Summary
Innate lymphoid cells are functionally diverse subsets of immune cells including the conventional natural killer cells, lymphoid tissue inducers, type 1, 2 and 3 with significant roles in immunity and pathogenesis of inflammatory diseases. Type 2 innate lymphoid cells (ILC2s) resemble type 2 helper (Th2) cells in cytokine production and contribute to anti-helminth immunity, maintaining mucosal tissue integrity and adipose tissue browning. ILC2s play important roles in the pathogenesis of allergic diseases and asthma. Studying the pathways of activation and regulation of ILC2s are currently a priority for giving a better understanding of pathogenesis of diseases with immunological roots. Recently, our laboratory and others have shown several pathways of regulation of ILC2s by costimulatory molecules such as ICOS, regulatory T cells and by compounds such as nicotine. In this review, we summarize the current understanding of the mechanisms of activation and regulation of ILC2s and the role of these cells in health and disease.
Keywords: Asthma, Eosinophils, Th1/Th2/Th17 Cells, Parasitic-Helminth, Fungal
Innate lymphoid cells
Innate lymphoid cells are a recently identified heterogeneous but developmentally related group of lymphocytes1–7. Innate lymphoid cells are categorized into three major subsets of type 1, 2 and 3, by stereotyping the differentiated CD4+ T helper (Th) cells based on their cytokine production profile and the master transcription factors that regulate their development1–7. Type 1 innate lymphoid cells (ILC1s), similar to Th1 cells, produce interferon (IFN)-γ, required T-box transcription factor (T-bet) for their development and are further subdivided into NK cell and ILC1s8, 9. NK cells are perhaps the first members of innate lymphoid cells that have been identified more than four decades ago1. Type 3 innate lymphoid cells (ILC3s) resemble Th17 cells in producing IL-17 and IL-22, require Retinoid-Related Orphan Receptor-Gamma (ROR)-γt for their development and are subdivided into lymphoid tissue inducer cells (LTi) and ILC3s10–12. Mirroring Th2 cells, type 2 innate lymphoid cells (ILC2s) produce IL-5, IL-13 and required the transcription factor retinoid acid-related orphan receptor alpha (RORα) and high levels of Gata-3 for their development5, 6, 13.
Definition of Type 2 innate lymphoid cells
ILC2s were initially identified as type 2 cytokine producing non-B, non-T lymphocytes in response to alarmins, IL-33 and IL-255, 6, 13. The initial reports referred to ILC2s with names such as natural helper cells or nuocytes5, 6, 13. The collective nomenclature of innate lymphoid cells and its subsets was first suggested by a consortium of pioneers in the field in 201314. To date, there is no specific cell surface marker for innate lymphoid cells, and these cells are generally defined by the lack of expression of the markers of the previously identified immune cell lineages and the expression of the leukocyte common antigen, CD451–7. Lineage markers include CD3, B220, CD11b, Gr-1, CD11c, Ter-119 and FCεRI in mice and CD3, CD14, CD16, CD19, CD20, CD56, CD235a, CD1a, CD123 in humans15. Since ILC2s have been identified as IL-33 or IL-25 responsive innate lymphoid cells, all murine innate lymphoid cells, lineage-negative CD45+ cells, that express the IL-33 receptor, ST2 or IL-25 (IL-17RB) receptor would be considered as ILC2s5, 6, 13. However, several other cell surface markers have been described for further distinction of both murine and human ILC2s among all innate lymphoid cell population, including Inducible T cell Costimulator (ICOS), Killer cell lectin-like receptor subfamily G member 1 (KLRG-1) and cytokine receptors IL-7 receptor-α, and IL-2 receptor-α16. Murine but not human ILC2s also express CD90 (Thy1)16, 17. Human ILC2s express CD161, CCR6 and Prostaglandin D2 receptor 2 (CRTH2)17, 18. ST2 is not detectable in freshly isolated human ILC2s, however, these cells respond to IL-33 after in vitro stimulation for at least 12 hours18.
ILC2s have been detected in the lungs, gastrointestinal tract, adipose tissue, skin, oral mucosa, secondary lymphoid organs and blood of humans and mice15, 17, 19. ILC2s constitute a significant proportion of murine pulmonary innate lymphoid cells whereas, in humans ILC2s are less than 3% of total innate lymphoid cells in the lungs15, 17, 19. In the skin of humans and mice, ILC2s constitute the majority of innate lymphoid cells20.
Activation of ILC2s and their cytokine repertoire
Cytokine repertoire of ILC2s
Although ILC2s were initially identified as IL-5 and IL-13 producing cells5, 6, 21, they can produce several other cytokines including, IL-9, and IL-4. Recent transcriptional analysis of ILC2s and the evidence from the Immunological Genome Consortium show that murine ILC2s express genes of IL-2, Granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-622, 23. Production of IL-4, IL-5, IL-9, IL-13, GM-CSF and IL-6 by human ILC2s has been also shown at the protein level17. IL-10 production by a subset of CD127+ Sca-1+ CD25+ innate lymphoid cells has been reported in a murine model of contact hypersensitivity24. Whether these IL-10 producing cells are a subset of ILC2s or a distinct subset of innate lymphoid cells remains to be elucidated.
Activation of ILC2s by cytokines
ILC2s are terminally differentiated effector lymphocytes, armed with multiple mechanisms to sense a variety of danger signals which enables them to rapidly evoke an immune response by producing large quantities of cytokines. Unlike conventional lymphocytes, ILC2s lack antigen specific receptors and are activated through danger signals. Tissue damage and stress upon pathologic conditions lead to the production of danger signals including cytokines such as IL-33, IL-25, thymic stromal lymphopoietin (TSLP), prostaglandins and leukotrienes, all of which could be sensed by murine and human ILC2s. In addition, human ILC2s are activated by IL-1β and IL-1817, 18. Activated human ILC2s by IL-1β produce IL-5 and IL-1318.
Although, IL-33 and IL-25 can both activate ILC2s to produce cytokines and proliferate, IL-33 seems to be more potent in activating ILC2s than IL-2525. To date, subsets of ILC2s that distinctly differ in phenotype and function have not been identified and it remains to be identified whether cytokine responsiveness can determine distinct subsets of ILC2s. There is evidence suggesting that IL-25-responsive ILC2s can develop to IL-33-reponsive ILC2 in vivo and in vitro26. However, further studies are required to clearly determine whether IL-25-responsive ILC2s are different from the previously described multipotent progenitor type 2 cells27.
IL-9 is produced by several immune cells including Th9 and mast cells and contribute to worm expulsion and the asthma pathogenesis28, 29. There is evidence indicating that IL-9 plays a major role in the survival of ILC2s and their production of IL-5 and IL-1330, 31. Interestingly, ILC2s produce significant amount of IL-9 and has been shown that autocrine IL-9 is an important component in the homeostasis and optimal function of ILC2s32. Depending on the environmental factors and pathologic conditions, however, different factors seem to stimulate the production of IL-9 by either ILC2s or other cells such as mast cells32, 33. Several pathways have been described to stimulate IL-9 production by ILC2s. IL-2 is the first cytokine that has been reported to be required for IL-9 production by ILC2s30. It has been shown that IL-2 plays a major role in ILC2-mediated induction of type 2 lung inflammatory conditions in mice by acting as a survival factor and as a cofactor for their cytokine production34. IL-2 is also an essential cytokine for ex vivo stimulation and culture of ILC2s30, 35. So, IL-2 stimulates the production of IL-9 by ILC2s which is in turn required for the proliferation and cytokine production by these cells. It remains to be investigated whether IL-2-stimulation is dispensable in the presence of exogenous IL-9.
A different line of research suggests that IL-4 can enhance production of IL-9 by ILC2s36. This pathway seems to be an important factor in response to papain and allergens with protease activity36. IL-9 is such an important cytokine for proliferation and activation of ILC2s that the lack of T-bet, an ILC1 associated transcription factor, caused exaggerated ILC2s response by unleashing the production of IL-937. There is evidence suggesting that TSLP in synergy with IL-33 plays a role in IL-9 production by ILC2s32. This pathway seems to be critical in efficient ILC2-mediated anti-helminth infection. Apart from the effects of IL-9 production, TSLP stimulation has been found to activate ILC2s independent of IL-33 or IL-25 in the skin38.
Recently, two lines of evidence suggested a role for TL1A, a member of tumor necrosis factor superfamily, in activating ILC2s and developing type 2 pathology independent of IL-33 or IL-2539, 40. Human ILC2s also express IL-18 receptor and respond to IL-18 stimulation in a fashion similar to IL-33 and produce IL-4, IL-5, IL-9, IL-13, GM-CSF and IL-617.
Activation of ILC2s by eicosanoids
Under inflammatory conditions, a family of lipid mediators called “eicosanoids” are generated from arachidonic acid which itself is a product of phospholipids41. The four sub-families of eicosanoids including prostaglandins, leukotrienes, lipoxins and prostacyclin play important roles in development and resolution of inflammation41. Leukotrienes are generated by lipoxygenase enzymes whereas generation of prostaglandins and prostacyclin are mediated by cyclooxygenase enzymes41. Given the therapeutic potentials of eicosanoids, their effects on ILC2s, particularly in asthma pathogenesis have been well studied. As discussed below, prostaglandins, leukotrienes, lipoxins and prostacyclin have opposing effects on ILC2s.
Prostaglandin D2 (PGD2) has been shown to activate human ILC2s to produce IL-4, IL-5 and IL-1342. PGD2 also increases the expression of IL-33 and IL-25 receptors and synergizes the effects of IL-33 and IL-25 in cytokine production by human ILC2s42. One important effect of PGD2 on ILC2s is inducing chemotaxis. It has been shown that the frequency of CRTH2 expressing ILC2s are lower in the lungs than in the peripheral blood of non-asthmatic humans and that PGD2 is a strong chemoattractant for human ILC2s42–44. Further studies are required to determine whether the frequency of CRTH2 expressing ILC2s are higher in the lungs of asthmatic patients than non-asthmatic individuals. Unlike PGD2, prostaglandin I2 (PGI2), and lipoxins A4 have regulatory effects of ILC2s which are discussed below.
Cysteinyl leukotrienes (cysLT) are inflammatory mediators generated by eosinophils, basophils, mast cells, macrophages, and myeloid dendritic cells and play important roles in the pathogenesis of asthma and allergy through incompletely understood mechanisms45–47. Activation of ILC2s may be an important mechanism of contribution of cysLTs to asthma pathogenesis. Human and murine ILC2s express cysLT receptor-1 and can be activated by cysLTs C4, D4, E4 or cysLT-R1 agonists48, 49. Recently it was shown that peripheral ILC2s from patients with atopic dermatitis express higher level of cysLT receptor than those of non-atopic individuals and that cysLTs, in particular, LTE4 augment the activation of human ILC2s by IL-25, IL-33, TSLP or PGD249. The same study showed that the effects of cysLTs were inhibited by antagonist to cysLT receptor.
Regulation of ILC2s
Regulation of ILC2s by co-stimulatory molecule
Like other components of immune system, ILC2s homeostasis and function need to be regulated to prevent deleterious immune responses. The mechanisms of regulation of ILC2s have been studied at different levels of extrinsic and intrinsic molecular pathways as well as the regulatory effects of other cells on ILC2s. One of the most described co-stimulatory molecules that plays an important role in the homeostasis and regulation of function of ILC2s is ICOS15, 50–53. ILC2s express ICOS and we were the first to discover that ILC2s also express ICOS-L15, 50, 51. We showed that the genetic ablation of ICOS increases the apoptosis rate and impairs the cytokine production by ILC2s15. We further showed that genetic ablation of ICOS or blocking ICOS/ICOS-L interaction leads to reduced ILC2-mediated airway hyperreactivity and lung inflammation. Adoptively transferred ICOS-deficient ILC2s resulted in an attenuated airway hyperreactivity and lung inflammation in alymphoid hosts compare to wild type ILC2s15, 50, 51. We introduced the first humanized murine model for ILC2-mediated asthma which provides a unique platform for studying the role of human ILC2s in asthma pathogenesis and for identifying novel therapeutic approaches for asthma15. Mechanistically, we showed that in the absence of ICOS, the amount of phosphorylated Signal transducer and activator of transcription 5 (STAT5) and the amount of anti-apoptotic molecule, B-cell lymphoma 2(Bcl2) are decreased15. Since both ICOS and ICOS-L possess cytoplasmic domains, further studies are required to segregate the contribution of ICOS from ICOS-L downstream signaling to the homeostasis and function of ILC2s. In human dendritic cells, Protein Kinase C molecules (PKCs) have been found essential for the intracellular signaling through ICOS-L54. There is evidence suggesting that PKCθ is expressed by human and murine ILC2s and that PKCθ is required for the survival and function of murine ILC2s in vivo55. Whether PKCθ requirement is associated with ICOS-L signaling in ILC2s remains to be elucidated.
Regulation of ILC2s by regulatory T cells
Regulatory T (Treg) cells, the indispensable components of immune regulation, have been shown to suppress lung ILC2s56, 57. Recently we showed that adoptively transferred in vitro-generated FOXP3+ CD4 T cells but not naturally occurring Treg cells suppress ILC2-mediated airway inflammation and hyperreactivity in a murine model57. We further showed that autologous human Treg cells can suppress ILC2-mediated airway hyperreactivity and inflammation in a humanized murine model57. Treg cells can suppress effector cells through different mechanisms. Neutralizing IL-10 or TGF-β partially reversed the suppressive effects of Treg cells on ILC2s suggesting the presence of an alternative pathway of suppression. We showed that suppression of ILC2s by in vitro-generated Treg cells requires ILC2-Treg cell contact through ICOS-ICOS-L interaction57. A different line of research suggests that de novo generated Treg cells limit ILC2s activation and promote resolution of ILC2-mediate lung inflammation in a TGF-β dependent manner56.
Regulation of ILC2s by cytokines
Regulation of Th2 cells by type 1 cytokines has been an interesting issue in understanding the regulation of immune response to viral and bacterial infections and helminth and allergic responses. It has been well documented that Th1 cytokines can counterbalance Th2 cells58–62. Innate lymphoid cells mimic many but not all aspects of differentiated T helper cells. Whether type 1 cytokines can impact ILC2s in a similar fashion to their impact on Th2 cells, has attracted the attention of many lines of research in the field. A prototypic Th1 cytokine is IFN-γ, the only member of type-II interferons. Interferons are crucial components of antiviral immunity and are classified into two categories of type-I and type-II interferons63. Type 1 interferons such as IFN-α are rapidly produced by plasmacytoid dendritic cells (pDCs) upon viral infections64. Murine bone marrow ILC2s and human peripheral ILC2s express Interferon alpha receptor -1 (IFNAR-1) and using an influenza infection in mice it has been shown that in the lack of IFNAR-1 leads to the augmented ILC2 response65. IFNAR-1 is the shared receptor for type-I interferons, IFN-α and IFN-β63. IFN-β was reported to suppress the proliferation, cytokine production and induce apoptosis in ILC2s in vivo and in vitro65, 66. Mechanistically, the suppressive effects of IFN-β was found to be mediated by interferon-stimulated gene factor 3 (ISGF3). The source of IFN-β however, was not identified in the mentioned studies.
IFN-γ plays important roles in antiviral and antibacterial immunity as well as in the pathogenesis of obesity-related diabetes. Gene expression analyses in innate lymphoid cells by the Immunological Genome Consortium have revealed that ILC2s express at least one of the two chains of IFN-γ receptor23. Three lines of research have confirmed that murine ILC2s are responsive to IFN-γ52, 65, 66. One study showed that IFN-γ inhibits IL-33-mediated activation of murine ILC2s in the lung and visceral adipose tissue52. That study did not find a significant effect for IFN-γ in the lungs at the steady state52. Other studies have reported that IFN-γ suppress ILC2 activations in vivo in the lungs and in vitro65, 66. The effects of IFN-γ on ILC2s was found to be mediated by STAT165, 66. Whether IFN-γ has similar effects of human ILC2s is unknown to date.
IL-27 is a member of IL-12 family and an early product of antigen presenting cells in response to interferons and is mediating the effects of type-I interferons67, 68. IL-27 was found to suppress cytokine production by lung ILC2s in response to IL-33 in vitro and in response to Alternaria alternata in vivo65, 66. Based on these findings it could be concluded that viral infections counterbalance and suppress ILC2s through production of interferons, however, as discussed in the section of role of ILC2s in disease pathogenesis, evidence suggest that respiratory viral infections activate ILC2s.
PGI2 and Lipoxin A4 are eicosanoids that suppress ILC2s. Addition of PGI2 to purified bone-marrow murine ILC2s has been shown to inhibit IL-33-induced production of IL-5 and IL-13 in WT but not in PGI2-receptor deficient murine ILC2 cultures69. Similar findings were reported in purified peripheral human ILC2s cultures69. In a mouse model of Alternaria alternata-driven severe asthma, PGI2-receptor deficiency augmented whereas administration of PGI2 analog suppressed IL-5 and IL-13 production by lung ILC2s, airway eosinophilia and mucus production score in the airways69. The findings of this study, however, were conflicted by a more recent study in which they showed that in the absence of PGI2-receptor, IFN-γ producing pulmonary NK cells were expanded, house dust mite-induced airway eosinophilia and airway mucus production were reduced70. It remains to be addressed whether the seeming discrepancy between the two studies are caused by the type of allergen and allergen exposure protocol.
It has been shown that human peripheral blood ILC2s express receptor for Lipoxin A4 and that Lipoxin A4 suppresses IL-13 production by human peripheral ILC2s ex vivo stimulated with PGD271. Further studies are required to identify the mechanisms of the effects of Lipoxin A4 on ILC2s.
Intrinsic factors in ILC2 regulation
Arginase 1 (Arg1) is an enzyme that plays an important role in cellular metabolism of L-Arginine. Arg1 has been shown to be expressed by fetal liver and adult murine ILC2s72, 73. Recent evidence suggests that human lung ILC2s express Arg1 and that this enzyme is crucial for the optimal proliferation of ILC2s during chronic and acute lung inflammation in mice74. Arg1 inhibition was found to disrupt amino acid metabolism in ILC2s74. These findings suggest that Arg1 is one of the intrinsic regulators of ILC2s. Further studies are required to identify other potentially critical intrinsic factors that regulate ILC2s activation and proliferation.
Effects of smoking on ILC2s
Cigarette smoke has been associated with lung inflammatory conditions such as asthma and chronic obstructive pulmonary disorder (COPD)75, 76. Evidence suggests that cigarette smoke augments pathogen-induced COPD exacerbations by deviating the type-2 towards type-1 immune response77. It has been shown that cigarette smoke exposure reduces the expression of the IL-33 receptor (ST2) by ILC2 whereas it increased the expression of ST2 by pulmonary macrophages and NK cells in a murine model77. Cigarette smoke also increased the expression of IL-33 in the lungs77. That study did not show which of the many components in the cigarette smoke mediates the observed results. Recently, we showed that murine and human ILC2s express α7-nicotinic acetylcholine receptor and that a nicotine agonist inhibits ILC2s cytokine production78. We showed that nicotine agonist decreases the amount of Gata-3 and dampens the activation of NF-kB pathway. We found a nicotine agonist that alleviates the airway hyperreactivity and inflammation in human ILC2-derived murine model78. These two lines of evidence suggest that cigarette smoke may impact the pathogenesis of COPD and asthma in opposing directions and through two mechanisms of deviating towards type 1 immune response and direct inhibition of ILC2s which may have implications for clinical interventions for asthma.
The effects of gut flora and micronutrients on ILC2s
Transcriptional and epigenetic analysis of innate lymphoid cells has shown that depletion of gut flora with antibiotics or the absence of gut flora in germ-free mice is associated with upregulation of ILC3-associated genes such as Atf5, Gpx1, and Cxcl9 in gut ILC2s22. It remains to be studied whether these ILC3-associated genes are actually elevated at the protein level in ILC2s upon loss of gut flora. Moreover, the functional significance of increased expression of such genes in the activation, homeostasis and plasticity of ILC2s is unknown to date.
Micronutrients including vitamins have an impact on the regulation of the immune system and lymphocytes79. Deficiency of vitamin A is one of the common deficiency that leads to defects in immune system. Evidence suggests that deficiency in vitamin A leads to the diminished ILC3s number but increased number of IL-13 producing ILC2s in the intestines of mice and causes resistance to nematode infections80. It has been shown that retinoic acid, a vitamin A metabolite, regulates gut-homing receptor expression by ILC1s and ILC3s but not ILC2s in mice81. Deficiencies in vitamin A impaired the expression of gut-homing receptor only in ILC1 and ILC3s and as a results the number of these cells was diminished in vitamin A deficiency81. Whether vitamin A deficiency has such dramatic effects on human innate lymphoid cells is unknown.
Memory-like phenotype in ILC2s
Remembering the previously-encountered pathogens and eliciting a more potent response in the subsequent encounters is the definition of immunological memory which is mediated by long-lived T and B memory lymphocytes. Immunological memory is usually associated with antigen specificity and therefore, innate immunological memory is out of the scope of the definition of immunological memory. However, a recent evidence suggests that ILC2s may acquire memory-like phenotype82. Using a papain or IL-33 to activate pulmonary ILC2s, it was shown that upon stimulation, the number of ILC2s in the lungs or draining lymph nodes increases dramatically and gradually decline but even after a period more than 125 days they do not decline to the pre-stimulation number82. Four weeks after stimulation, ILC2s in IL-33 challenged mice found to be more responsive to re-stimulation with IL-33, expanded in higher number and produced more cytokine than those in naïve mice82. As expected, memory-like ILC2s found to dramatically respond to other allergens than originally exposed but poise the adaptive immunity to the new allergen more efficiently82. Further studies may be needed to further identify the phenotype and significance of these cells in worm expulsion and pathogenesis of allergic diseases.
Transcriptional regulation and ontogeny
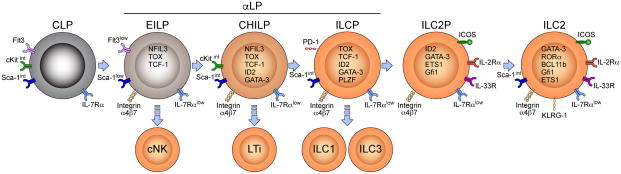
ILC2s are significant components of the immune system and their developmental process has received considerable attention from the scientists in the field. Ontogeny of innate lymphoid cells has been described in more details in mice than in humans. Murine Innate lymphoid cells are generated in fetal liver during embryonic development and in bone marrow from hematopoietic stem cells in adults72, 83. Based on the developmental cytokine dependency innate lymphoid cells can be categorized into two major subsets of IL-7 dependent and IL-7 independent innate lymphoid cells84. IL-7 dependent innate lymphoid cells include ILC1, ILC2 and ILC3 and IL-7 independent innate lymphoid cells include conventional natural killer (cNK) cells85–87. Interestingly, unlike cNK cells, IL-7 dependent innate lymphoid cells do not have cytotoxic activities10. Like conventional lymphocytes, all innate lymphoid cells derive from lymphoid progenitors 83. Common lymphoid progenitors (CLPs) derive all subsets of innate lymphoid cells88–91. It should be noted that development of differentiated ILC2s from their precursors is a continuum and all the developmental stages described in the literature are transient and may be overlapping. Currently, there is no generally accepted nomenclature for some of the developmental stages of ILC2s.
In the murine bone marrow, CLPs express intermediate to low amounts of stem cells antigen-1 (Sca-1), c-Kit, and high amount of fms like tyrosine kinase 3 (Flt3), IL-7Rα and do not express any of lineage markers89. Expression of Flt3 distinguishes CLPs from downstream precursors89. CLPs can give rise to B and T lymphocytes and all subsets of innate lymphoid cells including the conventional NK cells88, 89, 92. In the next developmental stage, CLPs acquire integrin-α4β7 (Itgα4β7) and by downregulating Flt3, Itgα4β7+ CLPs develop to innate lymphoid cell committed progenitors collectively called α-lymphoid precursors (αLP)16, 93. αLP cells include early ILC progenitors (EILPs), common helper innate lymphoid cell progenitor (CHILP), and ILC precursor (ILCP). Stages after EILPs are committed innate lymphoid cells that can only develop to IL-7 dependent innate lymphoid cells including ILC1, ILC2, ILC3 but not B or T cells93. αLP are lineagenegative, cKitint, Sca-1intermediate, Flt3−, and Itgα4β7+ cells in the bone marrow16. EILPs develop to CHILP which then further differentiated to ILCPs. ILCPs can take two different paths, either acquire IL-2Rα and develop into ILC2 progenitors (ILC2p) or differentiate to ILC1 and ILC3s85. Figure 1 shows different developmental stages for ILC2s and their associated transcriptional requirement.
Figure 1. Ontogeny of ILC2s.
Common lymphoid progenitors (CMPs) differentiated to early innate lymphoid cell progenitors (EILP) which express NFIL3, TCF-1 and TOX. EILPs have the potential to differentiate to conventional NK cells or further develop to common progenitor to all helper-like ILCs (CHILP). CHILPs express the key transcriptional regulator Id2 and can generate lymphoid tissue inducers (LTi) and ILC1, ILC2 and ILC3. By Expression of PLZF CHIPs differentiate to innate lymphoid cell progenitor (ILCP) which can develop to all three subsets of innate lymphoid cells. ILCPs further differentiate to ILC2 progenitors (ILC2P) by expressing ETS1, Gfi1and increasing the expression of Gata-3. ILC2Ps can only generate ILC2s which express Bcl11b, RORα and high levels of Gata-3.
Several transcription factors play important roles in different stages of generation of ILC2s from its precursors. One of the most important transcription factors is the nuclear factor interleukin-3-regulated protein (NFIL3) which has been shown to be crucial for the development of all innate lymphoid cells94, 95. NFIL3 is reported to be critically important for development of earlier precursors of innate lymphoid cells, αLP from CLPs88, 96. A recent study showed that NFIL3 is crucial only between the developmental stage of CLPs to Itgα4β7+ CLPs and NFIL3 is no longer crucially required when its expression proceeds by the expression of T cell factor (Tcf) 7 (encoding TCF-1 protein), Thymocyte selection-associated high mobility group box protein (TOX) and Inhibitor of DNA binding (Id2)16.
The requirement for TCF-1 for development all subsets of innate lymphoid cells has been shown by several studies97–99. The first study identifies that TCF-1 is required for development of ILC2s but subsequent studies found a crucial role for development of earlier precursors of innate lymphoid cells97–99. Based on the expression of TCF-1, an early precursor of innate lymphoid cells was labeled as EILPs99. EILPs which are transitioning precursor between CLP, and CHILP stages, however, are heterogeneous and consist of IL-7Rαlow and negative cells99. IL-7Rα expressing EILPs express Id2 and develop into CHILP cells and IL-7Rα negative cells differentiate to cNK cells99. Currently, it is not clear however, whether all EILPs are differentiated from CLPs and IL-7Rα negative EILPs derive from IL-7Rα expressing ones or there may be an alternative developmental pathway for cNK cells.
Id2 is a transcription regulatory protein that has been shown to be crucial for the development of all IL-7 dependent (IL-7Rα expressing) innate lymphoid cells85. Id2 is expressed in innate lymphoid cell progenitors concurrent or shortly after the expression of Tcf-716, 99. Evidence suggests that CHILP derives all IL-7 dependent innate lymphoid cells and in fact CHILP was defined based on the high expression of Id285. Id2 seems to be required for development of CHILP from its precursors and for later stages16. It has been shown that transient expression of NFIL3 drives the expression of Id2 which in turn represses the genes that can drive differentiation to B or T cells such as Gfib, Tal1, Lmo2 and Gata216. A different line of evidence suggests that Notch signaling in CLPs inhibits development of αLP cells and drives the differentiation of CLPs towards T cells90. Evidence suggests that TOX is another important transcript factor for developmental stages from EILP to CHILP16, 92.
CHILP cells are a heterogeneous population including a population that expresses transcription factor promyelocytic leukemia zinc finger (PLZF encoded by Zbtb16) and a population that is PLZF negative16. PLZF is required for the development of IL-7Rα expressing innate lymphoid cells87, 93, 100, 101. Evidence suggest that PLZF+ CHILP cells express Programmed cell death protein 1 (PD-1) which can be used to distinguish these cells, however, PD-1 seems to be functionally redundant for transition of CHILPs to the next developmental stage16, 102. A recent study targets PD-1 to deplete mature ILC2s which leads to reduced lung pathology in murine models of influenza and papain-induced lung inflammation102. More recent studies label PLZF+ CHILP cells as ILPs and PLZF− CHILP cells as LTi precursor (LTiP)101.
Several transcription factors have been identified to be specifically required for the development and/or maintenance of ILC2s including Gata3, Bcl11b, Rorα, ETS1, Gfi1 and G9a. Transcription factor Gata-3 is a master regulator for Th2 cell differentiation and has been shown to be also critically required for development, maintenance and function of human and murine ILC2s5, 21, 103–106. Although initially Gata-3 was identified as a transcriptional regulator for ILC2s, soon it was discovered that Gata-3 is required for other innate lymphoid cells as well. Later, it was found that Gata-3 drives development of ILC3s12. Further studies showed that although ILC2s express high levels of Gata-3 for their homeostasis and function, lower level of Gata-3 is required for the development of all IL-7-dependent innate lymphoid cells107. Recently it was shown that innate lymphoid cell progenitors express Gata-3 as early as αLP stage maintained high throughout differentiation to ILC2p16.
Growth factor independence 1 (Gfi1) is a transcription factor that is involved in multilineage hematopoiesis and has been implicated in many pathological conditions including allergic diseases108. Gfi1 is required for development and sustained phenotype of ILC2s by driving the expression of Gata-3 and ST2109. Loss of Gfi1 in activated ILC2s causes impaired expression of Gata-3 and co-production of IL17A and IL-13 by ILC2s109. A more recent evidence suggests that Gfi1 represses Sox4 which drives the expression of RORγt110. That finding explains the production of IL-17A by ILC2s in the absence of Gfi1.
Evidence suggests that B-cell lymphoma/leukemia 11B (Bcl11b) is expressed in mature ILC2s and plays a major role for their development110–112. The expression of Bcl11b has been also found in ILC2 progenitors at the stages as early as CHILP111, 112. Conditional depletion and chimera experiments have shown that development of ILC2s requires the expression of Bcl11b and that loss of Bcl11b is associated with the expansion of RORγt+ ILC3s111. Bcl11b plays an indispensable role in maintaining the mature ILC2 phenotype and their function110.
One the most important transcription factors for ILC2 development is RORα113. RORα deficient mice do not have ILC2s in any of their organs and fail to mount an ILC2-mediate inflammation113, 114. Evidence indicate that Rorα is upregulated at early progenitor stages such as CHILP suggesting that the commitment to ILC2 subset occurs at early stages of development16, 101.
Recent evidence suggests that transcription factor ETS1 is expressed by mature ILC2s as well as ILC2 precursors including CHILP115. In the absence of ETS1, the number of CHILP and ILC2s were decreased possibly due to instability of Id2 expression115. In addition to developmental requirement, ETS1 was found to be necessary for the optimal cytokine production by mature ILC2s115.
Euchromatic histone-lysine N-methyltransferase 2 (EHMT2 or G9a) is an epigenetic modulator that was found to be important in the development of ILC2s by repressing ILC3-associated genes116. Mice lacking G9a in hematopoietic cells showed decreased number of ILC2s in the peripheral organs and bone marrow116. Repressing ILC3-associated genes through G9a-dependent dimethylation of histone 3 lysine 9 (H3K9me2) was found to underlie the requirement for G9a in ILC2 development116. Further studies may be needed to identify the developmental stage in which G9a plays a crucial role.
Despite the notion that priming towards subsets of innate lymphoid cells may occur at the final stages of development of these cells, recent evidence suggests that this priming may happen at the stages as early as CHILP16, 90, 101. There is evidence suggesting that CHILP cells are heterogeneous and genes associated with ILC2s such as RORα, Bcl11b and ICOS are upregulated at those early stages of development16, 90, 101. The mechanisms that drive such an early stage priming remain to be identified.
Role of ILC2s in disease pathogenesis
Asthma
Asthma is a heterogeneous inflammatory disease of the airways characterize by reversible bronchoconstriction and airway hyperreactivity (AHR) in response to specific and unspecific stimuli117–119. Type 2 immune responses mediate the eosinophilic-dominant asthma in patients117–119. Evidence from experimental murine models and clinical findings suggest that ILC2s play important roles in the pathogenesis of asthma through the following major pathways: I) ILC2s can directly and rapidly cause asthma symptoms in an innate and allergen-unspecific manner. II) ILC2s contribute to the induction of allergen-specific type 2 adaptive immunity.
Experiments using animal models have shown that LC2s can directly elicit AHR, a cardinal feature of asthma, and airway eosinophilic inflammation by rapid production of IL-13 and IL-5 independent of adaptive immunity120, 121. The first report documenting the identification of IL-25 showed that adenovirus-mediated expression of IL-25 in the lungs increased the expression of IL-5 gene 5-fold and of IL-13 gene 172-fold higher than the control adenovirus-treated Rag-1-deficient mice and caused eosinophilia and lung inflammation in these mice that lack B or T cells2. Further studies showed that IL-33 is more potent than IL-25 in activating ILC2s in the lungs25, 122. A single dose intranasal administration of IL-33 was shown to be sufficient to activate ILC2s, induce large amounts of IL-5 and IL-13 in the lungs within 12 hours and cause airway eosinophilia within two days in wild type as well as in Rag2-deficient mice121. Additional doses of intranasal IL-33 augmented these effects causing a robust lung pathology and mucus hyperplasia121.
Clinically relevant allergens such house dust mite (HDM), Alternaria alternata, and some respiratory viruses have been shown to activate pulmonary ILC2s and cause lung pathology and asthma-like symptoms in mice48, 121, 123–127. The effects of these allergens in activating ILC2s seem to be indirect and through activation of lung epithelial and antigen presenting cells which in turn produce IL-33, IL-25 and TSLP128. It remains to be investigated whether allergens can directly activate ILC2s. One of the mechanisms by which allergens activate epithelial cells is the protease activity and papain, a plant-derived cysteine protease, mimics this effect of allergens in vivo129. In addition to protease activity, chitin a polysaccharide in the structure of fungi, arthropods and helminths offers another mechanism for activation of type 2 immune response by allergens and parasites and induction of lung eosinophilic inflammation3, 130. This shared structural compound between helminth and allergens may explain the induction of anti-helminth immune response to allergens.
Although there are multiple cytokines that can activate ILC2s, different allergens or pathogenic conditions may preferentially elicit specific cytokines. HDM, papain, fungal allergens and influenza virus have been shown to activate IL-33 pathway whereas Respiratory syncytial virus (RSV) activates ILC2s through TSLP123, 127, 129, 131–133. Lung epithelial cells, macrophages and monocytes have been shown to be the source of IL-33 production123, 127, 129, 131–133. Fungal allergens such as A. alternata and Aspergillus species can also activate ILC2s by inducing the production of TSLP in lung epithelial cells125. Respiratory exposure to chitin or migratory helminths infection activate ILC2s through induction of IL-33 and TSLP in alveolar type-II cells32. Rhinovirus activates ILC2s through production of IL-25 in the lungs of neonatal mice124. Whether distinct pathway of activation gears ILC2s towards a more efficient function under different inflammatory conditions remains to be elucidated.
In addition to directly causing lung eosinophilic pathology, several lines of evidence indicate that ILC2s can also contribute to the pathogenesis of asthma by enhancing the induction of Th2 response and suppressing the Treg cells134–139. ILC2s can facilitate the induction of Th2 cells and IgE production in response to intranasal HDM exposure135. Further studies indicate that mechanistically IL-13 production from ILC2s promotes activation and migration of pulmonary dendritic cells (DCs) to the lymph nodes where they facilitate the differentiation of Th2 cells134. Further production of IL-13 by ILC2s was found to be necessary for the expression of the Th2-attracting-CCL17 by a subset of pulmonary dendritic cells to recruit the differentiated Th2 cells to the lungs and to induce memory Th2 cells136. In addition to the effects of ILC2-derived IL-13, ILC2s and Th2 cells have positive impact on each other. A subset of ILC2s express IL-4, IL-13, MHC-II and through co-stimulatory molecules such as OX40L and possibly ICOS-L can potentiate a Th2 response in a ILC2-Th2 cells contact dependent manner137, 138, 140, 141. Th2 cells can also enhance ILC2 response by producing IL-2137, 138, 140.
ILC2s can also promote antibody production by B cells in response to respiratory antigen through secretion of IL-5137. In chronic models of asthma, over 6 month, depletion of T cells could only resolve airway inflammation and depletion of both T cells and ILC2s were required for resolution of AHR and remodeling142. Moreover, it has recently been shown that ILC2s can block regulatory T cells by producing IL-4, albeit in a mouse model of food allergy139.
In addition to the aforementioned pathways, activation of pulmonary ILC2s may enhance the bone marrow generation of eosinophils. It has been shown that intranasal Alternaria alternata induces eosinopoiesis in the BM in ST2-dependent manner127. Under inflammatory conditions, caused by intranasal HDM extract, TGF-β1 has been shown to be required for optimal type 2 immune response in the lungs143. TGF-β1 was required for augmented induction of AHR, airway eosinophilia, IL-5 and IL-13 levels in the lungs and BAL by increasing the number of ILC2s in the lungs143.
Asthma in children is a significant public health problem worldwide and the predisposing factors for childhood development of asthma are incompletely understood. Recent findings suggest that embryonic exposure to allergens and neonatal respiratory viral infections may augment the contribution of ILC2s to the development of asthma. Evidence suggests that ILC2s accumulate in the developing lungs in an IL-33-dependent manner and HDM exposure increased IL-33 in the lungs leading to the activation of ILC2s and cytokine production from these cells144. A different line of evidence suggests that rhinovirus causes production of IL-25 in murine neonatal but not adult lungs leading to activation of ILC2s and IL-13 production. Anti-IL-25 has been shown to attenuate these effects124.
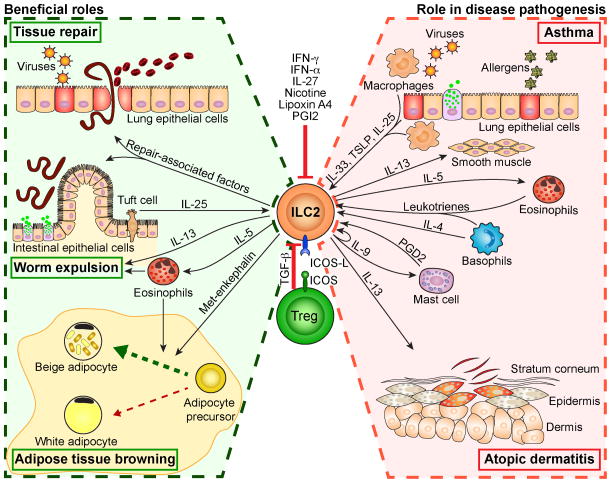
In addition to allergens, ambient air pollutants such as ozone can also cause asthma. ILC2s have been shown to mediate ozone-induce AHR and airway inflammation145, 146. In this allergen-independent asthma, both eosinophils and neutrophils are increased in the lungs and there is evidence that both ILC2s and γδ T cells are involved145, 146. Interestingly, there has been a strain difference in ozone sensitivity with BALC/c being more sensitive than B6145. Figure 2 summarizes the functional roles of ILC2s in immunity and disease pathogenesis.
Figure 2. Activation, regulation and the role of ILC2s in health and disease.
ILC2s can be activated by several stimuli including cytokines, IL-33, TSLP, IL-25, eicosanoids, PGD2 and leukotrienes. ILC2s play important roles in the pathogenesis of asthma and atopic dermatitis by producing IL-5 and IL-13. Beneficial effects of ILC2s include contribution to repair mucosal tissue damage due to worm migration or viral infections through IL-13 and repair associated genes. ILC2s play an important role in worm expulsion through production of IL-5 and IL-13. ILC2s contribute to controlling obesity by converting the white to beige adipocytes. ILC2s can be suppressed by nicotine, interferons, Lipoxin A and PGI2 and regulatory T cells through ICOS/ICOS-L interaction and TGF-β.
Effects of Respiratory Viruses on ILC2s
Different respiratory viruses activate pulmonary ILC2s through different pathways. Respiratory syncytial virus (RSV) activates ILC2s through induction of TSLP in the lungs causing AHR and mucus production. RSV induces TSLP in the lungs within 12h post infection and cause increased amounts of IL-13 production and ILC2-number in the lungs as early as 4 days post infection133. Neutralizing TSLP using antibody reversed the RSV-induced IL-13 in the lungs and genetic ablation of TSLP inhibits RSV-induced AHR, mucus and IL-13 production in the lungs.
Two lines of evidence suggest that experimental infection with influenza A virus (IAV) in mice causes IL-33 production in the lungs and activation of ILC2s123, 147. The first study shows that infection with a H3N1 strain of IAV causes increased number of ILC2s in the lungs, AHR, airway neutrophilia but not eosinophilia by day five post infection123. The second study, however, shows a clear production of IL-5 by ILC2s and increased number of eosinophils in the lungs at day 8 after infection with a H1N1 strain of IAV147. The seeming discrepancy between the findings of these studies can be due to the difference in the time of analyses after infection or may suggest that different strains of IAV may activate ILC2s through distinct pathways.
Evidence suggests that respiratory viral infections can enhance the subsequent response to allergens. Exposure of mice to pneumonia virus of mice (PVM) three days prior to Cockroach (CR) extract has led to higher level of eosinophilic inflammation and mucus production in the lungs than PVM or CR extract alone which was associated with higher viral load and lower weight gain148.
Evidence of involvement of ILC2s in respiratory allergies in Humans
Several lines of evidence suggest that ILC2s are associated with allergic respiratory inflammation and asthma in humans. One study found that the percentage of ILC2s in the peripheral blood mononuclear cells (PBMCs) was higher in patients with HDM-allergic rhinitis than patients mugwort allergic rhinitis149. Ex vivo stimulation of PBMCs with IL-33, IL-25 and IL-2 induced significantly higher amount of IL-5 and IL-13 in patients with HDM-allergic rhinitis149. Another study found that the frequency and absolute number of ILC2s are increased in the PBMC of allergic asthmatics compare to allergic rhinitis or healthy controls35. Total PBMCs of allergic asthmatic patients produced higher amount of IL-5 and IL-13 in response to IL-25 or IL-33 than those of allergic rhinitis or healthy controls35. A different study found that the total number of ILC2s in the blood and sputum of patients with severe asthma was higher than in patients with mild asthma150. This study showed that the number of IL-5+IL-13+ ILC2s was higher in the sputum of patients with severe asthma than patients with mild asthma150. The number of ILC2s in the biopsies of sinus mucosa was found to be higher in patients with chronic rhinosinusitis and nasal polyps who concurrently had asthma151, 152. Higher number of ILC2s has been observed in the induced sputum of children with severe therapy resistant asthma compared to children with control children who didn’t have asthma153.
A study on the effects of rhinovirus infection in asthmatic patients found evidence for the activation of ILC2s by rhinovirus infection154. That study showed that in vitro infection of human primary lung epithelial cells caused production of IL-33 by these cells. They found that the amount of IL-33 in the airways of asthmatic patients infected with rhinovirus positively correlated with the viral load. They also showed that rhinovirus infection increases the amount of IL-4, IL-5 and IL-13 in the airways of asthmatic patients154.
Allergen-specific IgE are produced in allergic asthmatic patients. A recent study provides evidence that ILC2s can contribute to the production of IgE by B cells155. That study showed that upon stimulation with IL-25 or IL-33, human peripheral ILC2s can enhance IgE production from autologous B cells through expression of CD154 and IL-4 production in vitro155. The functional significance of ILC2s for IgE production by B cells in allergic and asthmatic patients is unknown.
ILC2s in pulmonary fibrosis
Idiopathic pulmonary fibrosis is a serious chronic fibrosing interstitial pneumonia due to unknown causes156. Experimental evidence suggests that ILC2s play a role in the pathogenesis of pulmonary fibrosis by producing IL-13157, 158. Bleomycin-induced pulmonary fibrosis in mice was found to be associated with increased pulmonary amounts of IL-25 in one study and IL-33 in another study157, 158. The first study found that IL-25 receptor and the second showed that IL-33 receptor deficiencies were associated with reduced bleomycin-induced fibrosis in the lungs of mice157, 158. Both studies showed that activation of ILC2s and their IL-13 production play a major role in the observed pathology.
Atopic dermatitis
The first evidence for contribution of ILC2s to the pathogenesis of atopic dermatitis (AD) in human comes from a study that showed ILC2s reside in the human skin and their number is increased in AD patients compare to healthy individuals20. This study showed that ILC2s are recruited to the skin of HDM-sensitized patients upon skin allergen challenge and that skin ILC2s produce large quantities of IL-5 and IL-13 in response to ex vivo stimulation with IL-3320. In mice, dermal ILC2s were identified and it was shown that that skin specific expression of IL-33 activates skin ILC2s and causes AD-like pathology in the skin of mice159, 160. Loss of filaggrin is a cardinal feature in AD. Interestingly, it has been shown that filaggrin mutant mice spontaneously develop AD which was mediated by IL-5 producing ILC2s in the skin and independent of adaptive immunity161. The effects of tolerance on ILC2s are mainly unknown, however, a recent study showed that oral tolerance inhibited the type-2 inflammation including ILC2s in an ovalbumin-driven-AD model in mice162.
Beneficial effects of ILC2s in immunity
Anti-helminth immunity
Anti-helminth immunity is perhaps one of the most studied beneficial effects of ILC2s. Most of our understanding about the role of ILC2s in anti-helminth immunity comes from the experimental infections with nematodes such as Nippostrongylus brasiliensis in mice. In fact, pioneer studies that identified ILC2s addressed a crucial for these cells in expulsion of N. brasiliensis6, 21. These studies showed that the worm infection causes activation of intestinal ILC2s by IL-25 and IL-33 which in turn produce IL-13 and clear N. brasiliensis6, 21. Several subsequent studies provided further evidence that at least murine ILC2s play vital roles in the clearance of intestinal nematodes39, 40, 163. The source of IL-25 production and activation of ILC2s upon nematode infection was recently found to be intestinal Tuft cells which contribute to the homeostasis of ILC2s in the intestines at the steady state164. In addition to IL-13 production, ILC2s can contribute to worm expulsion by potentiating Th2 response through production of IL-4 and MHC-II-mediated interplay with T helper cells140, 141. It has been suggested that IL-25 induces a subset of IL-25-responsive ILC2s that they further develop into IL-33 responsive ILC2s and clear intestinal N. brasiliensis infection26.
Intestinal helminth infections cause micro malnutrition, such as vitamin A, and it is important that ILC2s maintain their functional efficiency during malnutrition because of their critical role in anti-helminth immunity. Studies have shown that ILC2s are predominantly dependent of fatty acid metabolism and are less sensitive to vitamin A deficiency during helminth infections80, 165.
Helminth infections have been associated with protection against allergic diseases166, 167. Interestingly, it has been reported that administration of secreted product by nematode Heligmosomoides polygyrus inhibited ILC2 and Th2 mediated allergic lung inflammation by suppressing IL-33 production168.
Role of ILC2s in adipose tissue and obesity
One of the initial reports that identified ILC2s described these cells as adipose tissue-associated lineage-negative c-Kit+Sca-1+ lymphoid cells5. Following studies not only isolated ILC2s from murine adipose tissues but also found that ILC2-derived IL-5 sustain eosinophils in the adipose tissue169, 170. Adipose tissue consists of white or beige adipocytes. White adipocytes store the excess energy in the form of lipids and expand in response to positive energy balance causing obesity171. In contrast, beige adipocytes dissipate energy for thermogenesis and maintaining body temperature171. Conversion of white adipose tissue (WAT) to beige adipose tissue (BAT) is considered beneficial to limit obesity. The low-grade chronic inflammation associated with obesity is an underling mechanism for development of diabetes and cardiovascular diseases. It has been shown that IL-25 activated ILC2s increase the number of eosinophils and alternatively activated macrophages in the adipose tissue and lead to weight loss and improve glucose tolerance in obese mice169. Subsequent studies showed that activation of ILC2s can promote the conversion of WAT to BAT in the so-called “process of browning”172, 173. These studies showed that ILC2s promote generation of beige adipocytes independent of adaptive immunity. However, one study showed a role for eosinophils and IL-4 receptor for stimulating the proliferation of bipotential adipocyte precursor to potentiate the generation of beige adipocytes173. The other study found that ILC2s produce methionine-enkephalin peptides that directly induces the beige adipocyte-associated uncoupling protein 1 independent of IL-4 or eosinophils172. The findings of these studies may a suggest multiple pathways by which ILC2s can contribute to browning of the WAT and limiting obesity.
There is an interesting interplay between asthma and obesity. Asthma can lead to obesity and obesity can cause asthma through incompletely understood mechanisms. Although, obesity-associated asthma is distinct from allergic asthma, evidence suggests that obesity plays an important role in asthma exacerbations174, 175. A recent study using diet-induced obesity has shown that obesity leads to increased production of IL-5 by ILC2s that caused elevated number of eosinophils in the lungs176. This study showed that intranasal HDM exposure causes a higher level of AHR and airway eosinophilic inflammation in obese than lean mice176.
Tissue repair
Different lines of evidence from murine studies suggest that ILC2s are involved in the process of tissue repair. In murine models of infection with helminth, N. brasiliensis, ILC2s were found to alleviate the lung tissue damage during the pulmonary transition stages of the worm31, 32. Mechanistically, production of IL-13 by ILC2s was associated with expression of genes such as Muc5a, Clca3, and Tff2, in lung epithelial cells, however, none of the suggested genes were functionally examined31. ILC2s were found to play a role in cutaneous wound healing in a mouse model of splinted excisional wound177. Depletion of ILC2s in an influenza experimental infection was found to aggravate the loss of epithelial integrity cause by IAV in mice178. This study found that ILC2s were enriched in genes associated with wound healing and identified Amphiregulin as a mechanism underlying ILC2-mediated regaining epithelial integrity178. Same group has also shown that ILC2s play a protective role in dextran sulfate sodium (DSS)-induced colitis and alleviate its intestinal pathology in mice through Amphiregulin179.
Plasticity of ILC2s
As mentioned earlier, innate lymphoid cells are terminally differentiated cells and their cytokine and transcription factor signature identifies the three subsets of ILC1, ILC2 and ILC3 resembling the Th1, Th2 and Th17 CD4+ T helper cells. Evidence indicates that some differentiated subsets of CD4+ T helper cells can show the cytokine profile of other subsets under certain stimulatory conditions and display a capacity for so-called “T cell plasticity”62. Given the similarity between innate lymphoid cells and CD4+ T helper cells, it is intriguing to know whether innate lymphoid cells have plasticity capacities. Plasticity of innate lymphoid cells can be defined by the capacity of any subset of innate lymphoid cells to produce the signature cytokines of other subsets. There are, however, fundamental differences between development of differentiated CD4+ T helper cells and innate lymphoid cells. T helper cells are generated in thymus as mature naïve cells and differentiate into Th1, Th2 and Th17 cells in response to T cell receptor stimulation, co-stimulatory signal and cytokine milieu in secondary lymphoid organs. However, differentiation of ILC1, ILC2 and ILC3 occur as a part of their development. As discussed earlier priming towards different subsets of innate lymphoid cells occur in early development stages.
Nevertheless, several lines of evidence indicate that human and murine ILC2s show plasticity towards IFN-γ producing ILC1s18, 180, 181. The first line of evidence showed a partial loss of CRTH2 in in vitro expanded human peripheral ILC2s four days after adoptive transfer to NSG mice, which lack B and T cells and innate lymphoid cells18. It was further shown that human ILC2s downregulated CRTH2 in response to IFN-γ18. This study found that activation of human ILC2s by IL-1β primes these cells to respond to type 1 cytokine IL-12 by downregulating CRTH2 and producing IFN-γ18. ILC2 responsiveness to IL-12 was inhibited by IL-4 and it was suggested that eosinophil-derived IL-4 enforces ILC2 phenotype in a cross-talk between ILC2s and eosinophils18. A different study found a role for basophil-derived IL-4 in controlling the function of ILC2s in murine model of lung inflammation36.
The second line of evidence found that a proportion of human purified ILC2s co-produce IFN-γ with IL-13 in vitro only when cultured in the presence of stromal cells180. They found that plastic clones of human ILC2s co-express ILC1 transcription factor T-bet180. It was observed that ILC2s from IL-12Rβ1-deficient donors do no show plasticity in vitro and it was concluded that the plasticity of ILC2s towards ILC1-like cells was mediated by IL-12180. A different study provides similar evidence for plasticity of human and murine ILC2s towards ILC1s181. This study also showed that influenza infection causes loss of Gata-3 and gain of T-bet by murine ILC2s, and production of IFN-γ by these cells which contributed to antiviral immunity181. Although these findings provide interesting bases for understanding plasticity of ILC2s, they are in clear contrast with prior findings that influenza infection causes activation of murine ILC2s and induces IL-5 and IL-13 production by these cells123, 147, 178. Further studies are required to identify the mechanisms of regulation of ILC2s under infectious and inflammatory conditions.
Deficiency in Bcl11b has been shown to developmentally cause ILC3-like phenotype in ILC2s110–112. A recent study suggests that Notch signaling causes a ILC3-like phenotype in a subset of ILC2s and promotes co-production of IL-17 with IL-13 in these cells182. Although these findings seem to be interesting, the study heavily relies on the expression level of KLRG-1 for defining ILC2s182. Without evaluating the expression level of IL-33R, IL-2Rα or IL-25R it is unclear whether the described cells can be categorized as true ILC2s.
Conclusions / Concluding remarks
ILC2s are a significant subset of immune cells that play important roles in immunity and the pathogenesis of mucosal inflammatory diseases. These cells are found in the skin, lungs, gastrointestinal tract, peripheral blood and even in secondary lymphoid organs in humans and mice. Despite their low number, ILC2s respond rapidly to mucosal danger signals by robust proliferation and cytokine production. ILC2s closely mimic cytokine production profile of Th2 cells and murine studies have provided ample evidence that ILC2s are crucial for anti-helminth immunity as well as for the resolution of mucosal tissue damage especially during resolution of influenza infection. Although, one recent study has suggested an overlapping function for human ILC2s in protective immunity183, the important role of ILC2s in the pathogenesis of allergic diseases and asthma should not be under estimated. As discussed, a large body of evidence from human and murine studies indicates that ILC2s play crucial roles in the pathogenesis of allergic diseases and asthma and associated with virus-associated asthma exacerbations. ILC2s can directly and rapidly induce allergic manifestation while priming the induction of Th2 cells. ILC2s may be the missing link in understanding the pathogenesis of human diseases with the bases of type 2 immune responses. Targeting ILC2s in the next generation therapeutic strategies for type 2 immune related disease may offer more efficient and personalized treatment regimens for such diseases.
Acknowledgments
We would like to thank Dr. Hirohito Kita for kindly reviewing our manuscript prior to submission. This article was financially supported by National Institutes of Health public health service grants R01 ES025786, R01 ES021801, R21 ES024707, R21 AI109059 (O.A.). H.M. is supported by American Heart Association postdoctoral fellowship #16POST27770125.
Footnotes
Conflict of interest: Authors declare that they do not have any conflict of interest
References
- 1.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 2.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 3.Reese TA, Liang HE, Tager AM, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 6.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawa S, Cherrier M, Lochner M, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 8.Bernink JH, Peters CP, Munneke M, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs A, Vermi W, Lee JS, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teunissen MB, Munneke JM, Bernink JH, et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol. 2014;134:2351–2360. doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- 11.Villanova F, Flutter B, Tosi I, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014;134:984–991. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini N, Klein Wolterink RG, Satoh-Takayama N, et al. Gata3 drives development of RORgammat+ group 3 innate lymphoid cells. J Exp Med. 2014;211:199–208. doi: 10.1084/jem.20131038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyasu S, Moro K, Tanabe M, Takeuchi T. Natural helper cells: a new player in the innate immune response against helminth infection. Adv Immunol. 2010;108:21–44. doi: 10.1016/B978-0-12-380995-7.00002-1. [DOI] [PubMed] [Google Scholar]
- 14.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 15.Maazi H, Patel N, Sankaranarayanan I, et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seillet C, Mielke LA, Amann-Zalcenstein DB, et al. Deciphering the Innate Lymphoid Cell Transcriptional Program. Cell Rep. 2016;17:436–447. doi: 10.1016/j.celrep.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Simoni Y, Fehlings M, Kloverpris HN, et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2016 doi: 10.1016/j.immuni.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Bal SM, Bernink JH, Nagasawa M, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 19.Simmerman E, Qin X, Marshall B, et al. Innate lymphoid cells: a paradigm for low SSI in cleft lip repair. J Surg Res. 2016;205:312–317. doi: 10.1016/j.jss.2016.06.081. [DOI] [PubMed] [Google Scholar]
- 20.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gury-BenAri M, Thaiss CA, Serafini N, et al. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166:1231–1246.e1213. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Robinette ML, Fuchs A, Cortez VS, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HS, Jang JH, Lee MB, et al. A novel IL-10-producing innate lymphoid cells (ILC10) in a contact hypersensitivity mouse model. BMB Rep. 2016;49:293–296. doi: 10.5483/BMBRep.2016.49.5.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow JL, Peel S, Fox J, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Guo L, Qiu J, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saenz SA, Siracusa MC, Monticelli LA, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licona-Limon P, Henao-Mejia J, Temann AU, et al. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39:744–757. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerzerho J, Maazi H, Speak AO, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131:1048–1057. 1057 e1041–1042. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelm C, Hirota K, Stieglitz B, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner JE, Morrison PJ, Wilhelm C, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol. 2016;9:275–286. doi: 10.1038/mi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moretti S, Renga G, Oikonomou V, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. 2017;8:14017. doi: 10.1038/ncomms14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roediger B, Kyle R, Tay SS, et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol. 2015;136:1653–1663. e1651–1657. doi: 10.1016/j.jaci.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 35.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–678. e674. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motomura Y, Morita H, Moro K, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Matsuki A, Takatori H, Makita S, et al. T-bet inhibits innate lymphoid cell-mediated eosinophilic airway inflammation by suppressing IL-9 production. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, Pappu R, Ramirez-Carrozzi V, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–740. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meylan F, Hawley ET, Barron L, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7:958–968. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35:284–292. doi: 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901. e893. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojno ED, Monticelli LA, Tran SV, et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8:1313–1323. doi: 10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laidlaw TM, Boyce JA. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin Exp Allergy. 2012;42:1313–1320. doi: 10.1111/j.1365-2222.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee TH, Woszczek G, Farooque SP. Leukotriene E4: perspective on the forgotten mediator. J Allergy Clin Immunol. 2009;124:417–421. doi: 10.1016/j.jaci.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Duroudier NP, Strachan DP, Blakey JD, Hall IP. Association of the cysteinyl leukotriene receptor 1 gene with atopy in the British 1958 birth cohort. J Allergy Clin Immunol. 2009;124:566–572. 572 e561–563. doi: 10.1016/j.jaci.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salimi M, Stoger L, Liu W, et al. Cysteinyl leukotriene E4 activates human ILC2s and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.12.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamachi F, Isshiki T, Harada N, Akiba H, Miyake S. ICOS promotes group 2 innate lymphoid cell activation in lungs. Biochem Biophys Res Commun. 2015;463:739–745. doi: 10.1016/j.bbrc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Paclik D, Stehle C, Lahmann A, Hutloff A, Romagnani C. ICOS regulates the pool of group 2 innate lymphoid cells under homeostatic and inflammatory conditions in mice. Eur J Immunol. 2015;45:2766–2772. doi: 10.1002/eji.201545635. [DOI] [PubMed] [Google Scholar]
- 52.Molofsky AB, Van Gool F, Liang HE, et al. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maazi H, Akbari O. ICOS regulates ILC2s in asthma. Oncotarget. 2015;6:24584–24585. doi: 10.18632/oncotarget.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hedl M, Lahiri A, Ning K, Cho JH, Abraham C. Pattern recognition receptor signaling in human dendritic cells is enhanced by ICOS ligand and modulated by the Crohn’s disease ICOSLG risk allele. Immunity. 2014;40:734–746. doi: 10.1016/j.immuni.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madouri F, Chenuet P, Beuraud C, et al. Protein kinase Ctheta controls type 2 innate lymphoid cell and TH2 responses to house dust mite allergen. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 56.Krishnamoorthy N, Burkett PR, Dalli J, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194:863–867. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rigas D, Lewis G, Aron JL, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coomes SM, Pelly VS, Kannan Y, et al. IFNgamma and IL-12 Restrict Th2 Responses during Helminth/Plasmodium Co-Infection and Promote IFNgamma from Th2 Cells. PLoS Pathog. 2015;11:e1004994. doi: 10.1371/journal.ppat.1004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mocci S, Coffman RL. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J Immunol. 1995;154:3779–3787. [PubMed] [Google Scholar]
- 60.Tassi E, Braga M, Longhi R, et al. Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PLoS One. 2009;4:e7234. doi: 10.1371/journal.pone.0007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams CL, Schilling MM, Cho SH, et al. STAT4 and T-bet are required for the plasticity of IFN-gamma expression across Th2 ontogeny and influence changes in Ifng promoter DNA methylation. J Immunol. 2013;191:678–687. doi: 10.4049/jimmunol.1203360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 63.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 64.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duerr CU, McCarthy CD, Mindt BC, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17:65–75. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moro K, Kabata H, Tanabe M, et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17:76–86. doi: 10.1038/ni.3309. [DOI] [PubMed] [Google Scholar]
- 67.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sweeney CM, Lonergan R, Basdeo SA, et al. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;25:1170–1181. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Zhou W, Toki S, Zhang J, et al. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. Am J Respir Crit Care Med. 2016;193:31–42. doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simons B, Ferrini ME, Carvalho S, Bassett DJ, Jaffar Z, Roberts K. PGI2 Controls Pulmonary NK Cells That Prevent Airway Sensitization to House Dust Mite Allergen. J Immunol. 2017;198:461–471. doi: 10.4049/jimmunol.1600275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bando JK, Liang HE, Locksley RM. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol. 2015;16:153–160. doi: 10.1038/ni.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bando JK, Nussbaum JC, Liang HE, Locksley RM. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J Leukoc Biol. 2013;94:877–884. doi: 10.1189/jlb.0213084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monticelli LA, Buck MD, Flamar AL, et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol. 2016;17:656–665. doi: 10.1038/ni.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 76.Cooke A, Fergeson J, Bulkhi A, Casale TB. The Electronic Cigarette: The Good, the Bad, and the Ugly. J Allergy Clin Immunol Pract. 2015;3:498–505. doi: 10.1016/j.jaip.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 77.Kearley J, Silver JS, Sanden C, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity. 2015;42:566–579. doi: 10.1016/j.immuni.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Galle-Treger L, Suzuki Y, Patel N, et al. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat Commun. 2016;7:13202. doi: 10.1038/ncomms13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veldhoen M, Ferreira C. Influence of nutrient-derived metabolites on lymphocyte immunity. Nat Med. 2015;21:709–718. doi: 10.1038/nm.3894. [DOI] [PubMed] [Google Scholar]
- 80.Spencer SP, Wilhelm C, Yang Q, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim MH, Taparowsky EJ, Kim CH. Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut. Immunity. 2015;43:107–119. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez-Gonzalez I, Matha L, Steer CA, Ghaedi M, Poon GF, Takei F. Allergen-Experienced Group 2 Innate Lymphoid Cells Acquire Memory-like Properties and Enhance Allergic Lung Inflammation. Immunity. 2016;45:198–208. doi: 10.1016/j.immuni.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 83.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bjorklund AK, Forkel M, Picelli S, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 85.Klose CS, Flach M, Mohle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 86.Zhong C, Cui K, Wilhelm C, et al. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat Immunol. 2016;17:169–178. doi: 10.1038/ni.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Constantinides MG, Gudjonson H, McDonald BD, et al. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci U S A. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu X, Wang Y, Deng M, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife. 2014:3. doi: 10.7554/eLife.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baerenwaldt A, von Burg N, Kreuzaler M, et al. Flt3 Ligand Regulates the Development of Innate Lymphoid Cells in Fetal and Adult Mice. J Immunol. 2016;196:2561–2571. doi: 10.4049/jimmunol.1501380. [DOI] [PubMed] [Google Scholar]
- 90.Chea S, Schmutz S, Berthault C, et al. Single-Cell Gene Expression Analyses Reveal Heterogeneous Responsiveness of Fetal Innate Lymphoid Progenitors to Notch Signaling. Cell Rep. 2016;14:1500–1516. doi: 10.1016/j.celrep.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 91.Scoville SD, Mundy-Bosse BL, Zhang MH, et al. A Progenitor Cell Expressing Transcription Factor RORgammat Generates All Human Innate Lymphoid Cell Subsets. Immunity. 2016;44:1140–1150. doi: 10.1016/j.immuni.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seehus CR, Aliahmad P, de la Torre B, et al. The development of innate lymphoid cells requires TOX-dependent generation of a common innate lymphoid cell progenitor. Nat Immunol. 2015;16:599–608. doi: 10.1038/ni.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geiger TL, Abt MC, Gasteiger G, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211:1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seillet C, Rankin LC, Groom JR, et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014;211:1733–1740. doi: 10.1084/jem.20140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu W, Domingues RG, Fonseca-Pereira D, et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep. 2015;10:2043–2054. doi: 10.1016/j.celrep.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 97.Yang Q, Monticelli LA, Saenz SA, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mielke LA, Groom JR, Rankin LC, et al. TCF-1 controls ILC2 and NKp46+RORgammat+ innate lymphocyte differentiation and protection in intestinal inflammation. J Immunol. 2013;191:4383–4391. doi: 10.4049/jimmunol.1301228. [DOI] [PubMed] [Google Scholar]
- 99.Yang Q, Li F, Harly C, et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol. 2015;16:1044–1050. doi: 10.1038/ni.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verhoef PA, Constantinides MG, McDonald BD, Urban JF, Jr, Sperling AI, Bendelac A. Intrinsic functional defects of type 2 innate lymphoid cells impair innate allergic inflammation in promyelocytic leukemia zinc finger (PLZF)-deficient mice. J Allergy Clin Immunol. 2016;137:591–600. e591. doi: 10.1016/j.jaci.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishizuka IE, Chea S, Gudjonson H, et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol. 2016;17:269–276. doi: 10.1038/ni.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y, Tsang JC, Wang C, et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature. 2016;539:102–106. doi: 10.1038/nature20105. [DOI] [PubMed] [Google Scholar]
- 103.Furusawa J, Moro K, Motomura Y, et al. Critical role of p38 and GATA3 in natural helper cell function. J Immunol. 2013;191:1818–1826. doi: 10.4049/jimmunol.1300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoyler T, Klose CS, Souabni A, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Klein Wolterink RG, Serafini N, van Nimwegen M, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2013;110:10240–10245. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yagi R, Zhong C, Northrup DL, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Meer LT, Jansen JH, van der Reijden BA. Gfi1 and Gfi1b: key regulators of hematopoiesis. Leukemia. 2010;24:1834–1843. doi: 10.1038/leu.2010.195. [DOI] [PubMed] [Google Scholar]
- 109.Spooner CJ, Lesch J, Yan D, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat Immunol. 2013;14:1229–1236. doi: 10.1038/ni.2743. [DOI] [PubMed] [Google Scholar]
- 110.Califano D, Cho JJ, Uddin MN, et al. Transcription Factor Bcl11b Controls Identity and Function of Mature Type 2 Innate Lymphoid Cells. Immunity. 2015;43:354–368. doi: 10.1016/j.immuni.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walker JA, Oliphant CJ, Englezakis A, et al. Bcl11b is essential for group 2 innate lymphoid cell development. J Exp Med. 2015;212:875–882. doi: 10.1084/jem.20142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu Y, Wang C, Clare S, et al. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J Exp Med. 2015;212:865–874. doi: 10.1084/jem.20142318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Zook EC, Ramirez K, Guo X, et al. The ETS1 transcription factor is required for the development and cytokine-induced expansion of ILC2. J Exp Med. 2016;213:687–696. doi: 10.1084/jem.20150851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Antignano F, Braam M, Hughes MR, et al. G9a regulates group 2 innate lymphoid cell development by repressing the group 3 innate lymphoid cell program. J Exp Med. 2016;213:1153–1162. doi: 10.1084/jem.20151646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeiger RS, Yegin A, Simons FE, et al. Evaluation of the National Heart, Lung, and Blood Institute guidelines impairment domain for classifying asthma control and predicting asthma exacerbations. Ann Allergy Asthma Immunol. 2012;108:81–87. doi: 10.1016/j.anai.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 118.Fitzpatrick AM, Teague WG, Meyers DA, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389. e381–313. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Apter AJ. Advances in adult asthma diagnosis and treatment in 2009. J Allergy Clin Immunol. 2010;125:79–84. doi: 10.1016/j.jaci.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim HY, Chang YJ, Subramanian S, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. e211–216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klein Wolterink RG, Kleinjan A, van Nimwegen M, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 123.Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong JY, Bentley JK, Chung Y, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134:429–439. doi: 10.1016/j.jaci.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iijima K, Kobayashi T, Hara K, et al. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193:1549–1559. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy. 2016;71:977–988. doi: 10.1111/all.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pichery M, Mirey E, Mercier P, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 129.Agoro R, Piotet-Morin J, Palomo J, et al. IL-1R1-MyD88 axis elicits papain-induced lung inflammation. Eur J Immunol. 2016;46:2531–2541. doi: 10.1002/eji.201646366. [DOI] [PubMed] [Google Scholar]
- 130.Van Dyken SJ, Garcia D, Porter P, et al. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Piehler D, Eschke M, Schulze B, et al. The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal Immunol. 2016;9:937–949. doi: 10.1038/mi.2015.106. [DOI] [PubMed] [Google Scholar]
- 132.Tashiro H, Takahashi K, Hayashi S, et al. Interleukin-33 from Monocytes Recruited to the Lung Contributes to House Dust Mite-Induced Airway Inflammation in a Mouse Model. PLoS One. 2016;11:e0157571. doi: 10.1371/journal.pone.0157571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stier MT, Bloodworth MH, Toki S, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gold MJ, Antignano F, Halim TY, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133:1142–1148. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 136.Halim TY, Hwang YY, Scanlon ST, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Drake LY, Iijima K, Bartemes K, Kita H. Group 2 Innate Lymphoid Cells Promote an Early Antibody Response to a Respiratory Antigen in Mice. J Immunol. 2016;197:1335–1342. doi: 10.4049/jimmunol.1502669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 139.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oliphant CJ, Hwang YY, Walker JA, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pelly VS, Kannan Y, Coomes SM, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kamijo S, Takeda H, Tokura T, et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 2013;190:4489–4499. doi: 10.4049/jimmunol.1201212. [DOI] [PubMed] [Google Scholar]
- 143.Denney L, Byrne AJ, Shea TJ, et al. Pulmonary Epithelial Cell-Derived Cytokine TGF-beta1 Is a Critical Cofactor for Enhanced Innate Lymphoid Cell Function. Immunity. 2015;43:945–958. doi: 10.1016/j.immuni.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Kleer IM, Kool M, de Bruijn MJ, et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity. 2016;45:1285–1298. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 145.Yang Q, Ge MQ, Kokalari B, et al. Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2016;137:571–578. doi: 10.1016/j.jaci.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumagai K, Lewandowski R, Jackson-Humbles DN, et al. Ozone-Induced Nasal Type 2 Immunity in Mice Is Dependent on Innate Lymphoid Cells. Am J Respir Cell Mol Biol. 2016;54:782–791. doi: 10.1165/rcmb.2015-0118OC. [DOI] [PubMed] [Google Scholar]
- 147.Gorski SA, Hahn YS, Braciale TJ. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS Pathog. 2013;9:e1003615. doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lynch JP, Werder RB, Simpson J, et al. Aeroallergen-induced IL-33 predisposes to respiratory virus-induced asthma by dampening antiviral immunity. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 149.Fan D, Wang X, Wang M, et al. Allergen-Dependent Differences in ILC2s Frequencies in Patients With Allergic Rhinitis. Allergy Asthma Immunol Res. 2016;8:216–222. doi: 10.4168/aair.2016.8.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86. e78. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 151.Ho J, Bailey M, Zaunders J, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394–403. doi: 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- 152.Miljkovic D, Bassiouni A, Cooksley C, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–1161. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 153.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624–626. e626. doi: 10.1016/j.jaci.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 154.Jackson DJ, Makrinioti H, Rana BM, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maggi L, Montaini G, Mazzoni A, et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 156.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hams E, Armstrong ME, Barlow JL, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111:367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li D, Guabiraba R, Besnard AG, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134:1422–1432. e1411. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Imai Y, Yasuda K, Sakaguchi Y, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saunders SP, Moran T, Floudas A, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J Allergy Clin Immunol. 2016;137:482–491. doi: 10.1016/j.jaci.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baek JO, Roh JY, Jung Y. Oral tolerance inhibits atopic dermatitis-like type 2 inflammation in mice by modulating immune microenvironments. Allergy. 2016 doi: 10.1111/all.12960. [DOI] [PubMed] [Google Scholar]
- 163.Patel N, Wu W, Mishra PK, et al. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe. 2014;15:339–350. doi: 10.1016/j.chom.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 164.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilhelm C, Harrison OJ, Schmitt V, et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J Exp Med. 2016;213:1409–1418. doi: 10.1084/jem.20151448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smits HH, Hartgers FC, Yazdanbakhsh M. Helminth infections: protection from atopic disorders. Curr Allergy Asthma Rep. 2005;5:42–50. doi: 10.1007/s11882-005-0053-5. [DOI] [PubMed] [Google Scholar]
- 167.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 168.McSorley HJ, Blair NF, Smith KA, McKenzie AN, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–1078. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Molofsky AB, Nussbaum JC, Liang HE, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev. 2012;13(Suppl 2):83–96. doi: 10.1111/j.1467-789X.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- 172.Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee MW, Odegaard JI, Mukundan L, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rastogi D, Canfield SM, Andrade A, et al. Obesity-associated asthma in children: a distinct entity. Chest. 2012;141:895–905. doi: 10.1378/chest.11-0930. [DOI] [PubMed] [Google Scholar]
- 175.Okubo Y, Nochioka K, Hataya H, Sakakibara H, Terakawa T, Testa M. Burden of Obesity on Pediatric Inpatients with Acute Asthma Exacerbation in the United States. J Allergy Clin Immunol Pract. 2016;4:1227–1231. doi: 10.1016/j.jaip.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 176.Everaere L, Ait-Yahia S, Molendi-Coste O, et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 177.Rak GD, Osborne LC, Siracusa MC, et al. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J Invest Dermatol. 2016;136:487–496. doi: 10.1038/JID.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lim AI, Menegatti S, Bustamante J, et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213:569–583. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Silver JS, Kearley J, Copenhaver AM, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17:626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang K, Xu X, Asghar Pasha M, et al. Cutting Edge: Notch Signaling Promotes the Plasticity of Group-2 Innate Lymphoid Cells. J Immunol. 2017 doi: 10.4049/jimmunol.1601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vely F, Barlogis V, Vallentin B, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016;17:1291–1299. doi: 10.1038/ni.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]