal growth that occurs. Since our hypotheses were specific to the effects of ibuprofen, we did not make comparisons between exercise and cage activity groups. Significance was set to p≤0.05. Figures are shown with mean and standard deviation.
Results
Tendon Mechanics
24 hours after a single bout of exercise, ibuprofen decreased the tendon cross-sectional area and increased the modulus (Figure 2A,B). At 2 Hz, administration of ibuprofen increased tan(δ) (Supplemental Figure 1). No other mechanical properties were changed acutely, supporting the decision to not perform tendon mechanical analysis on CA24 and CA24IBU groups. Chronic administration of ibuprofen in the cage activity groups increased stiffness, modulus, maximum stress (Figure 2), and dynamic modulus (Figure 3) at 2 weeks and decreased tendon cross-sectional area and increased modulus (Figure 2) and dynamic modulus at 8 weeks (Figure 3). Ibuprofen had minimal effect on the viscoelastic properties of the cage activity groups, increasing tan(δ) at only 0.1 and 1 Hz after 2 weeks, increasing tan(δ) at 0.1 Hz after 8 weeks, and decreasing tan(δ) at 10 Hz after 8 weeks (Supplemental Figure 1) with no effect on percent relaxation.
Figure 2. Tendon Mechanical Properties.
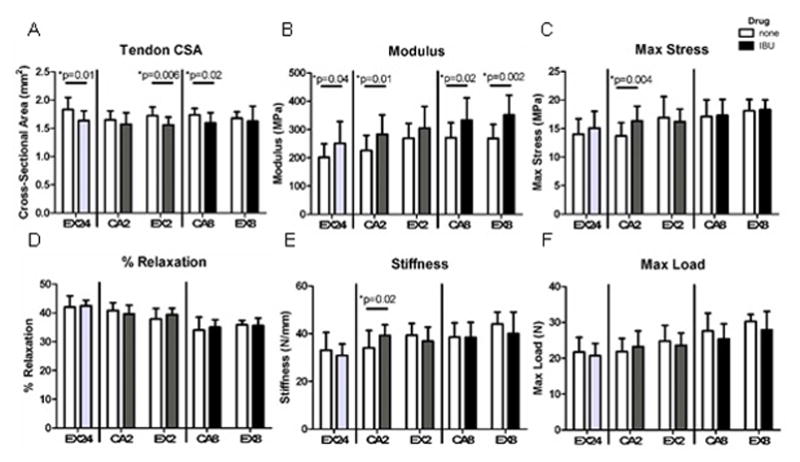
Following a single bout of exercise, ibuprofen decreased tendon cross-sectional area and increased modulus, returning the properties to baseline (dashed gray line). Chronic administration of ibuprofen did not negatively impact tendon mechanical adaptations to exercise or cage activity tendon mechanical properties. Open bars- no drug, Solid bars- administered ibuprofen (n=10–16 acute, n=8–17 at 2 weeks, n=9–13 at 8 weeks)
Figure 3. Dynamic Modulus.
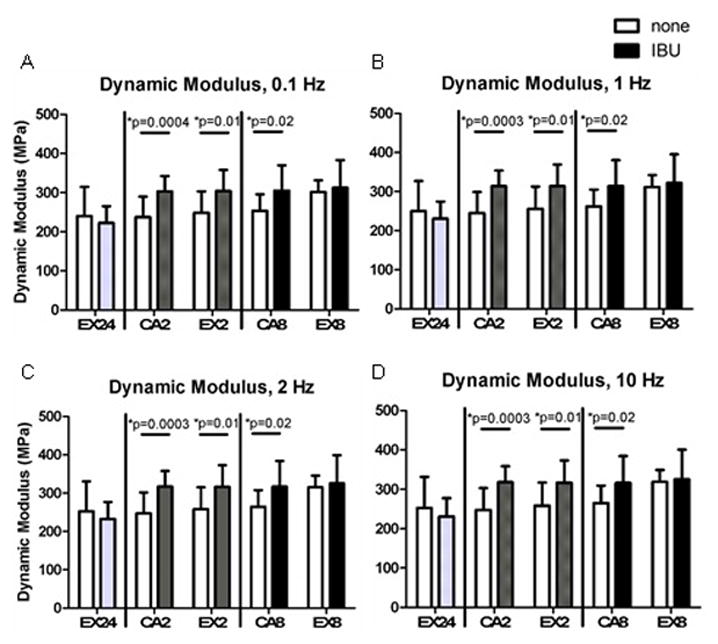
Administration of ibuprofen for 2 weeks increased dynamic modulus at all frequencies. Dynamic modulus also increased at 8 weeks in the cage activity group. Open bars- no drug, Solid bars- administered ibuprofen (n=10–11 acute, n=12–17 at 2 weeks, n=11–13 at 8 weeks)
Chronic administration of ibuprofen in the exercise groups led to decreased tendon cross-sectional area (Figure 2A) and increased dynamic modulus (Figure 3) after 2 weeks and increased modulus after 8 weeks (Figure 2B). Ibuprofen increased tan(δ) at 0.1 Hz after 2 weeks and at 0.1 and 1 Hz after 8 weeks (Supplemental Figure 1) with no effect on percent relaxation.
Tendon Histology
Acute administration of ibuprofen increased cell density in a cage activity group (Figure 5A) but had no effect on cell shape and no effect when combined with a single bout of exercise. Administration of ibuprofen for 2 weeks combined with exercise decreased cell density (Figure 4A). 8 weeks of ibuprofen combined with exercise led to increased cell density and rounder cells (Figure 4). Ibuprofen did not affect tendon collagen organization (Supplemental Figure 2). Representative tendon histology images are shown in Supplemental Figure 3. The mild changes in cellularity and cell shape and highly organized collagen are in contrast to that seen with injury.
Figure 5. Average Muscle Fiber Cross-Sectional Area.
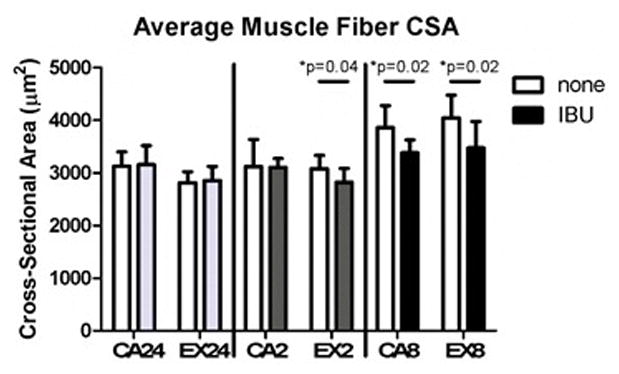
Administration of ibuprofen decreased average muscle fiber cross-sectional area in the 2 week exercise group and both of the 8 week groups. Open bars- no drug, Solid bars- administered ibuprofen (n=7–9 acute, n=6–9 at 2 weeks, n=6–8 at 8 weeks)
Figure 4. Tendon Histology.
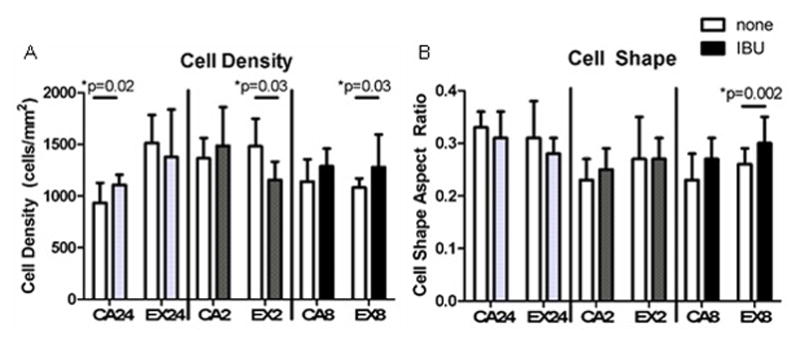
Ibuprofen had minimal effect on tendon cell density (A) and cell shape (B). Open bars- no drug, Solid bars- administered ibuprofen (n=7–9 acute, n=6–9 at 2 weeks, n=6–8 at 8 weeks)
Muscle Histology
For all groups, the average percent of centrally nucleated fibers was below 1%, and ibuprofen had no effect (Supplemental Table 1, Supplemental Figure 4). The average total fiber cross-sectional area decreased with administration of ibuprofen at 2 weeks in the exercise group and at 8 weeks in both the cage activity and exercise groups (Figure 5, Supplemental Figure 4).
Muscle fiber-specific changes were also evident with administration of ibuprofen. Similar to what has been shown previously,3 we found distinctions between the superficial and deep regions of the supraspinatus muscle, with the superficial region showing no MyHC-I staining, smaller MyHC-IIa fibers, and a greater proportion of MyHC-IIb fibers.
Specifically, in the superficial region of the muscle, ibuprofen decreased the percent of MyHC-IIb positive fibers and increased the percent of MyHC-IIx positive fibers in the 2 week cage activity group and decreased the percent of MyHC-IIa positive fibers in the 2 week exercise group (Table 1). In the deep region of the muscle, ibuprofen increased the percent of MyHC-IIx positive fibers in the 8 week exercise group (Table 1). Ibuprofen significantly decreased the cross-sectional area of MyHC-IIb positive fibers in the superficial region of the 2 week exercise group and the MyHC-IIa positive fibers in the superficial region of the 8 week cage activity group (Table 2). No ibuprofen-induced, fiber type-specific cross-sectional area measurements were found in the deep region of the supraspinatus muscle.
Table 1.
Fiber Type Distribution, %.
| Superficial | Deep | ||||||
|---|---|---|---|---|---|---|---|
| Group | MyHC-IIa | MyHC-IIb | MyHC-IIx | MyHC-I | MyHC-IIa | MyHC-IIb | MyHC-IIx |
| CA2 | 9±3 | 66±6 | 25±7 | 13±2 | 29±5 | 26±7 | 32±3 |
| CA2IBU | 8±5 | a50±17 | b41±19 | 11±2 | 28±7 | 27±9 | 34±8 |
| EX2 | 16±6 | 57±10 | 28±7 | 13±3 | 37±6 | 21±8 | 29±5 |
| EX2IBU | c9±3 | 52±17 | 39±18 | 14±4 | 36±7 | 19±8 | 31±7 |
| CA8 | 11±5 | 60±8 | 28±4 | 16±3 | 30±3 | 18±5 | 36±3 |
| CA8IBU | 9±2 | 60±7 | 31±6 | 14±5 | 32±5 | 15±5 | 39±5 |
| EX8 | 18±6 | 51±8 | 31±5 | 13±4 | 42±6 | 16±7 | 29±2 |
| EX8IBU | 13±6 | 50±18 | 38±21 | 12±2 | 40±6 | 15±7 | d32±4 |
Superficial and deep regions of the supraspinatus muscle were analyzed separately for percent fiber type. The superficial region had no MyHC-I staining. (n=6–9 at 2 weeks, n=6–8 at 8 weeks) Mean ± StDev, superscript indicates ibuprofen had a significant effect (p<0.05) compared to corresponding non-drug treated group:
p=0.01,
p=0.02,
p=0.01,
p=0.04
Table 2.
Fiber Type Cross-Sectional Area, μm2.
| Superficial | Deep | ||||
|---|---|---|---|---|---|
| Group | MyHC-IIa | MyHC-IIb | MyHC-I | MyHC-IIa | MyHC-IIb |
| CA2 | 1455±198 | 3614±510 | 1755±228 | 2127±204 | 4048±365 |
| CA2IBU | 1476±229 | 3440±418 | 1653±228 | 2018±367 | 3807±925 |
| EX2 | 1611±166 | 3848±547 | 1769±190 | 2220±196 | 4056±617 |
| EX2IBU | 1499±172 | a2985±342 | 2101±475 | 2459±619 | 3475±753 |
| CA8 | 1845±180 | 4225±485 | 2069±333 | 2783±481 | 4552±658 |
| CA8IBU | b1585±191 | 4048±495 | 2105±442 | 2600±531 | 4441±512 |
| EX8 | 1890±304 | 4669±415 | 2083±357 | 2720±291 | 5088±349 |
| EX8IBU | 1824±211 | 4333±751 | 2029±472 | 2613±606 | 4685±1275 |
Superficial and deep regions of the supraspinatus muscle were analyzed separately for fiber type cross-sectional area. The superficial region had no MyHC-I staining. (n=5–9 at 2 weeks, n=6–7 at 8 weeks) Mean ± StDev, superscript indicates ibuprofen had a significant effect (p<0.05) compared to corresponding non-drug treated group:
p=0.003,
p=0.02
Discussion
In contrast to our hypothesis, results suggest that chronic intake of ibuprofen at pharmacologic doses does not detrimentally affect supraspinatus tendon mechanical adaptations to exercise. Tendon mechanical properties were not diminished and in some instances increased with ibuprofen. This finding suggests that the arachidonic acid cascade may not play a major role in the adaptions of tendon to load in a non-injurious exercise model, in contrast to that seen with acute injury models.e.g.,1,8,10,18 On the other hand, total muscle fiber cross-sectional area decreased with ibuprofen at chronic time points, and some fiber type-specific changes were detected, indicating that chronic administration of ibuprofen impacts hypertrophy of sedentary and exercised supraspinatus muscles. Our results support that taking ibuprofen while engaging in exercise could interfere with muscle hypertrophy but does not negatively impact the supraspinatus tendon.
Before elucidating the mechanisms by which ibuprofen affects supraspinatus muscle and tendon, this initial study was necessary to first determine the changes specific to the supraspinatus muscle and tendon across a variety of time points, which has not been done previously. The results of this study confirm those found in prior literature using different models and tissues, and, importantly, the current study adds to the growing body of literature on the effects of NSAIDs on musculoskeletal tissues by examining simultaneously the supraspinatus muscle and tendon responses to both acute and chronic exercise.
Previous in vitro studies on tenocytes have shown that ibuprofen reduces proliferation34 and migration,33,34 increases MMP expression, and has no effect on collagen types I or III.32 A tendon-bone healing co-culture model has shown COX inhibition leads to reduced cell viability, proliferation, and migration.26 Although these studies suggest important effects of NSAIDs on tendon, they were not designed to replicate in vivo conditions. Current literature on the in vivo effects of NSAIDs on tendon and muscle is unclear. Previous studies on tendon have reported the acute effects of ibuprofen combined with exercise to include decreases in collagen synthesis6 and blood flow13 or minimal effects.21 In our study, we detected an acute decrease in tendon cross-sectional area and increase in tendon modulus when ibuprofen was combined with a single bout of exercise; however, since this study investigated only a single acute time point (24 hours after exercise), it is unknown whether these changes represent delayed or accelerated recovery.
Confirming a previous study,3 we detected apparent regional differences in muscle fiber type distribution within the supraspinatus, and future studies should investigate the functional implications of these two distinct regions. Our finding of decreased muscle fiber cross-sectional area supports a previous study that showed ibuprofen reduced muscle hypertrophy in rats.28 Furthermore, while some studies have found no changes in myofibrillar and collagen protein synthesis in muscle with administration of NSAIDs,16,20 in subjects given ibuprofen in conjunction with high-intensity eccentric resistance exercise, skeletal muscle fractional synthesis rates were attenuated, implying that the drug suppressed the beneficial muscle protein synthesis response to exercise.31 Other studies have shown that acute administration of NSAIDs led to fewer muscle satellite cells.15,17 Finally, prostaglandin F2α (PGF2α) is thought to be a regulator of muscle protein synthesis, contributing to muscle hypertrophy.30 Prostaglandins are a downstream product of COX activity, so ibuprofen may be decreasing PGF2α production. Following a single bout of exercise combined with ibuprofen, reduced muscle protein synthesis, decreases in satellite cells, which are thought to mediate hypertrophy,24 or reduced PGF2α may have led to the decreased muscle fiber cross-sectional area we measured chronically. Future studies should measure the acute effects of this exercise protocol these parameters. Overall, the highly organized structure of the tendon collagen and the very few muscle fibers with centrally located nuclei in this study suggest that these tissues in this study were healthy and were not damaged by ibuprofen.
In general, muscle is more responsive to exercise than tendon, implying that different processes regulate the adaptations of these tissues to load. Supporting this, in a gene screening study, we found distinct responses of supraspinatus muscle and tendon to both acute and chronic exercise.23 Therefore, treatments, like ibuprofen, would have tissue-dependent and activity-dependent effects that must be considered when making clinical recommendations for treatment.
This study is not without limitations. First, muscle fiber type was only measured in the chronic groups, and MyHC-IIx was left unstained and unanalyzed. Some of the decreases in total fiber cross-sectional area could be due to changes in MyHC-IIx, which were not measured; however, the decreases in total muscle fiber cross-sectional area do include MyHC-IIx fibers and therefore represent the global changes to the muscle. Additionally, it is unknown whether the measured decreases in muscle fiber cross-sectional area and altered fiber type distributions lead to functional differences of the whole muscle. Physiologic muscle assays were not performed in the current study in an effort to reduce the number of animals required. The results of this study provide evidence for future investigations on the physiologic properties of the muscle including force production, fatigue, and contractile properties, and more specific hypotheses with focused comparisons can be performed in these future studies. Satellite cell recruitment should be examined, as these cells have been implicated in muscle responses to exercise and some studies suggest that NSAIDs inhibit these cells. Additionally, this study investigates a single exercise protocol, and it is unknown how ibuprofen may affect supraspinatus tendon and muscle with a more intense exercise protocol; however, this protocol has previously been shown to induce beneficial adaptations to the rat shoulder without inducing chronic injury,22,23 indicating that it is a physiologically relevant model of non-injurious exercise. Future studies can investigate effects of ibuprofen when combined with alternate loading conditions such as overuse or under-loading by immobilization. The current study used young adult animals, but baseline inflammation increases with age; therefore, effects of ibuprofen may differ in aged populations and should be investigated further. Because this study investigated ibuprofen, a commonly consumed and clinically relevant nonspecific COX inhibitor, conclusions cannot be drawn regarding the implications of COX-1 or COX-2 specific inhibition; future studies could also investigate specific inhibitors. Furthermore, in the current study, ibuprofen was administered daily for the entire duration (2 or 8 weeks); however, biologic processes governing early and later adaptations to exercise may differ, supporting exploration of temporal administration of the drug. Finally, future studies should investigate the structural protein changes within the tissue that lead to the detected changes as well as the baseline and exercise-induced temporal expression of COX enzymes and other arachidonic acid cascade proteins, vascularity, blood flow, angiogenesis, MMPs and TIMPs, inflammatory cells, and cytokines and chemokines.
In conclusion, chronic administration of ibuprofen does not impair supraspinatus tendon mechanical properties in a rat model of exercise but does decrease supraspinatus muscle fiber cross-sectional area. Clinically, these findings suggest that ibuprofen does not detrimentally affect regulation of supraspinatus tendon adaptions to exercise but does decrease muscle growth. Individuals should be advised on the risk of decreased muscle hypertrophy when consuming ibuprofen. This fundamental study adds to the growing literature on the effects of ibuprofen on musculoskeletal tissues and provides a solid foundation on which future work can build.
Supplementary Material
Ibuprofen mildly increased tan(δ) at 2 Hz (C) 24 hours after a single bout of exercise. At 0.1 Hz (A), administration of ibuprofen for 2 and 8 weeks increased tan(δ) in the exercise and cage activity groups. Tan(δ) also increased with ibuprofen at 1 Hz (B) for the 2 week cage activity and 8 week exercise groups. Tan(δ) at 10 Hz (D) decreased in the 8 week cage activity group with administration of ibuprofen. Open bars- no drug, Solid bars- administered ibuprofen (n=11–16 acute, n=11–17 at 2 weeks, n=11–13 at
Ibuprofen had no effect on tendon collagen organization at 2 and 8 weeks. Open bars- no drug, Solid bars- administered ibuprofen (n=6–8)
Ibuprofen mildly increased cellularity acutely in the cage activity group and decreased cellularity with 2 weeks of exercise.
Ibuprofen decreased average muscle fiber cross-sectional area. Centrally nucleated fibers are rare. A) From EX8, mean fiber size 3902μm2 B) From EX8IBU, mean fiber size 3325 μm2 Acknowledgements: This study was funded by the Penn Center for Musculoskeletal Disorders (NIH/NIAMS P30 AR050950) and the U.S. Department of Veterans Affairs (VISN 4 CPPF).
Centrally Nucleated Fibers, %
What is known about the subject
Previous work suggests NSAIDs are detrimental to tissue healing following acute injury. Some studies have investigated the effects of NSAIDs in conjunction with exercise, but these effects are debated.
What this study adds to existing knowledge
This study uses a validated rat model of non-injurious exercise and simultaneously investigates supraspinatus tendon and muscle to show that ibuprofen has tissue-dependent effects.
References
- 1.Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med. 1986;14(4):303–308. doi: 10.1177/036354658601400411. [DOI] [PubMed] [Google Scholar]
- 2.Atcha Z, Rourke C, Neo AH, et al. Alternative method of oral dosing for rats. J Am Assoc Lab Anim Sci. 2010;49(3):335–343. [PMC free article] [PubMed] [Google Scholar]
- 3.Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23(2):259–265. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Burd NA, Dickinson JM, Lemoine JK, et al. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab. 2010;298(2):E354–61. doi: 10.1152/ajpendo.00423.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll CC, Dickinson JM, LeMoine JK, et al. Influence of acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. J Appl Physiol. 2011;111(2):508–515. doi: 10.1152/japplphysiol.01348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen B, Dandanell S, Kjaer M, Langberg H. Effect of anti-inflammatory medication on the running-induced rise in patella tendon collagen synthesis in humans. J Appl Physiol. 2011;110(1):137–141. doi: 10.1152/japplphysiol.00942.2010. [DOI] [PubMed] [Google Scholar]
- 7.Ciocca M. Medication and supplement use by athletes. Clin Sports Med. 2005;24(3):719–38. doi: 10.1016/j.csm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Connizzo BK, Yannascoli SM, Tucker JJ, et al. The detrimental effects of systemic ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014;472(8):2433–2439. doi: 10.1007/s11999-013-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37(5):739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Hammerman M, Blomgran P, Ramstedt S, Aspenberg P. COX-2 inhibition impairs mechanical stimulation of early tendon healing in rats by reducing the response to microdamage. J Appl Physiol. 2015;119(5):534–540. doi: 10.1152/japplphysiol.00239.2015. [DOI] [PubMed] [Google Scholar]
- 11.Holmes N, Cronholm PF, Duffy AJ, 3rd, Webner D. Nonsteroidal anti-inflammatory drug use in collegiate football players. Clin J Sport Med. 2013;23(4):283–286. doi: 10.1097/JSM.0b013e318286d0fa. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the united states: The slone survey. JAMA. 2002;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Langberg H, Boushel R, Skovgaard D, Risum N, Kjaer M. Cyclo-oxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J Physiol. 2003;551(Pt 2):683–689. doi: 10.1113/jphysiol.2003.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machida M, Takemasa T. Ibuprofen administration during endurance training cancels running-distance-dependent adaptations of skeletal muscle in mice. J Physiol Pharmacol. 2010;61(5):559–563. [PubMed] [Google Scholar]
- 15.Mikkelsen UR, Langberg H, Helmark IC, et al. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol. 2009;107(5):1600–1611. doi: 10.1152/japplphysiol.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikkelsen UR, Schjerling P, Helmark IC, et al. Local NSAID infusion does not affect protein synthesis and gene expression in human muscle after eccentric exercise. Scand J Med Sci Sports. 2011;21(5):630–644. doi: 10.1111/j.1600-0838.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- 17.Monda M, Vicidomini C, Viggiano A, et al. Inhibition of prostaglandin synthesis reduces the induction of MyoD expression in rat soleus muscle. J Muscle Res Cell Motil. 2009;30(3–4):139–144. doi: 10.1007/s10974-009-9182-0. [DOI] [PubMed] [Google Scholar]
- 18.Obremsky WT, Seaber AV, Ribbeck BM, Garrett WE. Biomechanical and histologic assessment of a controlled muscle strain injury treated with piroxicam. Am J Sports Med. 1994;22(4):558–561. doi: 10.1177/036354659402200420. [DOI] [PubMed] [Google Scholar]
- 19.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27(3):416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen SG, Miller BF, Hansen M, Kjaer M, Holm L. Exercise and NSAIDs: Effect on muscle protein synthesis in patients with knee osteoarthritis. Med Sci Sports Exerc. 2011;43(3):425–431. doi: 10.1249/MSS.0b013e3181f27375. [DOI] [PubMed] [Google Scholar]
- 21.Pingel J, Fredberg U, Mikkelsen LR, et al. No inflammatory gene-expression response to acute exercise in human achilles tendinopathy. Eur J Appl Physiol. 2013;113(8):2101–2109. doi: 10.1007/s00421-013-2638-3. [DOI] [PubMed] [Google Scholar]
- 22.Rooney SI, Loro E, Sarver JJ, et al. Exercise protocol induces muscle, tendon, and bone adaptations in the rat shoulder. Muscles Ligaments Tendons J. 2015;4(4):413–419. [PMC free article] [PubMed] [Google Scholar]
- 23.Rooney SI, Tobias JW, Bhatt PR, Kuntz AF, Soslowsky LJ. Genetic response of rat supraspinatus tendon and muscle to exercise. PLoS One. 2015;10(10):e0139880. doi: 10.1371/journal.pone.0139880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17(6):608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- 25.Schiaffino S, Gorza L, Sartore S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 26.Schwarting T, Pretzsch S, Debus F, Ruchholtz S, Lechler P. The effect of cyclooxygenase inhibition on tendon-bone healing in an in vitro coculture model. Mediators Inflamm. 2015:926369. doi: 10.1155/2015/926369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith LR, Barton ER. SMASH - semi-automatic muscle analysis using segmentation of histology: A MATLAB application. Skelet Muscle. 2014;4 doi: 10.1186/2044-5040-4-21. 21-5040-4-21. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soltow QA, Betters JL, Sellman JE, Lira VA, Long JH, Criswell DS. Ibuprofen inhibits skeletal muscle hypertrophy in rats. Med Sci Sports Exerc. 2006;38(5):840–846. doi: 10.1249/01.mss.0000218142.98704.66. [DOI] [PubMed] [Google Scholar]
- 29.Trappe TA, Carroll CC, Dickinson JM, et al. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R655–62. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol. 2013;115(6):909–919. doi: 10.1152/japplphysiol.00061.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002;282(3):E551–6. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- 32.Tsai WC, Hsu CC, Chang HN, Lin YC, Lin MS, Pang JH. Ibuprofen upregulates expressions of matrix metalloproteinase-1, -8, -9, and -13 without affecting expressions of types I and III collagen in tendon cells. J Orthop Res. 2010;28(4):487–491. doi: 10.1002/jor.21009. [DOI] [PubMed] [Google Scholar]
- 33.Tsai WC, Hsu CC, Chen CP, Chen MJ, Lin MS, Pang JH. Ibuprofen inhibition of tendon cell migration and down-regulation of paxillin expression. J Orthop Res. 2006;24(3):551–558. doi: 10.1002/jor.20069. [DOI] [PubMed] [Google Scholar]
- 34.Tsai WC, Tang FT, Hsu CC, Hsu YH, Pang JH, Shiue CC. Ibuprofen inhibition of tendon cell proliferation and upregulation of the cyclin kinase inhibitor p21CIP1. J Orthop Res. 2004;22(3):586–591. doi: 10.1016/j.orthres.2003.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ibuprofen mildly increased tan(δ) at 2 Hz (C) 24 hours after a single bout of exercise. At 0.1 Hz (A), administration of ibuprofen for 2 and 8 weeks increased tan(δ) in the exercise and cage activity groups. Tan(δ) also increased with ibuprofen at 1 Hz (B) for the 2 week cage activity and 8 week exercise groups. Tan(δ) at 10 Hz (D) decreased in the 8 week cage activity group with administration of ibuprofen. Open bars- no drug, Solid bars- administered ibuprofen (n=11–16 acute, n=11–17 at 2 weeks, n=11–13 at
Ibuprofen had no effect on tendon collagen organization at 2 and 8 weeks. Open bars- no drug, Solid bars- administered ibuprofen (n=6–8)
Ibuprofen mildly increased cellularity acutely in the cage activity group and decreased cellularity with 2 weeks of exercise.
Ibuprofen decreased average muscle fiber cross-sectional area. Centrally nucleated fibers are rare. A) From EX8, mean fiber size 3902μm2 B) From EX8IBU, mean fiber size 3325 μm2 Acknowledgements: This study was funded by the Penn Center for Musculoskeletal Disorders (NIH/NIAMS P30 AR050950) and the U.S. Department of Veterans Affairs (VISN 4 CPPF).
Centrally Nucleated Fibers, %


