Abstract
Addiction involves drug-induced neuroplasticity in the circuitry of motivated behavior, which includes the medial forebrain bundle and the lateral hypothalamic area. Emerging at the forefront of neuroplasticity regulation are specialized extracellular matrix (ECM) structures that form perineuronal nets (PNNs) around certain neurons, mainly parvalbumin positive (PV+), fast-spiking interneurons (FSINs), making them a promising target for the regulation of drug-induced neuroplasticity. Despite the emerging significance of PNNs in drug-induced neuroplasticity and the well-established role of the lateral hypothalamic area (LHA) in reward, reinforcement, and motivation, very little is known about how PNN-expressing neurons control drug-seeking behavior. We found that a discrete region of the anterior dorsal LHA (LHAad) exhibited robust PNN and dense ECM expression. Approximately 87% of parvalbumin positive (PV+) neurons co-expressed the PNN marker Wisteria floribunda agglutinin (WFA), while 62% of WFA positive (WFA+) neurons co-expressed PV in the LHAad of drug naïve rats. Removal of PNNs within this brain region via chrondroitinase ABC (Ch-ABC) administration abolished acquisition of cocaine-induced CPP and significantly attenuated the acquisition of cocaine self-administration (SA). Removal of LHAad PNNs did not affect locomotor activity, sucrose intake, sucrose-induced CPP, or acquisition of sucrose SA in separate groups of cocaine naïve animals. These data suggest that PNN-dependent neuroplasticity within the LHAad is critical for the acquisition of both cocaine-induced CPP and SA but is not general to all rewards, and that PNN degradation may have utility for the management of drug-associated behavioral plasticity and memory in cocaine addicts.
Keywords: perineuronal nets, extracellular matrix, dorsal anterior lateral hypothalamic area, conditioned place preference, self-administration, cocaine, addiction, drug-associated memory
Introduction
Central to a better understanding of and treatment for addiction is the further characterization of drug-induced neuroplasticity within the neurocircuitry of motivated behavior following drug exposure and dependence. Drug-induced neuroplasticity produces neurocircuitry that is hyporesponsive to natural reward-associated stimuli and hyperresponsive to drug-associated stimuli [1]. Brain regions regulating motivated behavior are modified by supraphysiological levels of neurotransmitters induced by drugs of abuse, supplanting motivation for natural reward with much more powerful motivation for drug reward.
The medial forebrain bundle (MFB) is one of the most highly differentiated fiber systems in the circuitry of motivated behavior [2], and its activation is essential for reinforcement by and motivation to obtain both natural and drug rewards [3, 4]. Activation of MFB fibers within the lateral hypothalamic area (LHA) are the most reinforcing, sensitive, and motivating [2, 5]. In this light, LHA neurocircuitry acts as a key integration site for motivation, reinforcement, and reward [6]; however, the function of the LHA is still poorly understood. This circuitry is highly susceptible to supraphysiological activation by drugs of abuse, which in turn, produces drug-induced neuroplasticity. Indeed, the LHA is one of the most transcriptionally-responsive brain regions following cocaine administration, with altered expression of genes associated with synaptogenesis, synaptic plasticity, and synaptic neurotransmission [7].
Advances in understanding how the extracellular matrix (ECM) contributes to a wide range of synaptic signaling has led to the evolution from the tripartite synapse theory of synaptic signaling (1: presynapse, 2: postsynapse, 3: astrocyte) [8] to the tetrapartite synapse theory (1–3 as above + 4: ECM) [9]. Perineuronal nets (PNNs) are a specialized form of the ECM consisting of chondroitin sulfate proteoglycans (CSPGs), hyaluronic acid, tenascin-R, and link proteins. In most brain areas (but not all), PNNs ensheath the soma, proximal dendrites, and initial axon segment of mainly parvalbumin-positive (PV+), fast-spiking interneurons (FSINs) [10]. PNNs play key roles in neural development, synaptogenesis, neuroprotection, and experience-dependent synaptic plasticity. Through several mechanisms, PNNs can inhibit, promote, and maintain synaptic plasticity. PNN removal is thought to promote structural plasticity by creating a more immature-like, unstable, and plastic state [11, 12] and in turn regulate long-term plasticity and facilitate the development of new synaptic inputs [13, 14]. Importantly, the LHA expresses high levels of PNNs [15], providing one possible mechanism whereby drug-induced neuroplasticity engages the circuitry of motivated behavior.
Targeting PNNs may be promising for the treatment and long-term management of addiction. Toward this end, PNN removal appears to create a ‘tabula rasa’ or a blank slate for neuroplasticity that has the potential to combat maladaptive cocaine-induced plasticity while also facilitating new adaptive plasticity. Cocaine-induced neuroplasticity of the ECM has been reported in both cocaine-dependent humans [16] and rodent models of cocaine addiction [17,18]. We recently found that PNN removal in the medial prefrontal cortex (mPFC) attenuated the acquisition and reconsolidation of cocaine-associated memory using a CPP task [19].
In the present study, we characterize the discrete anterior region, dorsal zone, of the lateral hypothalamic area (LHAad), which we found exhibited robust and dense expression of both PNNs and loose ECM, and investigate whether PNN removal in the LHAad prior to cocaine exposure can prevent cocaine-induced behavioral plasticity. Notably, the present study assesses LHAad PNNs and their role in drug-associated memory, testing the rewarding and reinforcing properties of cocaine by using the CPP task and self-administration (SA) acquisition tasks, respectively. Specifically, we hypothesized that destabilizing PNNs in the LHAad would decrease the rewarding and reinforcing properties of both noncontingent and contingent cocaine but not sucrose exposure. To test this hypothesis, LHAad PNNs were destabilized within the LHAad, using chondroitinase-ABC (Ch-ABC) [20] prior to the first day of both CPP and SA training. Determining the role of PNNs within the circuitry of motivated behavior and drug-associated behavioral plasticity may offer new insights into the etiology of drug addiction.
Materials and Methods
Subjects
Male Sprague-Dawley rats were obtained from Simonsen Laboratories (Gilroy, CA), weighing 300–330 g at the start of the experiment, singly housed in a temperature- and humidity-controlled room with a 12-h light/dark cycle, and given food and water ad libitum (unless stated otherwise) throughout experimentation with exception of the time they were placed in the CPP or self-administration apparatus. Animals for CPP experiments were run during their light cycle (lights on at 07:00 hr) and animals for SA experiments were run during their dark cycle (lights off at 07:00 hr). All experiments were approved by the Institutional Animal Care and Use Committee and according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to reduce animal suffering and to reduce the number of animals used in the experiments.
Surgery and microinjections
Rats were anesthetized with brief exposure to isoflurane followed by an intramuscular injection of zyket (ketamine 87 mg/kg + xylazine 13 mg/kg). Rats were anesthetized and implanted with a chronic indwelling iv catheter (cocaine SA cohort only) into the right jugular vein as previously reported [21]. Immediately following catheter surgery, both daily and immediately before and after self-administration, the catheter was injected with 0.1 ml of flushing solution (cefazolin 10 mg/ml, 0.1 ml heparin 1000 U/ml, in sterile saline) to prevent infection and catheter occlusion. During stereotaxic surgery (CPP and SA cohorts), rats were placed into a stereotaxic apparatus and bilateral stainless steel cannulae (26 gauge) were implanted 1 mm above the lateral hypothalamic area, anterior region, dorsal zone (LHAad) using the following coordinates determined from Paxinos and Watson (1998): 0° angle away from midline; A/P −1.8 mm from bregma; M/L ± 1.3 mm from midline; D/V −6.4 mm from skull surface) [22, 23] and fixed with dental acrylic cement. Obturators (33 gauge) measuring the same length as the cannulae were inserted into the cannulae following surgery and remained in place until the time of microinjection. Following surgery, all animals received an intramuscular injection of ketoprofen (10mg/kg). Rats recovered for 5–7 days before the start of the experiment. Stainless steel needles (1 mm longer than cannulae, 33 gauge) were connected to tubing attached to a 1.0 μL Hamilton syringe and inserted into the cannulae. Using an infusion pump, a volume of 0.6 μL of the enzyme Ch-ABC or vehicle (sterile water) was injected bilaterally over a period of 108 sec. Following injection, the needles remained in place for an additional 60 sec to allow for fuller drug diffusion.
Drugs
Cocaine hydrochloride was a gift from the National Institute on Drug Abuse. The cocaine salt was dissolved in saline by the weight of the salt. For CPP experiments, the training dose of cocaine was 10 mg/kg, and for self-administration (SA) experiments, the training dose was 0.125 mg/kg/infusion. Cocaine for CPP was non-contingently given intraperitoneally (IP) and cocaine for SA was contingently given intravenously (IV). Protease-free Ch-ABC was obtained from Sigma-Aldrich and was dissolved in sterile water (vehicle) to a final concentration of 0.09 U/μL, as previously reported [19] and has been found not to be toxic to neurons [19]. Ch-ABC degrades the glycosaminoglycan side chains of chondroitin sulfate proteoglycans [20] and is widely used to disrupt PNNs.
Conditioned place preference (CPP)
All CPP experiments were conducted during the light phase. Figure 2A shows the time course for cocaine-induced CPP training and testing. The CPP apparatus comprises three Plexiglas compartments (Med Associates, St. Albans, VT), including two main outer compartments (28 × 21 × 21 cm), one of which has black walls with a wire mesh floor and the other of which has white walls with a metal rod floor. The central compartment (12 × 21 × 21 cm) has gray walls with solid gray flooring. Locomotor activity and side preference were automatically recorded with infrared photocell beams within the apparatus. A manual guillotine door separates each compartment, allowing for the confinement of the rat to one side of the apparatus. Rats were handled for at least 2 days prior to the start of each experiment. Animals then received two initial preference (IP) days, the first serving as a habituation day (IP1) and the second serving as a test for initial preference (IP2). At the beginning of the initial preference days, animals were placed in the central compartment of the apparatus and allowed free access to all compartments for 15 min. At the end of each session, the compartments were cleaned and dried, and time spent within each compartment was recorded. All cocaine- and sucrose-induced CPP experiments were performed in separate groups of subjects. Sucrose CPP subjects were food restricted to 85% of ad libitum weight [24] and allowed access to 4.5 g total of 45 mg sucrose pellets (BioServ, Frenchtown, New Jersey, USA).
Figure 2. Injection of Ch-ABC within the LHAad blocks the acquisition of cocaine- but not sucrose-induced CPP.
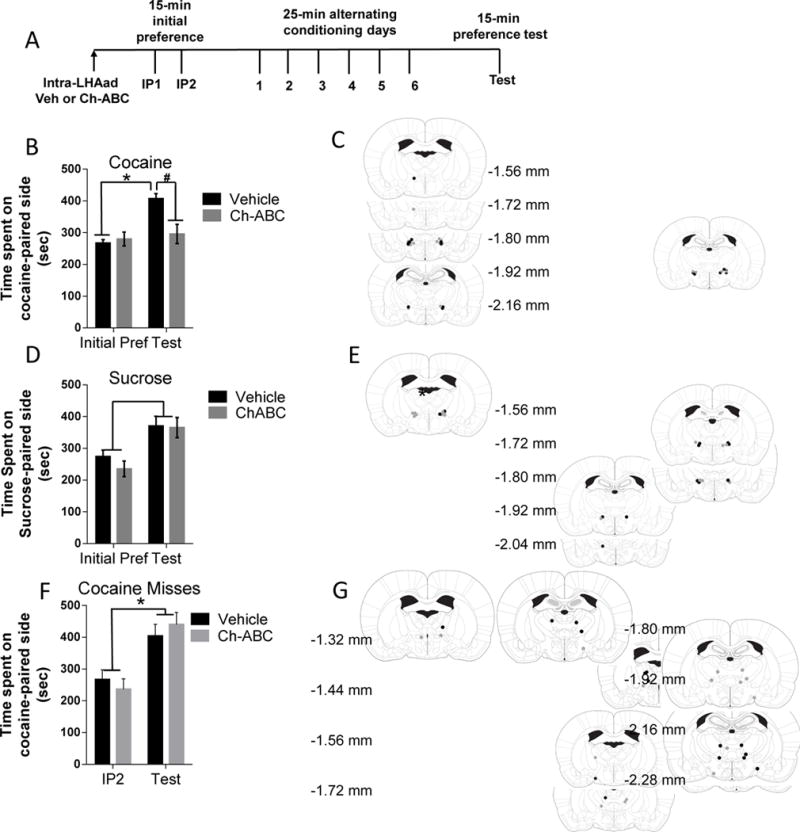
Data represent time spent on cocaine- or sucrose-paired side (mean ± SEM) recorded during initial preference (IP) and test day following injections of vehicle and Ch-ABC (0.054 U/side). (A) Timeline of the CPP experiment. (B) Acquisition of cocaine-induced CPP was observed following vehicle pretreatment but not following injections of Ch-ABC into the LHAad (P < 0.05). (C) Injections sites in cocaine CPP trained animals that received vehicle (black circles) and Ch-ABC (gray circles) microinjections within the LHAad. (D) Sucrose-induced CPP was observed following injection of both vehicle and Ch-ABC into the LHAad. (E) Injection sites in sucrose CPP trained animals that received vehicle (black circles) and Ch-ABC (gray circles) microinjections within the LHAad. (F) Ch-ABC injections into brain regions adjacent, outside, but not within the LHAad failed to block the acquisition of cocaine-induced CPP. (G) Injection sites in rats tested for cocaine CPP that received vehicle (black circles) and Ch-ABC (gray circles) microinjections.
The reward-paired compartment was determined by counter-balancing the preferred and non-preferred sides as well as the two distinct compartments (black vs. white sides). Rewards (cocaine, 10 mg/kg IP; sucrose pellets, 4.5 g) were administered on alternate days for six consecutive training days. Non-rewards (saline, 1 ml/kg IP; absence of sucrose) were delivered on the opposite days during the six training days. For training, rats were confined for 25 min. One day following training, a single place preference test for 15-min was conducted following training in a reward-free state in which time spent accessing all three compartments was determined. Total locomotor activity and time spent within the reward- and the non-reward-paired compartment were recorded for both initial preference and test days to determine CPP acquisition. Animals that spent significantly more time in the reward-paired compartment on test day compared to the second initial preference day were considered to have acquired conditioned place preference.
Self-administration Training: acquisition, extinction, cue-induced reinstatement
Figures 3A, 4A show the timeline of the course of the cocaine and sucrose SA experiments, respectively. Acquisition of cocaine and sucrose SA was conducted in separate groups of animals to produce drug-experienced and drug-naïve cohorts for direct comparison. The evening before the first day of SA, a single intra-LHAad microinjection of Ch-ABC (0.09 U/μL) or vehicle was administered. During training, each animal was placed in sound-attenuating operant-chambers (Med Associates, St. Albans, VT), which were equipped with both active and inactive levers on the same side of the SA chamber, a conditioned stimulus (CS) cue light over each lever, and a house light on the opposite wall. A press on the active lever resulted in an IV infusion of cocaine or delivery of a single sucrose pellet with concurrent activation of both the cue light, a conditioned stimulus (CS), above the active lever, and the house light, which was immediately followed by a 20-s time-out period at which time pressing the active lever resulted in no consequences. Following this time-out period, the house light was extinguished, and the next active lever press resulted in reward delivery. Responding on the inactive lever resulted in no consequences but was recorded. Self-administration began 5–7 days following surgery and animals were not food restricted for any portion of the SA experiments. All training and testing occurred during the dark phase of the light cycle (between 08:00 and 16:00 h) and rats were trained in daily 2-hr sessions, 5 days a week, using a fixed-ratio of 1 (FR1; one lever press = 1 reward) for a total of 20 SA sessions to self-administer IV cocaine (0.125 mg/kg in 0.10 ml over 6 s) or IO sucrose pellets (BioServ, Frenchtown, New Jersey, USA; 45 mg pellets). On the 21st day of self-administration, a single 5-hr extinction session (21A) was conducted in which lever pressing had no consequences. Immediately following the extinction session, a single 1-hr cue-induced reinstatement session occurred, in which an active lever press resulted in the activation of the cue-light but no cocaine was delivered (Figures 3A, 4A). Total active/inactive lever responses and rewards were recorded. The cocaine dose (0.125 mg/kg/infusion) to observe Ch-ABC mediated effects upon drug-seeking behavior was optimized using a protocol previously reported by others [25] and a standard dose used to assess the acquisition of cocaine self-administration [25–27].
Figure 3. Injection of Ch-ABC within the LHAad attenuates the acquisition of cocaine-induced self-administration.
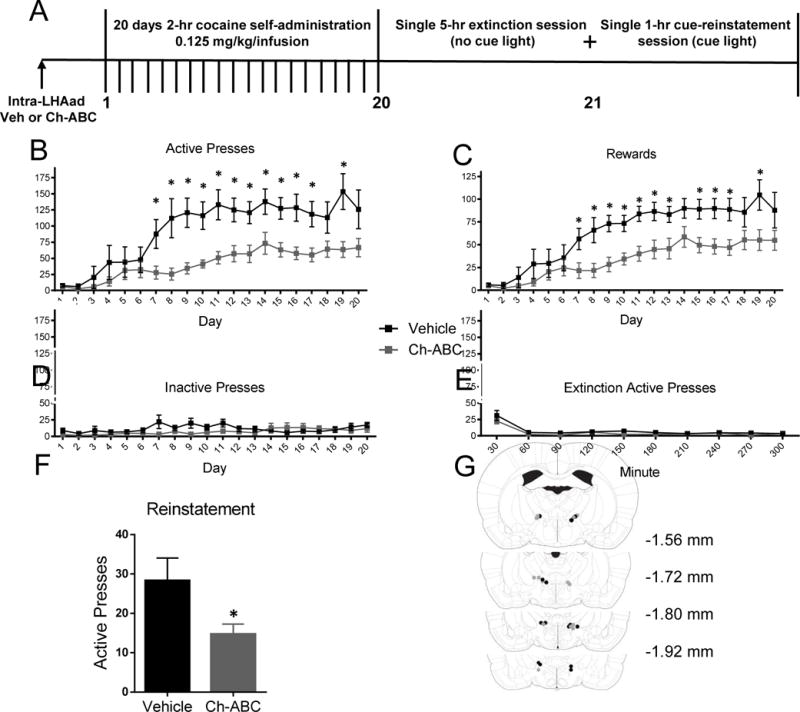
(A) Timeline of the experiment. Data are mean ± SEM of (B) active lever presses, (C) rewards, (D) inactive lever presses, (E) active lever presses during extinction, and (F) active lever presses during the first 30-min of cue-induced reinstatement. (G) Injection sites in the LHAad for vehicle (black circles) and Ch-ABC (gray circles) cohorts.
Figure 4. Injection of Ch-ABC within the LHAad does not attenuate the acquisition of sucrose-induced self-administration.
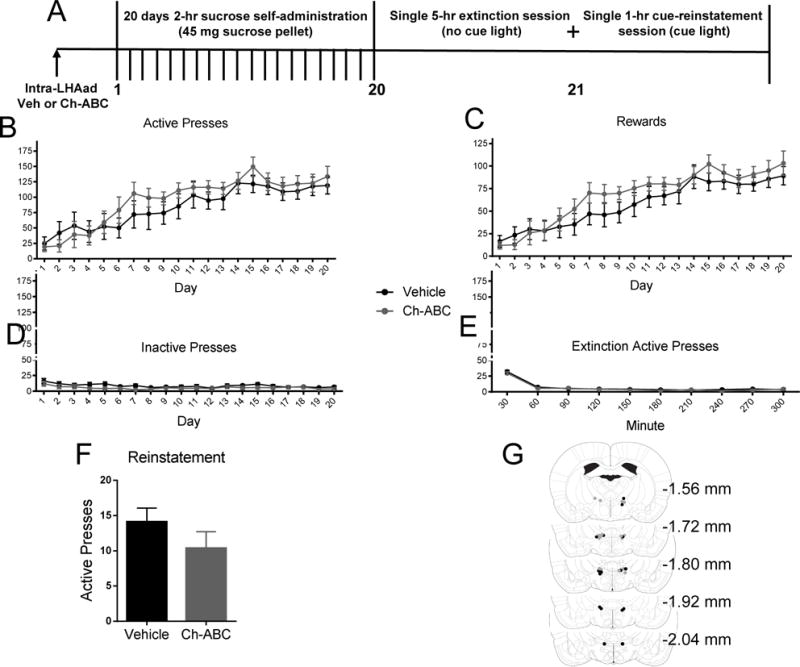
(A) The timeline of the experiment. Data are mean ± SEM of (B) active lever presses, (C) rewards, (D) inactive lever presses, (E) active lever presses during extinction, and (F) active lever presses during the first 30-min of cue-induced reinstatement. (G) Injection sites in the LHAad for vehicle (black circles) and Ch-ABC (gray circles) cohorts.
Histochemistry
Coronal brain sections (30 μm) through the LHAad were made on a freezing microtome. Cannula placement was verified following all experiments; placements not including the LHAad were analyzed as a separate missed cohort. Immunohistochemistry was performed as previously described [19] by washing free-floating sections three times for 5 min in PBS. Tissue was then placed in 50% ethanol for 30 min. After a set of three 5 min washes in PBS, the sections were placed in 3% goat or donkey blocking serum (Vector Labs) for 1 h. Free-floating slices were then incubated overnight at 4°C on a shaker table with primary antibodies. The primary antibody used was mouse anti-parvalbumin (PV; 1:1000; Thermo Scientific). The following day and after three 10 min washes in PBS, sections were incubated for 2 h with goat anti-mouse AlexaFluor594 (1:200; Life Technologies, Carlsbad, CA, USA) secondary antibody. After another PBS wash, the tissue was incubated overnight at 4°C on a shaker table but with fluorescein-labeled Wisteria floribunda agglutinin (WFA; 1:500; Vector Laboratories, Burlingame, CA, USA) in PBS containing 2% goat serum. The tissue was washed three additional times for 10 min each in PBS and mounted onto Frost plus slides as previously described [19]. After drying, the tissue was coverslipped with ProLong (Vector Labs). Images of the LHAad were photographed using a Leica SP8 confocal microscope. For the PV labeled cells that were double-labeled with WFA, the images were photographed in the red and green channels and the microscope switched between the two fields to evaluate double labeling using Leica SP8 laser scanning confocal microscope with Leica Application Suite. An HCX PL apo CS, dry, 20X objective with 0.70 numerical aperture was used for all images. WFA-bound fluorescein for PNN expression and Alexa Fluora 594 for PV expression were excited using a 488 laser and 552 laser, respectively, using a photomultiplier tube, which detected emission photons within the ranges of the utilized fluorophores. The number of single-labeled WFA-positive cells and those co-localized with PV within the frame at 20X were assessed by counting all cells surrounded by WFA and/or containing PV immunolabeling.
Histological confirmation of injection sites
The accuracy of cannula implantation was confirmed in each rat after cardiac perfusion with 150-ml NaCl followed by 150-ml 4% paraformaldehyde under isoflurane anesthesia. Brains were removed and stored in 4% paraformaldehyde for at least one day, transferred to 20% sucrose for at least one day, then frozen at −80 °C. 30-μm sections were cut using a microtome, collected and kept in storage buffer until fluorescein-labeled WFA staining (1:500) to confirm both cannula placement and PNN expression within the LHAad. Cannula placement and Ch-ABC spread targeting the LHAad were verified at the end of all experiments. Rats with cannula placement Ch-ABC spread outside of the LHAad were excluded from the primary analysis and placed into a separate “missed placement” group for the assessment of anatomical specificity of drug effects on both cocaine-induced conditioned place preference and self-administration. Injection sites for rats tested for the effects of drug injections inside and outside the LHAad of both cocaine- and sucrose-conditioned rats are depicted in Figures 2C, E, and G. Injection sites for rats tested for the effects of drug injections inside the LHAad of both cocaine- and sucrose SA acquisition are shown in Figures 3G and 4G, respectively.
Statistics
All statistical tests were conducted using Prism 6 (GraphPad Software, Inc.). All CPP and SA experiments were analyzed using a two-way ANOVA (vehicle vs. Ch-ABC treatment as the between-subjects measure and CPP and SA day as the within-subjects measure). Further analyses of main effects were conducted using an unpaired Student’s two-tailed t-test, Bonferroni’s post-hoc test, simple main effect analysis, and simple Neuman-Keuls post-hoc test, when appropriate, in the case of a significant interaction. Differences were considered significant when p < 0.05. Group sizes are reported in the results section.
Results
A discrete subregion of the LHA is characterized by robust PNN/ECM expression
The lateral hypothalamic area (LHA) has been previously reported to express significant levels of perineuronal nets (PNNs) [15]. Considering the established role of the lateral hypothalamic area (LHA) and the emerging role of PNNs in drug-induced neuroplasticity, we first sought to determine areas of high expression using Wisteria floribunda agglutinin (WFA), a standard PNN visualization method [28]. A discrete region of the anterior LHA (LHAa) expressed high-density PNN-containing neurons, which corresponds well with specific zones of the LHAa, including the dorsal (LHAad) and intermediate zones (LHAai) (Figures 1A, B, C) [29]. The LHAad/i are the two most dorsal zones of the LHAa; therefore, the abbreviation LHAad is used to refer to the PNN-positive LHAa, which includes both the LHAai and the LHAad. Comparatively sparse PNN expression is found in the ventral zone of the anterior LHA (LHAav; G) and just caudally in the dorsal zone of the LHA (LHAd; H-I). These results suggest that this newly discovered patch of high-density PNN-containing neurons that are specifically expressed in the lateral hypothalamic area, anterior region, dorsal/intermediate zones, is a promising target for PNN-dependent cocaine-induced behavioral plasticity.
Figure 1. Characterization of the lateral hypothalamic area, anterior region, dorsal/intermediate zones expressing robust PNNs [107].
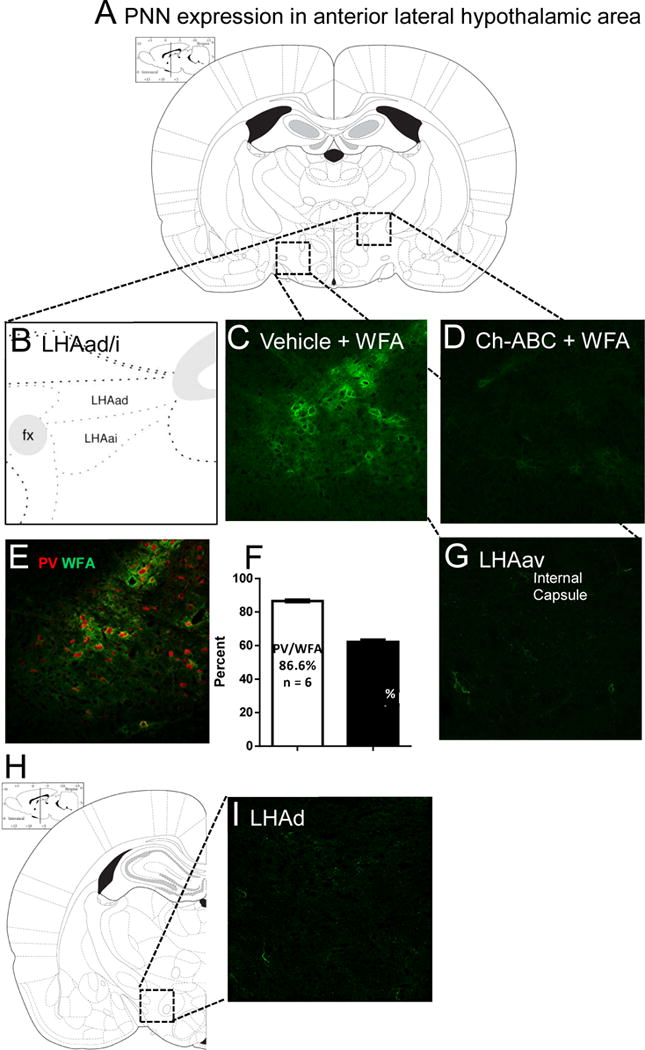
(A) Depiction of the targeted region characterized by robust PNN expression using the PNN marker WFA. (B) This area correlates with the lateral hypothalamic area (LHA), anterior region (LHAa), including the dorsal (LHAad) and intermediate zones (LHAai) found dorsolateral to the fornix [29]. (C) Areas of strong PNN expression in the LHAad [29] (D) is abolished by Ch-ABC administration. (E) Patterns of distribution of WFA and PV in the rat lateral hypothalamic area, anterior region, dorsal zone (LHAad). (F) Approximately 87% of PV+ neurons colocalize with WFA and 62% of WFA+ neurons colocalize with PV (n = 6). (G) PNN expression in the LHAa ventral zone (LHAav) is comparatively sparse. (H-I) Similar patterns of sparse expression are also found just caudally in the lateral hypothalamic area (LHA) dorsal region (LHAd) of the middle LHA [29, 94].
LHAad PNN surrounded neurons co-localize with parvalbumin
To further characterize PNN-surrounded neurons of the LHAad, we used WFA as a marker for PNNs and measured double labeling for PV. Although PNNs surround primarily PV+ fast-spiking interneurons (PV+-FSNs) in the cortex [28], a similar region recently discovered in the mouse reported that the majority of PNN+ neurons were not PV+ [30]. This suggests the possibility that PNN-surrounded neurons in the LHAad may lack PV. We report that within the PNN-rich LHAad, 87% of parvalbumin-positive (PV+) neurons coexpressed WFA, while 62% of WFA positive (WFA+) neurons coexpressed PV in the LHAad of drug- naïve rats (PV+/WFA+ 86.6 ± 0.7, n = 6; WFA+/PV+ 62.2 ± 1.4, n = 6; Figures 1E, F). These findings suggest that, within the LHAad, the majority of PV+ neurons are surrounded by PNNs, and while the majority of PNN-surrounded neurons co-express PV, a significant PNN+ neuronal population lacks PV.
Removal of PNNs within the LHAad blocks acquisition of cocaine-induced CPP
To test whether PNN-surrounded neurons in the LHAad were involved in the acquisition of cocaine-induced CPP, we removed PNNs from the LHAad using Ch-ABC prior to initial preference and CPP training. The timeline of the experiment is shown in Figure 2A. The effects of injection of Ch-ABC (0.054 U/side) and vehicle into the LHAad on the acquisition of cocaine-induced CPP seeking are shown in Figure 2B. Infusion of Ch-ABC completely blocked the acquisition of cocaine-induced CPP. A two-way repeated measures ANOVA (CPP day × drug treatment) showed a main effect of CPP day (F1,14 = 33.29, p < 0.0001; n = 8 vehicle, 8 Ch-ABC) and a CPP × Ch-ABC interaction (F1,14 = 21.04, p = 0.0004). Post-hoc testing (Bonferroni’s multiple comparisons) showed that significant cocaine-induced CPP was observed in rats treated with vehicle but not Ch-ABC (P < 0.05 vs. initial preference),with Ch-ABC treated rats spending less time on the cocaine-paired side compared to vehicle treated rats (vehicle 407.3 ± 29.76, n = 8; Ch-ABC 267.1 ± 29.76, n = 8; p < 0.05; Figure 2B). No differences in locomotor activity (infrared photocell beam breaks) between the treatment groups was observed during either the initial preference (vehicle 1329 ± 238.5, n = 8; Ch-ABC 1136 ± 114.8, n = 8; p = 0.4765) or the CPP test (vehicle 1480 ± 168.4, n = 7, Ch-ABC 1330 ± 119.8 n = 6; p = 0.4983; data not shown). Locomotor activity was not different between initial preference and test days for either vehicle treated rats (Initial Preference 1329 ± 238.5, n = 8; Test 1480 ± 168.4, n = 7; p = 0.6244; data not shown) or Ch-ABC treated rats (Initial Preference 1136 ± 114.8, n = 8; Test 1330 ± 119.8, n = 6; p = 0.2705; data not shown) treated animals. Figure 2C shows the microinjection injection sites within the LHAad for the vehicle and the Ch-ABC treated animals included in the analysis. These findings suggest that destabilizing PNNs within the LHAad prior to conditioning abolishes the acquisition of cocaine-induced CPP.
Removal of PNNs within the LHAad does not attenuate acquisition of sucrose-induced CPP
To test whether the observed Ch-ABC effects upon cocaine-induced CPP acquisition were specific to cocaine or could more broadly be attributed to the regulation of either general reward such as sucrose or to nonspecific behavioral suppression, a separate cohort of rats was tested for the effect of Ch-ABC (0.054 U/side) or vehicle administration into the LHAad on the acquisition of sucrose-induced CPP. PNNs were removed within the LHAad using Ch-ABC prior to initial preference and CPP training similarly to the cocaine-induced CPP cohort (Figure 2A). Time spent on the sucrose-paired side prior to conditioning on initial preference day was not different between vehicle and Ch-ABC treated animals (vehicle 274.5 ± 20.12, n = 6; Ch-ABC 235.3 ± 24.54, n = 8; p = 0.2625). Two-way repeated measures ANOVA (CPP day × drug treatment) showed a main effect of CPP day (F1,12 = 15.49, *p = 0.0020; n = 6 vehicle, 8 Ch-ABC) but not a CPP × Ch-ABC interaction (F1,12 = 0.3630, p = 0.5581) or LHAad treatment effect (F1,12 = 0.6226, p = 0.4454). No differences in locomotor activity between the treatment groups were observed during either the initial preference (vehicle 1134 ± 61.67, n = 6; Ch-ABC 1098 ± 59.42, n = 8; p = 0.6886) or the CPP test (vehicle 1345 ± 99.84, n = 6, Ch-ABC 1234 ± 71.41 n = 8; p = 0.3712; data not shown). Moreover, locomotor activity was not different between initial preference and test days for either vehicle treated rats (Initial Preference 1134 ± 61.67, n = 6; Test 1345 ± 99.84, n = 6; p = 0.6244; data not shown) or Ch-ABC treated rats (Initial Preference 1098 ± 59.42, n = 8; Test 1234 ± 71.41, n = 8; p = 0.1639; data not shown) treated animals. Importantly, sucrose consumption (grams ± SEM) during the conditioning phase of the study was not different between vehicle and Ch-ABC treated animals (vehicle 7.58 ± 1.55 g, n = 6; Ch-ABC 9.25 ± 1.04 g, n = 8; p = 0.3708). Figure 2E shows the injection sites for the vehicle and the Ch-ABC treated animals included in the analysis. Two sucrose rats, both receiving vehicle injections, were excluded from sucrose analysis because of injection sites outside the PNN-expressing LHAad. These data suggest that PNN expression in the LHAad is not necessary for acquisition of sucrose-induced CPP or for consumption of sucrose.
Removal of PNNs outside the LHAad does not attenuate acquisition of cocaine-induced CPP
To determine whether the observed Ch-ABC effects were the result of LHAad PNN degradation, we independently examined the acquisition of cocaine-induced CPP in rats that received vehicle and Ch-ABC injections in adjacent, yet outside, of the PNN-expressing LHAad (Figures 2F, G). Two-way repeated measures ANOVA (CPP day × drug treatment) showed a main effect of CPP day (F1,15 = 21.47, p = 0.0003; n = 9 vehicle, 8 Ch-ABC) but not treatment (F1,15 = 0.0084, p = 0.9281; n = 9 vehicle, 8 Ch-ABC), and no CPP × treatment interaction (F1,15 = 0.8230, p = 0.3787; n=9 vehicle, 8 Ch-ABC). Cocaine-induced CPP was observed in both Ch-ABC (Initial Preference 237.0 ± 32.68, n = 8; Test 440.4 ± 37.62, n = 8; p = 0.0011) and vehicle treated rats (Initial Preference 267.5 ± 30.55, n = 9; Test 404.1 ± 36.79, n = 9; p = 0.0113; Figure 2F). No differences in locomotor activity between treatment groups were observed during either the initial preference (vehicle 1165 ± 161.7, n = 9; Ch-ABC 1199 ± 121.3, n = 8; p = 0.8696) or the CPP test (vehicle 1373 ± 81.11, n = 9, Ch-ABC 1368 ± 94.33 n = 8; p = 0.9684). Moreover, locomotor activity was not different between initial preference and test days for both vehicle (Initial Preference 1165 ± 161.7, n = 9; Test 1373 ± 81.11, n = 9; p = 0.2661; data not shown) and Ch-ABC (Initial Preference 1199 ± 121.3, n = 8; Test 1368 ± 94.33, n = 8; p = 0.290; data not shown) treated animals. Figure 2G shows the injection sites for the vehicle and Ch-ABC treated animals that were included in the ‘missed’ LHAad group in the analysis. These findings suggest that destabilizing PNNs outside the LHAad prior to conditioning fails to block the acquisition of cocaine-induced CPP and does not cause secondary locomotor effects. Together, the data within Figures 2B and F highlight the importance of PNN removal within this relatively small subregion of the LHA in the acquisition of cocaine-induced CPP. Injection sites for rats tested for the effects of drug injections inside the LHAad of both cocaine- and sucrose-conditioned rats and outside the LHAad of cocaine-conditioned rats are depicted in Figures 2C, E, and G, respectively.
Removal of PNNs within the LHAad reduces the acquisition of cocaine self-administration
Acquisition of drug self-administration has been used as an animal model of vulnerability to drug addiction [31]. To test whether PNN surrounded neurons in the LHAad were involved in the acquisition of cocaine self-administration, we removed PNNs within the LHAad using Ch-ABC one day prior to the first self-administration session. The effects of Ch-ABC (0.054 U/side) and vehicle injection into the LHAad on the acquisition of cocaine-induced active lever presses, rewards, inactive lever presses, extinction, and active lever presses during the first 30-min of a single cue-induced reinstatement session are shown in Figures 3B, C, D, E, and F, respectively. Self-administration lever pressing and reward delivery data were analyzed by repeated measures analysis of variance (ANOVA), with treatment as the between-groups factor, and time as a within-subject factor (repeated measure). Significant two-way interactions were examined using simple main effect analysis, and the Neuman–Keuls test for post hoc mean comparisons was applied, when appropriate. Two-way repeated measures ANOVA of active lever presses (self-administration day × drug treatment) showed a main effect of self-administration day (F19,228 = 16.21, p < 0.0001; n = 7 vehicle, 7 Ch-ABC), drug treatment (F1,12 = 9.183, p = 0.0105; n = 7 vehicle, 7 Ch-ABC), and a self-administration × drug treatment interaction (F19,228 = 3.006, p < 0.0001; n = 7 vehicle, 7 Ch-ABC; Figure 3B). Simple main effect analysis of active lever presses showed that on days 7–17 and 19 active lever presses were significantly different between vehicle and Ch-ABC treated animals (Figure 3B). Newman-Keul’s post hoc analysis demonstrated that starting on day 7 for vehicle and day 10 for Ch-ABC treated animals, active lever presses were significantly greater than day 1 of SA Acq. Similarly to active lever presses, two-way repeated measures ANOVA of rewards (self-administration day × drug treatment) showed a main effect of self-administration day (F19,228 = 21.00, p < 0.0001; n = 7 vehicle, 7 Ch-ABC), drug treatment (F1,12 = 8.428, p = 0.0132; n = 7 vehicle, 7 Ch-ABC), and a self-administration × drug treatment interaction (F19,228 = 2.012, p = 0.0088; n = 7 vehicle, 7 Ch-ABC; Figure 3C). Simple main effect analysis of rewards showed that on days 7–13, 15–17, and 19, rewards were significantly greater compared to day 1 of self-administration (Figure 3C). Newman-Keul’s post hoc analysis demonstrated that starting on day 7 for vehicle and day 9 for Ch-ABC treated animals, rewards were significantly greater than day 1 of SA Acq. By contrast, two-way repeated measures ANOVA of inactive lever presses (self-administration day × drug treatment) did not show a main effect of self-administration day (F19,228 = 1.383, p = 0.1364 0.0001; n = 7 vehicle, 7 Ch-ABC), or drug treatment (F1,12 = 1.947, p = 0.1882; n = 7 vehicle, 7 Ch-ABC), but a trend towards a self-administration × drug treatment interaction (F19,228 = 1.611, p < 0.0549; n = 7 vehicle, 7 Ch-ABC; Figure 3D) was observed, indicating that Ch-ABC treated animals trended toward decreased inactive lever pressing. No differences in active lever presses during extinction were found (Figure 3E). In summary, these data are indicative that Ch-ABC treated animals self-administer less cocaine and acquire self-administration more slowly than do Vehicle treated animals.
During cue-induced reinstatement, a student’s two-tailed t-test showed that the Ch-ABC treated group pressed the active lever less than the vehicle treated group within the first 30-min of a single 1-hr cue-induced reinstatement session immediately following the 5-hr extinction session (vehicle 28.29 ± 5.760, n = 7; Ch-ABC 14.71 ± 2.561 n = 7; p = 0.05; Figure 3F). Injection sites for cocaine hits in rats tested for self-administration acquisition are shown in Figure 3G. Data reporting active and inactive lever presses and rewards are supportive of the hypothesis that PNN destabilization within the LHAad significantly decreases acquisition of cocaine self -administration (Figure 3). In summary, these data suggest that Ch-ABC significantly attenuates acquisition of cocaine self-administration, which perhaps not surprisingly, is mirrored by a significant reduction in cue-induced reinstatement.
Removal of PNNs within the LHAad does not attenuate the acquisition of sucrose self-administration
To test whether the observed Ch-ABC effects upon acquisition of cocaine SA were specific to cocaine or could more broadly be attributed to the regulation of either general reward, such as sucrose, or to nonspecific behavioral suppression, a separate cohort of rats was tested for the effect of Ch-ABC (0.054 U/side) or vehicle administration into the LHAad on the acquisition of sucrose SA, similarly to sucrose CPP. PNNs were destabilized within the LHAad using Ch-ABC similarly to both cocaine- and sucrose-induced CPP and cocaine SA cohorts (Figures 2A, 3A, and 4A) one day prior to the first self-administration session. The effects of Ch-ABC (0.054 U/side) and vehicle injection into the LHAad on the acquisition of sucrose-induced active lever presses, rewards, inactive lever presses, extinction, and active lever presses within the first 30-min of a single cue-induced reinstatement session are shown in Figures 4B, C, D, E, and F, respectively. Self-administration lever pressing and reward delivery data were analyzed by repeated measures analysis of variance (ANOVA), with treatment as the between-groups factor, and time as a within-subject factor (repeated measure). Significant two-way interactions were examined using simple main effect analysis, and the Neuman–Keuls test for post hoc mean comparisons was applied, when appropriate. Two-way repeated measures ANOVA of active lever presses (self-administration day × drug treatment) showed a main effect of self-administration day (F19,133 = 8.523, p < 0.0001; n = 8 vehicle, 8 Ch-ABC), but not drug treatment (F1,7 = 1.148, p = 0.3196; n = 8 vehicle, 8 Ch-ABC), or a self-administration day × drug treatment interaction (F19,133 = 0.7868, p = 0.7192; n = 8 vehicle, 8 Ch-ABC; Figure 4B). Similarly to active lever presses, two-way repeated measures ANOVA of rewards (self-administration day × drug treatment) showed a main effect of self-administration day (F19,133 = 13.45, p < 0.0001; n = 8 vehicle, 8 Ch-ABC), but not drug treatment (F1,7 = 1.199, p = 0.3098; n = 8 vehicle, 8 Ch-ABC), or a self-administration × drug treatment interaction (F19,133 = 0.9087, p = 0.5731; n = 8 vehicle, 8 Ch-ABC; Figure 4C). Two-way repeated measures ANOVA reported no differences in inactive lever presses during acquisition (Figure 4D), active lever presses during extinction (Figure 4E). In summary, these data are indicative that Ch-ABC treated animals do not self-administer less sucrose and do not acquire self-administration more slowly than do Vehicle treated animals.
During cue-induced reinstatement, a student’s two-tailed t-test showed that the Ch-ABC treated group did not press the active lever less than the vehicle treated group within the first 30-min of a single 1-hr cue-induced reinstatement session immediately following the 5-hr extinction session (vehicle 14.13 ± 1.941, n = 8; Ch-ABC 10.38 ± 2.345 n = 8; p = 0.2382; Figure 4F). Figure 4G shows the injections sites for the vehicle and the Ch-ABC treated animals included in the analysis. 8 rats, 4 receiving vehicle and 4 receiving Ch-ABC injections, were excluded from analysis because of injection sites outside the PNN expressing LHAad. These data suggest that PNN expression in the LHAad is not necessary for acquisition of sucrose-induced SA in ad libitum fed animals.
Discussion
Drugs of Abuse and PNNs
Cocaine-induced neuroplasticity of the ECM has been reported in both cocaine-dependent humans [16] and rodent models of cocaine addiction [17, 18]. Cocaine-induced plasticity can restrict the formation of new plasticity [32, 33], and PNNs may play a role in this restriction [17, 19, 34] in essence, setting in place new neural connectivity. Furthermore, exposure to drugs of abuse changes the structure and function of PNNs thought to facilitate drug-seeking behavior [17, 18, 34].
Recent studies indicate that drug-induced changes in PNN intensity occur, suggesting that PNNs are labile in nature; these changes are dependent on salient external stimuli, the brain region, and the extent of drug exposure and withdrawal time [17, 18, 34–36]. The intensity of the PNN marker WFA is altered after cocaine, ethanol, and nicotine exposure throughout the brain [34, 37–39]. More specifically, cocaine has been shown to both increase and decrease WFA intensity in the cerebellum, which is dependent on the time course, stage of withdrawal, and a cocaine challenge [34, 37]. Collectively, these findings suggest that exposure to drugs of abuse changes PNN expression in a time dependent manner within multiple brain regions.
Endogenous and exogenous PNN regulation and drugs of abuse
Restoration of PNNs via inhibition of MMPs that can degrade PNNs inhibits the reinstatement of cocaine- or heroin-seeking behavior [17, 18, 35]. Interestingly, decreases in the expression of PNN/ECM components were found during abstinence or extinction from heroin self-administration, while increases were found after cue-induced reinstatement [17], consistent with reported increases in MMP activity during withdrawal but inconsistent with increases during cue-induced reinstatement [18]. Although both increases in MMP activity and PNN expression have both been reported following drug exposure, it is likely that these increases are dependent on both the brain region and the stage of the drug treatment and withdrawal times.
Enzymatic regulation of PNNs has shown promise for the treatment of drug-induced neuroplasticity and the prevention of both drug-induced CPP and reinstatement of drug seeking [17–19, 35]. Degradation of PNNs prior to cocaine exposure may prevent drug-induced neuroplasticity, and in turn, decreasing the rewarding and reinforcing properties of cocaine. PNN degradation is hypothesized to create a tabula rasa (blank slate) for neuroplasticity, with the potential to combat maladaptive cocaine-induced plasticity while also facilitating new, adaptive plasticity.
Lateral hypothalamus and addiction
The LHA exhibits control over intracranial self-stimulation of the medial forebrain bundle, reward-seeking behavior, and codes for reward expectation [16] for both drug and non-drug rewards [16–20]. Activation of the medial forebrain is highly rewarding, reinforcing, and motivating [21–24] producing conditioned place preference [25] and intracranial self-stimulation [23, 25]. Moreover, medial forebrain bundle intracranial self-stimulation and drugs of abuse synergistically and potently regulate reward [22] which is exerted via direct or indirect projections between the nucleus accumbens, caudal ventral pallidum, lateral hypothalamic area (LHA), and the ventral tegmental area (VTA) [22, 26–33]. Despite the emerging significance of PNNs in drug-induced plasticity and the well-established role of the LHA in the circuitry of motivated behavior, no studies to our knowledge have examined the role of LHA PNNs in drug-seeking behavior.
Lateral hypothalamus, drugs of abuse, and PNNs
In the present study, we report the first study of its kind to examine the role of a subregion of the lateral hypothalamic area (LHA) that has been parcellated anatomically by the presence of PNNs in cocaine-induced behavior. We found that a region of the anterior dorsal LHA (LHAad) exhibited robust PNN expression along with dense ECM staining by WFA, with approximately 90% of parvalbumin positive (PV+) neurons being co-expressed with the PNN marker WFA, and approximately 60% of WFA positive (WFA+) neurons being co-expressed with PV in drug naïve animals. This co-labeling of WFA/PV is consistent with previous studies of other brain regions [19, 39]. PNN removal with Ch-ABC within, but not outside, the LHAad prior to conditioning abolished acquisition of cocaine- but not sucrose-induced conditioned place preference and significantly attenuated acquisition of cocaine but not sucrose self-administration.
Our findings highlight the importance of PNN removal within this subregion of the LHA in the rewarding and reinforcing effects of cocaine without having secondary effects on locomotor activity or sucrose intake in separate groups of cocaine naïve animals. Recently, our laboratory has reported that PNN removal within the prelimbic cortex can prevent both the acquisition and expression of cocaine-induced CPP [19]. This is somewhat consistent with another recent report involving extinction of cocaine-induced behavior after targeting the amygdala with Ch-ABC [36]. The current findings that PNN removal within the LHAad prevents the acquisition of both cocaine-induced CPP and self-administration (SA) is consistent with PNNs being necessary for cocaine-induced plasticity.
The cocaine dose (0.125 mg/kg/infusion) used here to observe Ch-ABC mediated effects upon drug-seeking behavior was optimized using a protocol previously reported by others [25] and is a standard dose used to assess the acquisition of cocaine self-administration [25–27]. Acquisition of SA has been used as a model of vulnerability to the reinforcing effects of drugs [15]. Future studies are needed to conduct a full dose response curve to determine how intra-LHAad Ch-ABC administration alters dose-effect functions of cocaine for conditioned place preference and self-administration. The effects of LHAad Ch-ABC administration on the acquisition of cocaine seeking may be the result of interference with either drug-associated memories [42] and/or the reinforcing, rewarding, and motivational properties of cocaine itself. The finding that PNNs are required for the acquisition of cocaine CPP and self-administration point to a novel mechanism of initial cocaine-induced plasticity. Future studies will need to determine detailed contributions of PNNs and their components to initial cocaine-induced plasticity changes in the LHAad.
Lateral hypothalamic area, anterior region, dorsal and intermediate zones express robust PNN/ECM Expression
The expression of PNNs/dense ECM within the rat LH showed WFA staining of a well-defined cytoarchitectural pattern in what has been previously termed the lateral hypothalamic nucleus [76]. This area was later further parcellated into the lateral hypothalamic area, anterior region, specifically the dorsal (LHAad) and intermediate (LHAai) zones, and is dorsolateral to the fornix [29]. Comparably, the ventral anterior LHA (LHAav) and the more posterior dorsal LHA (LHAd) expressed sparse PNN/ECM expression when compared to the LHAad with abundant PNN/ECM expression surrounding the cell body and proximal dendrites of select neurons of the LHAad.
PNNs and the mouse anterior hypothalamus versus rat anterior lateral hypothalamus
Similarly to what was observed in the LHAad of the rat, Horii-Hayashi et al. (2015) recently reported both robust loose matrix and more organized PNN somatodendritic expression in the perifornical area of the anterior hypothalamus of the mouse; essentially what appears to be an equivalent (but medial rather than lateral) area in the mouse. Interestingly, the major neuronal phenotype in this PNN-defined region were not GABAergic or PV+ [30] which is in stark contrast to our findings within the LHAad. Recently, PV+ glutamate neurons have been reported in the ventral lateral hypothalamus of the rat and colocalize with the PNN marker WFA [77], providing further evidence, that in some brain regions, PNN+ neurons are not primarily GABAergic or PV+. These data are indicative that there are PNN+ brain regions within both mouse and rat that are more analogous than homologous, and these regions correspond to the anterior hypothalamic area of the mouse and the anterior lateral hypothalamic area of the rat [30]. Moreover, Ch-ABC appears particularly suitable to assess the role of this patch of dense ECM material, as compared to other brain regions lacking loose ECM, since it degrades CSPGs found within both loose ECM and PNNs. Future studies will need to determine the degree of conservation of the heterogeneous and species-specific nature of PNN expression within the hypothalamic area between the mouse and rat.
Possible Ch-ABC mechanism of action in the LHAad
The mechanism through which LHAad PNN destabilization inhibits acquisition of cocaine-seeking behavior is unclear but there are several possibilities. Endogenous ECM proteases belong to a large family of metzincin metalloproteinases [78]. These proteases are secreted into the extracellular space from PNN-surrounded neurons [79], which in turn, can remodel PNNs to regulate synaptic formation and plasticity [80–82]. MMPs appear to be both regulated by exposure to drugs of abuse and integral to drug-induced neuroplasticity [16–18, 35, 81]. Exogenous PNN degradation via Ch-ABC administration inhibits cocaine-induced pro-drug-seeking plasticity [19, 36]. This is consistent with others reporting increases in PNN expression following a cocaine challenge [34].
The LHA is one of the most transcriptionally responsive brain regions to cocaine exposure, regulating genes that are expressed during synaptogenesis and synaptic plasticity [7]. Synaptic puncta are located within the gaps of the PNN lattice [83] with Ch-ABC increasing the number of synaptic puncta [13] and lateral diffusion of AMPA receptors [84]. PNNs enhance neuronal activity-regulated pentraxin (NARP), which is involved in homeostatic plasticity and is highly enriched in excitatory synapses of PV interneurons [85]. NARP is presynaptically secreted in a PNN dependent manner, recruiting AMPA receptors to cluster upon postsynaptic PV interneurons [85]. Expression of syndecan-3, a heparan sulfate proteoglycan component of the ECM that regulates ligand-receptor signaling, is induced by cocaine within the LH and significantly inhibits the motivation to self-administer cocaine [86]. Other candidate neuroplastic agents known to interact with PNNs include semphorin-3A, BDNF, and Otx2 [87–89]. Destabilization of PNNs within the LHAad may mitigate these agents of plasticity and their effects upon PNNs, in turn, preventing cocaine-induced plasticity.
Cocaine specific effects of LHAad Ch-ABC administration
A decrease in place avoidance and/or anxiety could also be perceived as in increase in reward. However, we counterbalanced across treatment groups any initial preferences of each rat for the black or white chamber, assigning both preferred and non-preferred compartments and both black and white chambers to be paired with cocaine injections. By counterbalancing these factors, we minimize possible interpretation confounds [6]. We successfully demonstrated cocaine-induced CPP using the counterbalanced approach, consistent with the cocaine- and sucrose-paired chambers being reinforcing and with the ability of Ch-ABC treatment to block this cocaine- but not sucrose-associated reinforcement.
It is unknown why removal of PNNs had little impact on behavior associated with natural rewards (i.e. sucrose consumption) but profound effects on cocaine-associated behaviors. Non-overlapping neurocircuitry between feeding and drug seeking within the LHAad is one possible explanation. In contrast to the anterior LHA, orexin and melanin-concentrating hormone (MCH) neurons of the more caudal LHA powerfully regulate both feeding and drug seeking [43, 74, 90,91] through a projection to the VTA [92]. LHAad circuitry is distinct from the more caudal and highly orexinergic region of the LHA [93]. The LHAad does not express significant levels of melanin-concentrating hormone or orexin/hypocretin peptide mRNA in the rat [93, 94]. Moreover, retrogradely labeled VTA neurons that express orexin/hypocretin are not found in the LHAad [95]. Other potential LHAad neuropeptides include CRF, galanin, dynorphin A&B, and met-enkephalin which have all been reportedly expressed in a very similar brain region of the LHA [76, 96–98].
LHAad function is difficult to deduce from the literature, with complex [99–101] and inconsistent [102–105] previous characterization. Its exact function is still unknown but has been previously included in neurocircuitry involved in the integration of incentive-motivation and reinforcement [68–70, 106] and thought to provide reciprocal interconnectivity between both the nucleus accumbens and VTA [102–105]. Towards this end, the LHAad may represent a node within the circuitry of motivated behavior that is particularly susceptible to drugs of abuse, possibly due to robust PNN/ECM expression, functioning as a highly plastic integration site for motivationally relevant stimuli of only comparably high hedonic value (i.e. drugs of abuse vs. food reward).
Conclusions
In summary, despite the emerging significance of PNNs in drug-induced neuroplasticity and the well-established role of the LHA in reward, reinforcement, and motivation, very little is known about how PNN-expressing neurons in the LHA control drug-seeking behavior. This is the first study of its kind to examine the role of a subregion of the LHA that has been parcellated anatomically and functionally by the existence of PNNs in cocaine-induced behavior. The present study investigated a newly discovered patch of high-density PNN-containing neurons with surrounding dense ECM that is specifically expressed in a small region of the anterior dorsal lateral hypothalamic area (LHAad). PNNs around the neurons here were predominantly co-localized with parvalbumin and necessary for the acquisition of both cocaine-induced CPP and self-administration. Together, our results suggest that PNN destabilization within the LHAad prior to cocaine exposure prevents cocaine-induce behavioral plasticity, which may have implications for targeting PNNs in the development of new therapeutic approaches for the treatment of addiction.
Highlights.
A discrete subregion of the lateral hypothalamic area (the lateral hypothalamic area, anterior region, dorsal zone; LHAad) is characterized by robust PNN and loose ECM expression
PNN expression in the LHAad is predominantly co-localized with parvalbumin (PV).
PNN expression in the LHAad is necessary for acquisition of cocaine- but not sucrose-induced CPP
PNN destabilization in the LHAad does not produce secondary locomotor or ingestive effects
PNN expression in the LHAad is necessary for acquisition of cocaine but not sucrose self-administration
Acknowledgments
The authors wish to thank Dr. Daniel S. Zahm (Saint Louis University School of Medicine) for helpful discussions. This work was funded by National Institute of Health (NIH) grant number DA033404 to BAS and Washington State Initiative 171 (Washington State University Alcohol and Drug Abuse Research Program grant 124777 to JMB.
Abbreviations
- Ch-ABC
chondroitinase-ABC
- CPP
conditioned place preference
- CSPGs
chondroitin sulfate proteoglycans
- LHAad
the lateral hypothalamic area (LHA), anterior region (LHAa), dorsal (LHAad) zone
- ECM
extracellular matrix
- GABA
γ-Aminobutyric acid
- MFB
medial forebrain bundle
- PNNs
perineuronal nets
- PV
parvalbumin
- PV+-FSINs/FSNs
parvalbumin-positive fast-spiking interneurons and neurons, respectively
- SA
self-administration
- WFA
Wisteria floribunda agglutinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- 3.Valenstein ES, Campbell JF. Medial forebrain bundle-lateral hypothalamic area and reinforcing brain stimulation. Am J Physiol. 1966;210:270–274. doi: 10.1152/ajplegacy.1966.210.2.270. [DOI] [PubMed] [Google Scholar]
- 4.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 5.Olds J, Olds ME. The mechanisms of voluntary behavior. In: Heath RG, editor. The Role of Pleasure in Behavior. Hoeber; New York: 1964. [Google Scholar]
- 6.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 9.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartig W, Brauer K, Bigl V, Bruckner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994;635:307–311. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- 11.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 12.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pyka M, Wetzel C, Aguado A, Geissler M, Hatt H, Faissner A. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci. 2011;33:2187–2202. doi: 10.1111/j.1460-9568.2011.07690.x. [DOI] [PubMed] [Google Scholar]
- 14.Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104:359–369. doi: 10.1016/s0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 15.Seeger G, Brauer K, Hartig W, Bruckner G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience. 1994;58:371–388. doi: 10.1016/0306-4522(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 16.Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One. 2007;2:e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, Riga D, Wiskerke J, Binnekade R, Stegeman M, Schoffelmeer AN, Mansvelder HD, Smit AB, De Vries TJ, Spijker S. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2011;35:2120–2133. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA. Removal of perineuronal nets in the medial prefrontal cortex impairs the consolidation and reconsolidation of cocaine-induced conditioned place preference. J Neurosci. 2015;35:4190–4202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruckner G, Bringmann A, Hartig W, Koppe G, Delpech B, Brauer K. Acute and long-lasting changes in extracellular-matrix chondroitin-sulphate proteoglycans induced by injection of chondroitinase ABC in the adult rat brain. Exp Brain Res. 1998;121:300–310. doi: 10.1007/s002210050463. [DOI] [PubMed] [Google Scholar]
- 21.Sorg BA, Todd RP, Slaker M, Churchill L. Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology. 2015;92:25–33. doi: 10.1016/j.neuropharm.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates: Fifth Edition. Elsevier Academic Press; Amsterdam; Boston: 2005. [Google Scholar]
- 23.Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci U S A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 25.Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- 26.Flagel SB, Vazquez DM, Robinson TE. Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology. 2003;28:1741–1751. doi: 10.1038/sj.npp.1300228. [DOI] [PubMed] [Google Scholar]
- 27.Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- 28.Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Swanson LW. Brain maps: Structure of the Rat Brain A Laboratory Guide with Printed and Electronic Templates for Data, Models and Schematics. third. Elsevier; Amsterdam: 2004. [Google Scholar]
- 30.Horii-Hayashi N, Sasagawa T, Hashimoto T, Kaneko T, Takeuchi K, Nishi M. A newly identified mouse hypothalamic area having bidirectional neural connections with the lateral septum: The perifornical area of the anterior hypothalamus enriched in chondroitin sulfate proteoglycans. Eur J Neurosci. 2015 doi: 10.1111/ejn.13024. [DOI] [PubMed] [Google Scholar]
- 31.Deminiere JM, Piazza PV, Le Moal M, Simon H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev. 1989;13:141–147. doi: 10.1016/s0149-7634(89)80023-5. [DOI] [PubMed] [Google Scholar]
- 32.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Sanroman D, Leto K, Cerezo-Garcia M, Carbo-Gas M, Sanchis-Segura C, Carulli D, Rossi F, Miquel M. The cerebellum on cocaine: plasticity and metaplasticity. Addict Biol. 2015 doi: 10.1111/adb.12223. [DOI] [PubMed] [Google Scholar]
- 35.Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, Han HB, Luo YX, Xu LZ, Wu P, Lu L. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34:6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Sanroman D, Carbo-Gas M, Leto K, Cerezo-Garcia M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Rossi F, Miquel M. Cocaine-induced plasticity in the cerebellum of sensitised mice. Psychopharmacology (Berl) 2015;232:4455–4467. doi: 10.1007/s00213-015-4072-1. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez-Sanroman DB, Monje RD, Bardo MT. Nicotine self-administration remodels perineuronal nets in ventral tegmental area and orbitofrontal cortex in adult male rats. Addict Biol. 2016 doi: 10.1111/adb.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, He D, Lasek AW. Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcohol Clin Exp Res. 2015;39:1930–1938. doi: 10.1111/acer.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- 41.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 42.Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 45.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 46.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olds ME, Olds J. Approach-avoidance analysis of rat diencephalon. J Comp Neurol. 1963;120:259–295. doi: 10.1002/cne.901200206. [DOI] [PubMed] [Google Scholar]
- 48.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 49.Milner PM. Brain-stimulation reward: a review. Canadian journal of psychology. 1991;45:1–36. doi: 10.1037/h0084275. [DOI] [PubMed] [Google Scholar]
- 50.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of comparative and physiological psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 51.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 52.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 54.Markou A, Koob GF. Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology. 1992;7:213–224. [PubMed] [Google Scholar]
- 55.Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- 56.Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther. 1994;271:294–301. [PubMed] [Google Scholar]
- 57.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- 59.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 60.Lin D, Koob GF, Markou A. Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem Behav. 2000;65:407–417. doi: 10.1016/s0091-3057(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 61.Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- 62.You ZB, Chen YQ, Wise RA. Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience. 2001;107:629–639. doi: 10.1016/s0306-4522(01)00379-7. [DOI] [PubMed] [Google Scholar]
- 63.Kenny PJ, Koob GF, Markou A. Conditioned facilitation of brain reward function after repeated cocaine administration. Behav Neurosci. 2003;117:1103–1107. doi: 10.1037/0735-7044.117.5.1103. [DOI] [PubMed] [Google Scholar]
- 64.Shizgal P, Bielajew C, Corbett D, Skelton R, Yeomans J. Behavioral methods for inferring anatomical linkage between rewarding brain stimulation sites. Journal of comparative and physiological psychology. 1980;94:227–237. doi: 10.1037/h0077668. [DOI] [PubMed] [Google Scholar]
- 65.Gallistel CR, Gomita Y, Yadin E, Campbell KA. Forebrain origins and terminations of the medial forebrain bundle metabolically activated by rewarding stimulation or by reward-blocking doses of pimozide. J Neurosci. 1985;5:1246–1261. doi: 10.1523/JNEUROSCI.05-05-01246.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nauta WJH, Haymaker W. Hypothalamic nuclei and fiber connexions. In: Haymaker W, Andersson E, Nauta WJH, Thomas CC, editors. The Hypothalamus. Springfield; 1969. pp. 136–209. [Google Scholar]
- 67.Morgane PJ. Anatomical and neurobiochemical bases of the central nervous control of physiological regulations and behavior. In: Mogenson GJ, Calaresu FR, editors. Neural Integration of Physiological Mechanisms and Behavior. University of Toronto Press; Toronto: 1975. pp. 24–67. [Google Scholar]
- 68.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 69.Swanson LW. The neural basis of motivated behavior. Acta morphologica Neerlando-Scandinavica. 1988;26:165–176. [PubMed] [Google Scholar]
- 70.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 71.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 72.Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM. Decoding Neural Circuits that Control Compulsive Sucrose Seeking. Cell. 2015;160:528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Munzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell metabolism. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geeraedts LM, Nieuwenhuys R, Veening JG. Medial forebrain bundle of the rat: IV. Cytoarchitecture of the caudal (lateral hypothalamic) part of the medial forebrain bundle bed nucleus. J Comp Neurol. 1990;294:537–568. doi: 10.1002/cne.902940404. [DOI] [PubMed] [Google Scholar]
- 77.Meszar Z, Girard F, Saper CB, Celio MR. The lateral hypothalamic parvalbumin-immunoreactive (PV1) nucleus in rodents. J Comp Neurol. 2012;520:798–815. doi: 10.1002/cne.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;30:15337–15357. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T, Hawrylycz M, Cuenod M, Do K, Urban A, Lein ES. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015;20:154–161. doi: 10.1038/mp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 81.Wright JW, Harding JW. Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural plasticity. 2009;2009:579382. doi: 10.1155/2009/579382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geissler M, Gottschling C, Aguado A, Rauch U, Wetzel CH, Hatt H, Faissner A. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci. 2013;33:7742–7755. doi: 10.1523/JNEUROSCI.3275-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 85.Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Repunte-Canonigo V, Kawamura T, Lefebvre C, Shin W, Howell LL, Hemby SE, Harvey BK, Califano A, Morales M, Koob GF, Sanna PP. Hypothalamic proteoglycan syndecan-3 is a novel cocaine addiction resilience factor. Nature communications. 2013;4:1955. doi: 10.1038/ncomms2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernard C, Prochiantz A. Otx2-PNN Interaction to Regulate Cortical Plasticity. Neural plasticity. 2016;2016:7931693. doi: 10.1155/2016/7931693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Winter F, Kwok JC, Fawcett JW, Vo TT, Carulli D, Verhaagen J. The Chemorepulsive Protein Semaphorin 3A and Perineuronal Net-Mediated Plasticity. Neural plasticity. 2016;2016:3679545. doi: 10.1155/2016/3679545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 90.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 91.Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009;106:6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 94.Hahn JD. Comparison of melanin-concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2010;468:12–17. doi: 10.1016/j.neulet.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 96.Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- 97.Melander T, Hokfelt T, Rokaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 98.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 99.Tsai CT, Mogenson GJ, Wu M, Yang CR. A comparison of the effects of electrical stimulation of the amygdala and hippocampus on subpallidal output neurons to the pedunculopontine nucleus. Brain Res. 1989;494:22–29. doi: 10.1016/0006-8993(89)90139-x. [DOI] [PubMed] [Google Scholar]
- 100.Yang CR, Mogenson GJ. An electrophysiological study of the neural projections from the hippocampus to the ventral pallidum and the subpallidal areas by way of the nucleus accumbens. Neuroscience. 1985;15:1015–1024. doi: 10.1016/0306-4522(85)90250-7. [DOI] [PubMed] [Google Scholar]
- 101.Yang CR, Mogenson GJ. Hippocampal signal transmission to the pedunculopontine nucleus and its regulation by dopamine D2 receptors in the nucleus accumbens: an electrophysiological and behavioural study. Neuroscience. 1987;23:1041–1055. doi: 10.1016/0306-4522(87)90179-5. [DOI] [PubMed] [Google Scholar]
- 102.Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Res Bull. 1983;11:309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- 103.Swanson LW, Mogenson GJ, Gerfen CR, Robinson P. Evidence for a projection from the lateral preoptic area and substantia innominata to the ‘mesencephalic locomotor region’ in the rat. Brain Res. 1984;295:161–178. doi: 10.1016/0006-8993(84)90827-8. [DOI] [PubMed] [Google Scholar]
- 104.Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- 105.Wu M, Hrycyshyn AW, Brudzynski SM. Subpallidal outputs to the nucleus accumbens and the ventral tegmental area: anatomical and electrophysiological studies. Brain Res. 1996;740:151–161. doi: 10.1016/s0006-8993(96)00859-1. [DOI] [PubMed] [Google Scholar]
- 106.Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Academic Press. 2007 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]


