Abstract
Background
The majority of studies evaluating neurocognition in humans who had procedures under anesthesia early in life found long-term deficits even though the typical anesthesia duration normalized to the human life span is much shorter than that shown to induce developmental abnormalities in rodents. Therefore, we studied whether subsequent environmental stressors contribute to deficiencies programmed by a brief neonatal etomidate exposure.
Methods
Postnatal days (P) 4, 5, or 6, Sprague-Dawley rats, pretreated with vehicle or the Na+-K+-2Cl− (NKCC1) inhibitor, bumetanide, received two injections of etomidate resulting in anesthesia for 2 h. To simulate stress after anesthesia, the animals were exposed to a single maternal separation for 3 h at P10. 3–7 days after exposure to etomidate the rats had increased hypothalamic NKCC1 mRNA and corticotropin releasing hormone (CRH) mRNA and decreased K+-2Cl− (KCC2) mRNA levels with greater changes in males. In rats neonatally exposed to both etomidate and maternal separation, these abnormalities persisted into adulthood. These animals also exhibited extended corticosterone responses to restraint stress with increases in total plasma corticosterone more robust in males, as well as behavioral abnormalities. Pretreatment with the NKCC1 inhibitor ameliorated most of these effects.
Conclusions
Post-anesthesia stressors may exacerbate/unmask neurodevelopmental abnormalities even after a relatively short anesthetic with etomidate, leading to dysregulated stress response systems and neurobehavioral deficiencies in adulthood. Amelioration by bumetanide suggests a mechanistic role for etomidate-enhanced gamma-aminobutyric acid type A receptor-mediated depolarization in initiating long-lasting alterations in gene expression that are further potentiated by subsequent maternal separation.
Keywords: Etomidate, Maternal separation, Environmental factor, Developing brain, Behavior, Stress
1. Introduction
One and a half million neonates and infants are exposed to general anesthesia annually in the United States alone (Servick, 2014). Most retrospective epidemiological studies of patients, who had medical procedures requiring general anesthesia before 1–4 years of age, found significant subsequent neurocognitive deficiencies in late childhood or adolescence (for review see Creeley, 2016). The possibility of anesthetic-induced developmental abnormalities is strongly supported by the results of numerous laboratory studies of healthy animals, though the full range of neonatal anesthesia-induced changes and underlying mechanisms remain poorly understood even in animal models (for review see Walters and Paule, 2016). Many factors influence the developmental outcome of anesthetic exposure in animals, one of which is the duration of anesthesia. Given that life expectancy in humans is 20–35 times longer than in rodents, the typical anesthesia duration in humans, normalized to the species’ life span, is much shorter than that shown to induce developmental abnormalities in rodents (Stratmann et al., 2009). This observation suggests that additional factors contribute to developmental abnormalities initially programmed by early life exposures to general anesthetics.
We recently demonstrated that relatively long (5–6 h) exposures of neonatal rats to either sevoflurane or propofol, two different anesthetics that share the ability to enhance gamma-aminobutyric acid type A receptor (GABAAR) activity, induced long-term neurobehavioral and neuroendocrine abnormalities, including exacerbated endocrine responses to stress in adulthood (Tan et al., 2014; Xu et al., 2015). These abnormalities were reminiscent of those induced by excessive postnatal stress, such as repeated maternal separations throughout the first weeks of life (Patchev et al., 1997; Brunson et al., 2005), suggesting that the developmental effects of anesthesia and environmental stressors early in life share similar underlying mechanisms and that they may interact to induce a combined effect. The combined effect of early life exposure to general anesthesia and environmental stressors may be of special translational relevance because many infants, who require general anesthesia, are inevitably exposed in their lives to environmental stressors, such as diseases, pain, or psychosocial stress. Given the frequency of anesthesia in the youngest patients, elucidating how GABAergic anesthetics predispose to dysregulated stress responses is of even broader translational significance, because dysregulated stress responses were linked to a number of developmental neurocognitive and neuropsychiatric disorders (Sanchez et al., 2001).
To investigate the developmental effects of a combination of a relatively short exposure to general anesthesia and a subsequent environmental stressor that may not be sufficient to induce developmental abnormalities by themselves, we exposed neonatal rats to 2 h anesthesia with etomidate and then to a single maternal separation. To investigate the role of etomidate-induced enhancement of GABAAR-mediated depolarizing/stimulatory signaling in the developmental effects, a subgroup received the Na+-K+-2Cl− (NKCC1) transporter inhibitor, bumetanide, prior to administration of etomidate. Bumetanide exhibited promising therapeutic effects not only in animal models, but also in human studies of diseases, such as autism spectrum disorders (ASD), schizophrenia, Rett syndrome, and others, in which abnormal GABAAR-mediated depolarization - determined by an increased NKCC1/ K+-2Cl− (KCC2) ratio - may play an etiological role (Cellot and Cherubini, 2014; Tyzio et al., 2014; Lemonnier et al., 2013; Lemonnier et al., 2016). Similarly, we found a protective effect of bumetanide against the developmental effects of GABAergic anesthetics (Xu et al., 2015). Etomidate, despite its infrequent use in pediatric anesthesia, possesses ideal properties for the initial testing of this hypothesis. Like propofol, etamidate selectively enhances GABAAR activity, but unlike propofol disrupts the adrenal synthesis of corticosterone by inhibiting 11-β-hydroxylase (Vanlersberg and Camu, 2008). Because of these combined actions, etomidate may induce greater activation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis in neonatal rats by combining direct etomidate-induced enhancement of GABAAR-mediated depolarization/stimulation in the hypothalamus and reduced negative feedback because of inhibited synthesis of adrenal corticosterone. In addition, the etomidate-caused inhibition of 11-β-hydroxylase increases substrate availability for synthesis of GABAergic neuroactive steroids, which by enhancing GABAAR-mediated depolarization/stimulation in the hypothalamus of neonatal rats may further increase production of corticotropin-releasing hormone (CRH) (Kaminski and Rogawski MA, 2001). An increase in CRH production is not only a crucial initial step in the activation of the LHPA axis by stress, but may also contribute to a number of neurodevelopmental abnormalities by acting outside of the LHPA axis (Toth et al., 2014; Flandreau et al., 2015; Zhang et al., 2016).
2. Methods and Materials
2.1. Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Sprague-Dawley rats were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (23–24 °C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched groups of two for the rest of the study. To control for litter variability, pups from the same litter were used for different treatment conditions. Multiple sets of animals were used in the experiments. The data reported in this study were collected from 109 male and 111 female rats.
2.2. Treatment groups
Two cohorts of animals were studied. Neonatal rats in cohort one (5 animals per sex, per treatment, and per time period) were used for gene expression studies to determine etomidate-induced changes immediately and 3–7 days after exposure to etomidate, the time period at which maternal separation was administered (Fig. S1, Table S1). Rats in cohort two (15–17 animals per sex and per treatment) were used for behavioral and neuroendocrine studies, as well as for gene expression measurements in adulthood (Fig. S1, Table S2). Separate animals were used in Cohort 1 and 2. During etomidate anesthesia at P4, P5 or P6 or maternal separation at P10 male and female rat pups were kept in a temperature-controlled chamber (+37 °C) with a continuous supply of 30% oxygen in air. Gas monitoring was performed using a calibrated Datex side stream analyzer (Datex-Ohmeda, Helsinki, Finland). The Etomidate (ET) group received 8 mg/kg of etomidate intraperitoneally (IP) for induction of anesthesia followed by second injection of etomidate (4 mg/kg, IP) 50 min after the first administration. These two doses of etomidate together were sufficient to anesthetize the pups for 2 h, the period during which they did not exhibit a righting reflex, but responded to tail clamp. Based on measurement of the time to loss and return of righting, the presence or absence of a response to tail clamp, the absence of respiratory depression, and EEG activity, we found that the two injections of etomidate induced a depth of anesthesia for a total of 2 h that is in a similar range to that induced by 2.1% sevoflurane (Edwards et al., 2010; Cao et al., 2012; Xu et al., 2015). Sevoflurane at 2.1% is near 0.6 minimum alveolar concentration for P4–P6 rats (Orliaguet et al., 2001). Previously we have shown that blood glucose and gas levels after anesthesia with 2.1% sevoflurane for 6 h were in the normal range (Edwards et al., 2010). To simulate stress after anesthesia, half of the animals in the ET group were subjected to maternal separation for 3 h at P10 i.e., the ET plus maternal separation group (ET + SEP group). The control animals were subjected to animal facility rearing only. To study the role of etomidate-enhanced GABAAR-mediated depolarization, a subgroup of rats from the ET + SEP group received a single injection of the NKCC1 inhibitor, bumetanide (1.82 mg/kg, IP, Ben Venue Laboratories, Inc., Bedford, OH), 15 min prior to initiation of anesthesia with etomidate, the Bumetanide plus ET + SEP group (Bu + ET + SEP group). Bumetanide in this concentration/dose range is widely used as the most selective currently available inhibitor of NKCC1 in both animal and human studies (Cellot and Cherubini, 2014; Tyzio et al., 2014; Lemonnier et al., 2013; Lemonnier et al., 2016; Xu et al., 2015; Tan et al., 2014). In order to control for the injections of bumetanide prior to anesthesia, all treatment groups except the Control group received equal volumes of saline (IP) at P4, P5 or P6.
Adult rats were sequentially evaluated in the elevated plus maze (EPM) starting at P60, for prepulse inhibition (PPI) of the acoustic startle response at P70 and for the corticosterone response to physical restraint for 30 min at ≥P120 (Fig. S1). The animals in cohort two were sacrificed one week after the last in vivo test to collect brain tissue samples.
2.3. Basal and stress-induced activity of the LHPA axis
For measurements of basal diurnal corticosteroid secretion, blood was collected on two different days between 7:00 and 9:00 am and 7:00 and 9:00 pm, respectively. One week later, the acute stress-induced release of corticosterone was studied after subjecting the animals to physical restraint for 30 min. Blood samples (~300 μL) were collected at 10, 60, and 120 min after the restraint. Physical restraint was administered using rodent holders (Kent scientific Corporation, Torrington, CT). Blood sampling was done using the “tail clip” method. Serum corticosterone was measured using commercial ELISA kits (Cayman Chemical Company, Ann Arbor, MI) following the manufacturer’s instructions.
2.4. Behavioral Tests
Assessment of behavior in the EPM
The EPM studies were performed using the EPM apparatus and BIO-EPM 3C video tracking software (EB Instruments, Pinellas Park, FL) during the light phase of the dark-light cycle as previously described by our laboratory (Xu et al., 2015). If a fall occurred, the animal was removed from the study (3 males and 4 females were removed from the study).
Measurements of the acoustic startle response and PPI of startle
The PPI of startle tests were performed using the SR-Lab startle apparatus (San Diego Instruments, San Diego, CA) as previously described by our laboratory (Edwards et al., 2010; Cao et al., 2012). The %PPI for each prepulse level was calculated using the following formula: %PPI =100× [(pulse alone) − (prepulse + pulse)]/pulse alone (Geyer and Dulawa, 2003). Data were collected as Vmax amplitude.
Analyses of mRNA levels for NKCC1, KCC2 and CRH
The mRNA levels for CRH, NKCC1, and KCC2 in the hypothalamus were analyzed via reverse transcription-PCR (qRT-PCR) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). RNA was extracted from the samples using an RNeasy Plus Kit (Qiagen, Valencia, CA, USA), reverse transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Laboratories, Hercules, CA, USA), and then analyzed via qRT-PCR. Oligonucleotide primers and Taqman probes specific for the above genes were obtained from Applied Biosystems (Carlsbad, CA, USA): CRH (Rn01462137_m1), NKCC1 (Rn00582505_m1), KCC2 (Rn00592624_m1). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (Rn01775763_g1). Gene expression was calculated using the ΔΔCT method and data was presented as relative fold change from that of control animals.
2.5. Statistical Analysis
Values are reported as mean ± SEM. Statistical analyses were carried out on raw data using JMP Pro 12 software (SAS Institute Inc., Cary, NC). General linear models with experimental groups and sex as main effects, and a group X sex interaction, were run for outcomes. Mixed models for repeated measures were used to analyze the serum corticosterone data, with morning corticosterone concentration, time post stress, sex, and treatment as main effects. By including morning corticosterone concentration, the model is accounting for the effect of pre-stress serum corticosterone on the response to stress. Interactions among treatment group, sex, and time were modeled to assess how the time course of the corticosterone response after stress differed due to both treatment and sex. Additionally, to assess differences in total corticosterone concentrations, area under the curve in respect to ground (AUCg) was calculated and compared across experimental groups and sexes (Pruessner et al., 2003). Sex and group differences in baseline corticosterone were assessed with Student’s t-test and ANOVA. For all analyses, posthoc pairwise comparisons were done with the Tukey-Kramer method, which also adjusts analyses for multiple comparisons. All comparisons were run as two-tailed tests. P ≤ 0.05 was considered significant.
3. Results
3.1. Neonatal anesthesia with etomidate alters gene expression for Cl− transporters and CRH in the hypothalamus of rat pups
There were significant treatment effects on hypothalamic NKCC1 mRNA levels at P4–6 (F(2,23) = 3.951, P = 0.034) and at P9–11 (F(2,23) = 13.826, P < 0.001), but no significant sex X treatment interaction at either time point (Fig. 1A–D).
Figure 1.
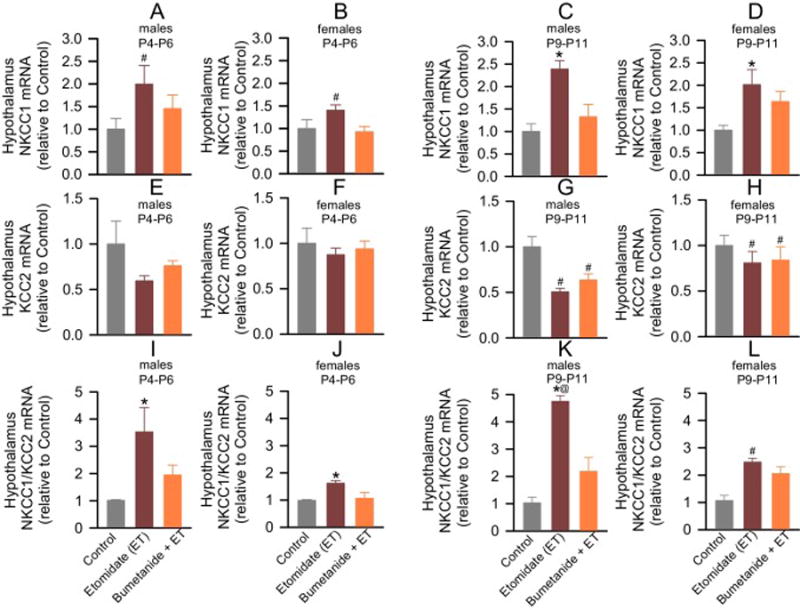
Anesthesia with etomidate for 2 h at postnatal days (P) 4, 5 or 6 increased hypothalamic levels of Na+-K+-2Cl− (NKCC1) mRNA, and reduced hypothalamic levels of K+-2Cl− (KCC2) mRNA in male and female rat pups. Shown are the respective NKCC1 and KCC2 mRNA levels (A–H) and the resulting NKCC1/KCC2 mRNA ratios (I–L). Etomidate increased hypothalamic NKCC1 mRNA levels in male and female pups 3–7 days after the anesthetic when compared to that of control pups (P < 0.001) and bumetanide-pretreated etomidate-anesthetized pups (P = 0.012), whereas the increase immediately after anesthesia was significant only compared to that of control pups (P = 0.034). Bumetanide alleviated the effects of etomidate on the NKCC1/KCC2 mRNA ratio 3–7 days after exposure to the anesthetic in males (P <0.001 vs Etomidate (ET), but P = 0.07 vs Control), but not in females (P = 0.016 vs Control, and P = 0.886 vs ET). Data normalized against Control are means ± SEM from 5 rats per treatment group. *P < 0.05 vs. Control, and Bumetanide (BU) + ET; #P < 0.05 vs. Control; @P < 0.001 vs. female ET group.
Rat pups exposed to etomidate anesthesia, exhibited reduced the KCC2 mRNA levels in the hypothalamus only at P9–P11 (F(2,24) = 7.337, P =0.003; Fig. 2E–H), specifically in comparison to control (P= 0.003); bumetanide did not decrease the effect of etomidate on KCC2 mRNA levels (P = 0.032 vs Control, and P = 0.583 vs ET; Fig. 1G,H). There was no significant sex X group interaction.
Figure 2.
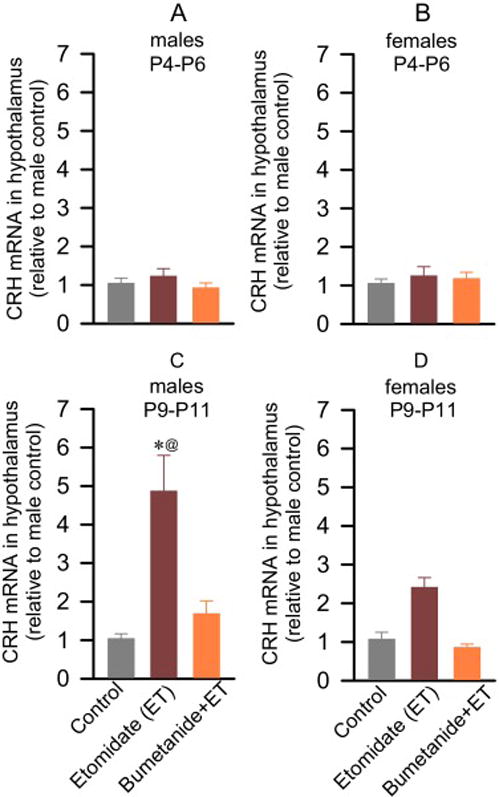
Anesthesia with etomidate for 2 h at postnatal days (P) 4, 5 or 6 increased hypothalamic levels of corticotropin releasing hormone (CRH) mRNA in male and female rat pups with greater increases in males. These effects were alleviated by pretreatment with bumetanide prior to anesthesia with etomidate. The brain hypothalamus tissue samples were collected ~2 h after the onset of anesthesia with etomidate (P4–P6) or 3–7 days later (P9–P11) for qRT-PCR analyses. Shown are the levels of CRH mRNA at P4–P6 (A–B) and P9–P11 (C–D). Bumetanide alleviated the effects of etomidate in males (P = 0.89 vs Control, and P < 0.001 vs Etomidate (ET)) but not in females (P = 0.999 vs Control, and P = 0.14 vs ET). Data normalized against control are means ± SEM from 5 rats per treatment group. *P < 0.05 vs. Control, and BU + ET; @P < 0.05 vs. female ET group.
The resulting NKCC1/KCC2 mRNA ratios, however, were increased in both sexes immediately after etomidate anesthesia (F(2,23) = 7.210, P = 0.004; Fig. 1I,J) and 3–7 days after exposure to the anesthetic (F(2,23) = 43.067, P < 0.001; Fig. 1K,L). The increases in the hypothalamic NKCC1/KCC2 mRNA ratios were greater in males than in females 3–7 days after exposure to the anesthetic (F(2,23) = 10.768, P < 0.001), but not immediately after anesthesia (F(2,23) = 2.475, P = 0.106). Bumetanide alleviated the effects of etomidate in males (P = 0.070 vs Control, and P < 0.001 vs ET), but not in females (P = 0.158 vs Control, and P = 0.886 vs ET; Fig. 1K,L).
There were no treatment effects on the hypothalamic levels of CRH mRNA immediately after etomidate anesthesia (F(2,24) = 0.782, P = 0.469; Fig. 2A,B). In contrast, a significant group X sex interaction in hypothalamic levels of CRH mRNA was observed at P9–P11 (F(2,24) = 4.330, P = 0.0248; Fig. 2C,D). Etomidate increased the hypothalamic levels of CRH mRNA only in males (P < 0.0001; Fig. 3C), with this increase attenuated in females (P =0.1466; Fig. 2D). Bumetanide alleviated the effects of etomidate in males (P = 0.891 vs Control, and P < 0.001 vs ET).
Figure 3.
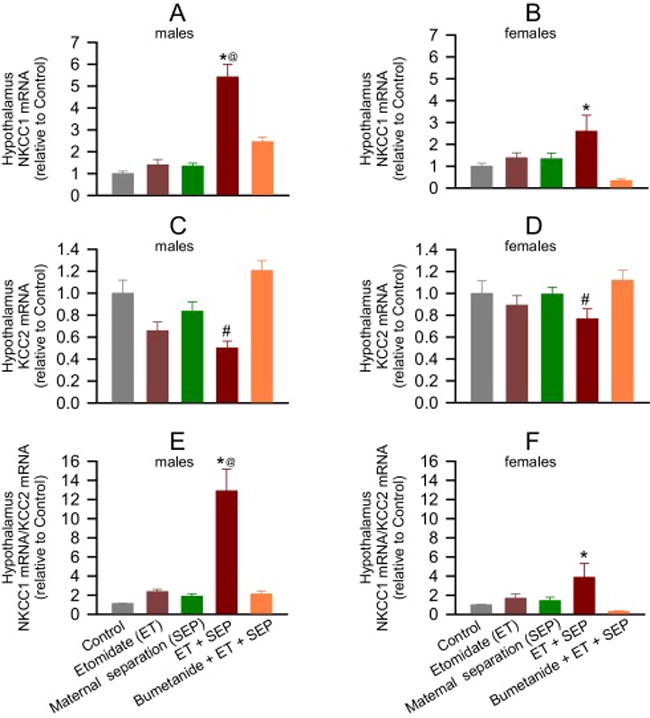
Anesthesia with etomidate for 2 h at postnatal days (P) 4, 5 or 6 followed by maternal separation for 3 h at P10 lead to increased hypothalamic levels of Na+-K+-2Cl− (NKCC1) mRNA reduced hypothalamic levels of K+-2Cl− (KCC2) mRNA in adult male and female rats. These effects were alleviated by pretreatment with bumetanide prior to anesthesia with etomidate. Shown are the respective levels of NKCC1 mRNA (A,B) and KCC2 mRNA (C,D), and the resulting NKCC1/KCC2 mRNA ratios (E,F). Bumetanide prevented the increases in the hypothalamic NKCC1/KCC2 mRNA ratios in males (P < 0.001 vs Etomidate (ET) + Maternal Separation (SEP), and P = 0.506 vs Control) and females (P < 0.001 vs ET + SEP, but P=0.0012 vs Control). Data normalized against control are means ± SEM from 5 rats per treatment group. *P < 0.05 vs. all treatment groups in males and vs. Control, and Bumetanide (BU) + ET + SEP in females; #P < 0.05 vs. all groups, except ET; @P < 0.05 vs. female ET + SEP group.
3.2. Anesthesia with etomidate at P4, P5 or P6 and subsequent maternal separation at P10 induce profound alterations in gene expression for the Cl− transporters and CRH in the hypothalamus of adult rats
Two way ANOVA revealed a significant group X sex interaction on hypothalamic NKCC1 mRNA levels in adult rats (F(4,40) = 8.078, P < 0.001, Fig. 3A, B). Compared to control, only the group exposed to both etomidate and maternal separation exhibited increased hypothalamic NKCC1 mRNA levels in males (P < 0.001), with a trend in females (P = 0.054). This increase was greater in males than females (P < 0.001). Bumetanide normalized these increases in males (P < 0.001 vs ET + SEP, P = 0.109 vs Control; Fig. 3A) and in females (P = 0.001 vs ET + SEP, and P=0.927 vs Control; Fig. 3B).
The hypothalamic levels of KCC2 mRNA were reduced in the etomidate-anesthetized maternally separated group (F(4,40) = 10.057, P < 0.001; Fig. 3C,D), with no group x sex interaction (F(4,40) = 1.417, P = 0.246). Bumetanide normalized the reduced hypothalamic KCC2 mRNA level (P < 0.001 vs ET + SEP, and P = 0.393 vs Control; Fig. 3C,D).
The resulting NKCC1/KCC2 mRNA ratios were increased in etomidate-anesthetized maternally separated rats (F(4,40) = 34.365, P < 0.001; Fig. 3E,F) with greater increases in males (F(4,40) = 8.457, P < 0.001; Fig. 4E,F). Bumetanide alleviated the increases in the hypothalamic NKCC1/KCC2 mRNA ratios in both males (P < 0.001) and females (P < 0.001) (Fig. 3E,F).
Figure 4.
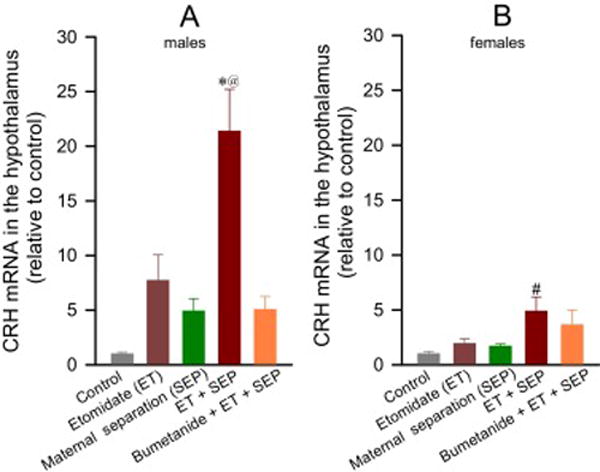
Anesthesia with etomidate for 2 h at postnatal days (P) 4, 5 or 6 followed by maternal separation for 3 h at P10 lead to increased hypothalamic levels of corticotropin releasing hormone (CRH) mRNA in adult male and female rats, with greater effects in male rats. Shown are the respective levels of CRH mRNA in male (A) and female (B) rats. Data normalized against control are means ± SEM from 5 rats per treatment group. *P < 0.05 vs. all treatment groups except Etomidate (ET) only group; #P < 0.05 vs. Control group; @P = 0.004 vs. female ET + Maternal separation (SEP) group.
Adult rats neonatally exposed to both etomidate and maternal separation exhibited increased hypothalamic CRH mRNA levels (F(4,40) = 21.514, P < 0.001; Fig. 4A,B), with greater increases in males than females (group x sex interaction: F(4,40) = 2.993, P = 0.030; Fig. 4A, B). Such increases were attenuated in male rats pretreated with bumetanide (P = 0.004 vs ET + SEP; Fig. 4A), but not in females (P = 0.976 vs ET + SEP; Fig. 4B).
3.3. Anesthesia with etomidate at P4, P5 or P6 and subsequent maternal separation at P10 induce extended endocrine responses to stress and behavioral abnormalities in adult rats
Female adult rats had higher corticosterone concentrations at rest and after stress than their male counterparts (P < 0.001; Fig. 5). Evening and morning serum levels of corticosterone at rest were similar among all experimental groups within the same sex (Fig. 5A,B). Analysis of the corticosterone responses to restraint stress revealed a significant time X group X sex interaction, indicating joint treatment and sex differences in the magnitude and time course of corticosterone responses (F(8,141) = 2.234, P = 0.023). Adult male rats neonatally exposed to both etomidate and maternal separation had the greatest total corticosterone response (measured as AUCg) in (P = 0.002 vs. Control, P < 0.001 vs. SEP only and BU + ET + SEP groups; Fig. 5C). Neonatal pretreatment with bumetanide normalized the adult stress response of etomidate-anesthetized maternally-separated rats (P=1.0 vs. Control). Similar to male rats, in female rats the total corticosterone response (AUCg) was also greater in etomidate-anesthetized maternally-separated rats (P < 0.001 vs. BU + ET + SEP; P = 0.035 vs. SEP, but P = 0.130 vs. Control; Fig. 5D). Peak corticosterone levels in female rats were not significantly different in any experimental group 10 min after restraint stress(P ≥ 0.05), except in the BU + ET + SEP group, which was lower when compared to all other experimental groups of female rats (P < 0.001).
Figure 5.
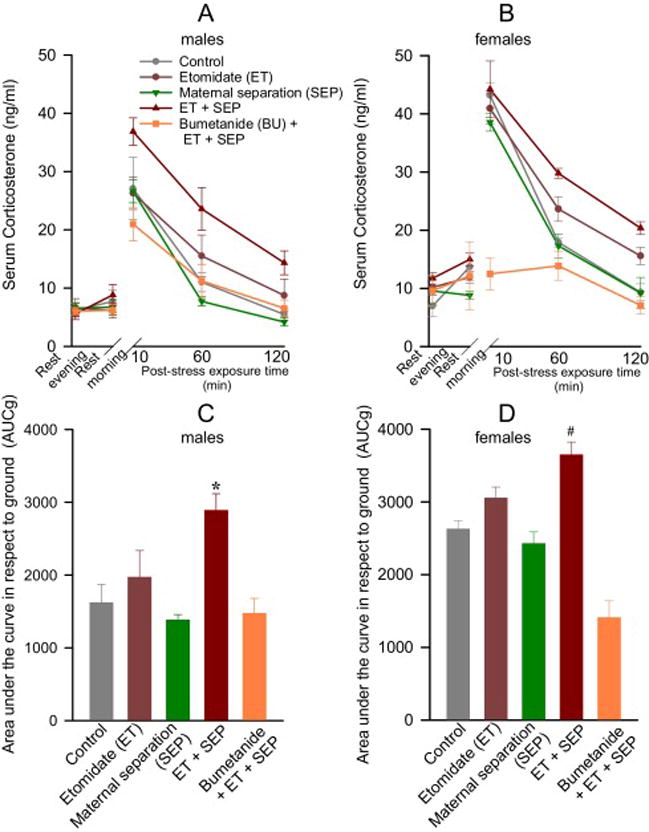
Anesthesia with etomidate for 2 h at postnatal days (P) 4, 5 or 6 followed by maternal separation for 3 h at P10 lead to increased serum levels of corticosterone after physical restraint for 30 min in adult male and female rats. These effects were alleviated by pretreatment with bumetanide prior to anesthesia with etomidate. Shown are the respective levels of serum corticosterone in male (A) and female (B) rats across each collection point, as well as the total corticosterone response (AUCg in male (C) and female (D) rats. Morning measurements were taken as baselines for calculations of the total corticosterone responses. Data are means ± SEM from 9 male rats per treatment group and from 6 female rats per treatment group, except the Bumetanide (BU) + Etomidate (ET) + Maternal separation (SEP) group, which had 7 female rats. *P<0.05 vs. Control, SEP, and BU + ET + SEP groups. #P<0.05 vs. SEP, and BU + ET + SEP groups.
Rats neonatally exposed to etomidate anesthesia and then to maternal separation at P10 exhibited reduced PPI of the acoustic startle response only at lowest prepulse intensity (3 dB) when compared to rats in the Control (P = 0.002), SEP (P = 0.006), and BU + ET + SEP (P = 0.002) groups, but not in the ET only group (P = 0.14) (Fig. S2). Group x sex interaction was not significant (F(4,150) = 0.622, P = 0.647). The PPI responses at prepulse intensities of 6 dB and 12 dB were similar across all treatment groups and between sexes.
In the elevated plus maze, adult male rats from all treatment groups traveled similar total distances, but the ET + SEP group spent a shorter time in open arms of the EPM when compared to male rats from the Control (P < 0.001) and BU + ET + SEP (P = 0.001) groups, with no treatment effects in distance covered (Fig. 6A,B). Adult female rats from all experimental groups were similar in terms of total distance covered and time spent in open arms of the EPM (Fig. 6C,D).
Figure 6.
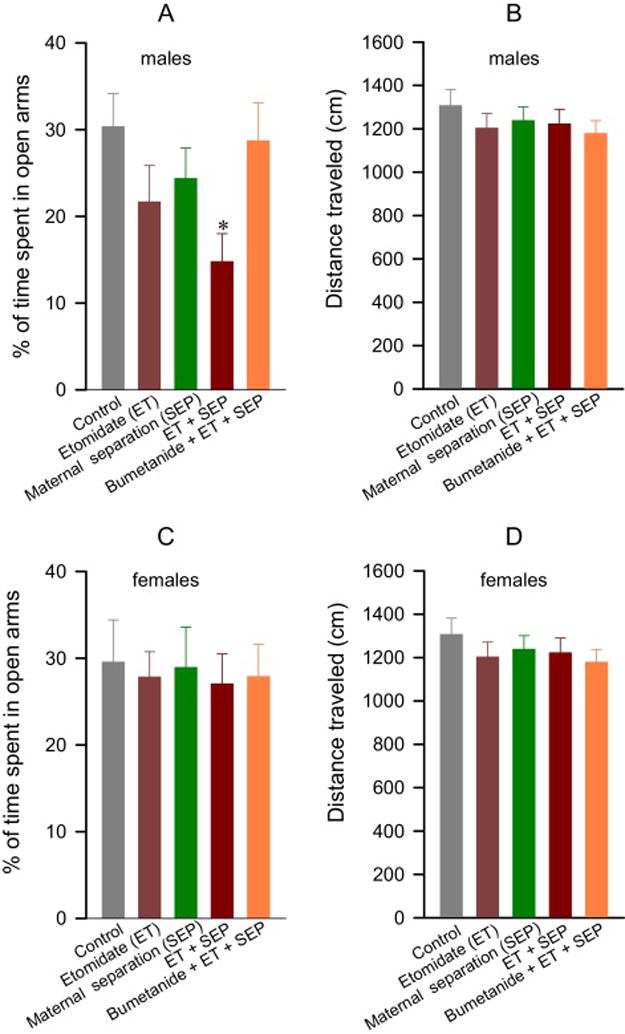
Anesthesia with etomidate for 2 h at postnatal days (P) 4, 5 or 6 followed by maternal separation for 3 h at P10 lead to reduced time spent in open arms of the elevated plus maze (EPM) in adult male, but not female rats, an effect that was alleviated by pretreatment with bumetanide prior to anesthesia with etomidate. The EPM tests were performed at P~60. Shown are % of time spent in open arms of the EPM and distance traveled by male (A,B) and female (C,D) rats. Data are means ± SEM from 14–16 male and 14–17 female rats per treatment group. #P < 0.05 vs. Control, and Bumetanide + Etomidate (ET) + Maternal separation (SEP) groups.
4. Discussion
We report profound, sex-dependent effects of etomidate in neonatal rats on NKCC1/KCC2 mRNA ratios and CRH mRNA levels in the hypothalamus and a further potentiation of these effects by a single stressor, an episode of maternal separation. These effects were more prominent in male rats. The persistent sex-dependent increases in the NKCC1/KCC2 mRNA ratio and CRH mRNA level in the hypothalamus of adult rats, neonatally exposed to etomidate anesthesia followed by maternal separation, were accompanied by extended neuroendocrine responses to acute stress and behavioral abnormalities, suggesting a causal link. Such a link is further supported by the finding that the NKCC1 inhibitor, bumetanide, largely prevented etomidate’s effects in neonatal rats. Likewise, bumetanide diminished the effects of neonatal etomidate exposure combined with maternal separation, at both the molecular and systemic levels in adult rats. Amelioration by bumetanide suggests that anesthetic-enhanced GABAAR-mediated depolarization/excitation may be one of the initial steps in these developmental effects. Significant alterations in gene expressions, neuroendocrine and neurobehavioral abnormalities in adult rats neonatally exposed to etomidate anesthesia and a single maternal separation suggest that even relatively mild environmental stressors may reveal long-term developmental abnormalities initiated by a relatively short exposure to a general anesthetic during an early period of life, and that a neonatally administered anesthetic may program abnormal functioning of the stress response systems and behavioral deficiencies in adulthood.
The increased hypothalamic NKCC1/KCC2 mRNA ratios resulted from increased levels of NKCC1 mRNA as well as decreased levels of KCC2 mRNA in male and female rat pups with greater effects 3–7 days after etomidate exposure than immediately after anesthesia. These findings imply that etomidate may induce developmental abnormalities through a previously unknown long-lasting alteration in gene expression. The depolarizing/stimulatory action of GABA in immature rat hypothalamic neurons (Gao and van den Pol, 2001), and preventive effects of bumetanide on the etomidate-induced alterations in gene expressions, suggest that etomidate induces these changes, at least in part, by enhancing GABAAR-mediated stimulation. The transition in GABAAR-mediated signaling from stimulatory to inhibitory occurs later in male than female rats (Akman et al., 2014), and thus may explain the more prominent etomidate-induced abnormalities in males. Different types of stress, including early life stress, may increase the NKCC1/KCC2 ratio, impair GABAAR-mediated inhibition and increase CRH levels (O’Malley et al., 2011; Veerawatananan et al., 2015; Gao et al., 2016). Hence, the increases in the NKCC1/KCC2 ratio and CRH mRNA in the hypothalamus caused by etomidate support the mechanistic plausibility of cumulative developmental effects of anesthesia with etomidate and subsequent early life stress.
The inhibitory control of the CRH-secreting hypothalamic paraventricular neurons (PVN) by GABAAR-mediated signaling and the positive modulation of this signaling by neuroactive steroids is one of the fundamental mechanisms of adaptation to stressful stimuli. Because of the depolarizing/stimulatory action of GABA in immature rat hypothalamic neurons (Gao and van den Pol, 2001), both neuroactive steroids and GABAeric anesthetics are likely to further up-regulate or in the case of GABAergic anesthetics initiate CRH release and systemic stress responses. Also, the delayed returns to baseline of restraint stress-induced corticosterone responses in male and female adult rats neonatally exposed to etomidate and maternal separation may be, at least in part, due to an increased hypothalamic NKCC1/KCC2 ratio and impaired neuroactive steroid-based GABAAR-mediated negative feedback. Higher sensitivity of adrenal glands to LHPA axis hormones in female rats (Figueiredo et al., 2007) may explain that corticosterone responses to acute stress were similarly extended in females, despite smaller increases in the NKCC1/KCC2 mRNA ratios. In line with significantly greater increases in the NKCC1/KCC2 mRNA ratios and CRH mRNA levels in hypothalamus of male rats neonatally exposed to etomidate and maternal separation, long-term alterations in behavior, such as reduction in the PPI and decrease in time spent in open arms of the EPM were more prominent in males, the effects that were ameliorated by pretreatment with bumetanide.
Dysregulation of the neuroendocrine response to stress and excessive CRH production, programmed by adverse early-life experiences, are thought to contribute to a wide spectrum of stress-related cognitive disorders, including depression, posttraumatic stress disorder, and schizophrenia (Lupien et al., 2009; Baram et al., 2012; Franklin et al., 2012). Our current findings add a new type of early life experience, namely neonatal exposure to general anesthesia, as an early programming event that may contribute to stress-related cognitive disorders. These effects may be further reinforced by subsequent life stressors. This can be a phenomenon of paramount importance given that every fourth newborn is exposed to general anesthesia during the first 12 months of life and, according to a UNICEF report, more than 50% of the world’s children live in conditions of chronic stress (UNICEF, 2005). Long-lasting and/or repeated episodes of stress caused by diseases, pain, maternal deprivation, psychological stress, etc. that children may experience in their lives may have a more profound exacerbating effect in comparison with the single episode of stress to otherwise healthy animals tested in this study. In future studies, it will be important to determine if different paradigms of post anesthesia stress that more closely model the stressful conditions human subjects may experience in their lives may exacerbate developmental effects, initially programmed by even shorter anesthetic exposures”.
This study provides experimental evidence that a combination of relatively short exposure to the GABAergic anesthetic etomidate and subsequent single maternal separation, induces long-term developmental sex-dependent abnormalities similar, but not identical, to those induced by relatively long exposures to sevoflurane (Xu et al., 2015) or propofol (Tan et al., 2014), suggesting the possibility of a general phenomenon. This is an initial basic science study designed to elucidate a tremendously complex translational problem. Even though etomidate is infrequently used in pediatric/neonatal surgery, comparison of the developmental effects of sevoflurane, the most widely used anesthetic in pediatric anesthesia, whose polyvalent actions include enhancement of GABAAR activity, propofol, the most frequently used intravenous anesthetic with a selective GABAAR-mediated action, and etomidate, an anesthetic with a GABAergic mechanism of action similar to propofol that, in contrast to propofol, is known to disrupt the adrenal synthesis of corticosteroids (Vanlersberg and Camu, 2008), but is likely to activate the remainder of the LHPA axis more than the other two anesthetics (see Introduction), represents a unique experimental tool to elucidate relative role of GABAAR signaling and stress response systems in the developmental effects of these three important general anesthetics. The current study suggests an important role for environmental stressors in determining the developmental outcome of exposure to general anesthetics early in life and for a contribution of neonatal anesthesia in the dysregulation of the stress response system and neurobehavioral deficiencies in adulthood. Our findings inform about specific molecular mechanisms that may be involved in the mediation of these effects. Etomidate-induced increases in the NKCC1/KCC2 mRNA ratio and in levels of CRH mRNA in the hypothalamus of neonatal rats that persist into adulthood may represent mechanisms that contribute to neuroendocrine and neurobehavioral abnormalities initially programmed by neonatal anesthesia and then further exacerbated by sub-threshold environmental stressors. Our findings provide support for the possibility that Cl− transporters, especially the NKCC1 Cl− importer, represent a promising therapeutic target for the prevention of neonatal anesthesia-programmed neurodevelopmental abnormalities and suggest new measurements, in addition to neurocognitive tests, to be performed in human studies, e.g. measurements of PPI and stress responses, to evaluate developmental effects of neonatal anesthesia.
Supplementary Material
Highlights.
Post-anesthesia stressor may exacerbate/unmask neurodevelopmental abnormalities even after a relatively short anesthetic with etomidate
Adult rats, neonatally exposed to etomidate and later to maternal separation, had increased hypothalamic Na+-K+-2Cl− (NKCC1) mRNA and corticotropin releasing hormone mRNA and decreased K+-2Cl− (KCC2) mRNA levels
The alterations in gene expression were accompanied by extended neuroendocrine responses to acute stress and behavioral abnormalities
The neuroendocrine and behavioral abnormalities were greater in males
Pretreatment with the NKCC1 inhibitor, bumetanide, ameliorated most of these effects
Acknowledgments
Role of the funding sources
This work was supported by the National Institutes of Health (R01GM93036 and R01NS091542 to A.E.M.) and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (to NG). The funding sources had no role in designing the study, collecting and analyzing the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
A.E.M. conceptualized and designed the study. L-S.J., J-J.Y., and T.V. acquired data and performed data analysis. Analyses and interpretation of the data and writing of the article were performed by A.E.M., C.N.S., N.G., T.E.M., C.S., and J-J.Y. All authors approved the final version of the article.
References
- Akman O, Moshé SL, Galanopoulou AS. Sex-specific consequences of early life seizures. Neurobiol Dis. 2014;72(Pt B):153–166. doi: 10.1016/j.nbd.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012;117:791–800. doi: 10.1097/ALN.0b013e318266c62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr. 2014;2:70. doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE. From Drug-Induced Developmental Neuroapoptosis to Pediatric Anesthetic Neurotoxicity-Where Are We Now? Brain Sci. 2016;6:3. doi: 10.3390/brainsci6030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum AJ, Akinola M, Martin A, Fath S. The role of stress mindset in shaping cognitive, emotional, and physiological responses to challenging and threatening stress. Anxiety Stress Coping. 2017;25:1–17. doi: 10.1080/10615806.2016.1275585. [DOI] [PubMed] [Google Scholar]
- Deidda G, Allegra M, Cerri C, Naskar S, Bony G, Zunino G. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat Neurosci. 2015;18:87–96. doi: 10.1038/nn.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J Neurophysiol. 2001;85:425–434. doi: 10.1152/jn.2001.85.1.425. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology. 2016 doi: 10.1159/000446114. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci Chapter. 2003;8:17. doi: 10.1002/0471142301.ns0817s24. Unit 8. [DOI] [PubMed] [Google Scholar]
- Flandreau E, Risbrough V, Lu A, Ableitner M, Geyer MA, Holsboer F, Deussing JM. Cell type-specific modifications of corticotropin-releasing factor (CRF) and its type 1 receptor (CRF1) on startle behavior and sensorimotor gating. Psychoneuroendocrinology. 2015;53:16–28. doi: 10.1016/j.psyneuen.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292:E1173–1182. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Rogawski MA. 11 β-Hydroxylase inhibitors protect against seizures in mice by increasing endogenous neurosteroid synthesis. Neuropharmacology. 2001;61:133–137. doi: 10.1016/j.neuropharm.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier E, Robin G, Degrez C, Tyzio R, Grandgeorge M, Ben-Ari Y. Treating Fragile X syndrome with the diuretic bumetanide: a case report. Acta Paediatr. 2013;102:e288–90. doi: 10.1111/apa.12235. [DOI] [PubMed] [Google Scholar]
- Lemonnier E, Lazartigues A, Ben-Ari Y. Treating schizophrenia with the diuretic bumetanide: A Case Report. Clin Neuropharmacol. 2016;39:115–117. doi: 10.1097/WNF.0000000000000136. [DOI] [PubMed] [Google Scholar]
- Li M, Xue X, Shao S, Shao F, Wang W. Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res. 2013;1518:82–90. doi: 10.1016/j.brainres.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci. 2012;6:4. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 2011;214:221–229. doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–739. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OF. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest. 1997;99:962–966. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Servick K. Biomedical Research. Researchers struggle to gauge risks of childhood anesthesia. Science. 2014;346:1161–1162. doi: 10.1126/science.346.6214.1161. [DOI] [PubMed] [Google Scholar]
- Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- Tan S, Xu C, Zhu W, Willis J, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE. Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology. 2014;121:1010–1017. doi: 10.1097/ALN.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, Mansuy IM, Merlo-Pich E, Geyer MA, Risbrough VB. Forebrain-specific CRF overproduction during development is sufficient to induce enduring anxiety and startle abnormalities in adult mice. Neuropsychopharmacology. 2014;39:1409–1419. doi: 10.1038/npp.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF. State of the world’s children 2006. New York: 2005. [Google Scholar]
- Vanlersberghe C, Camu F. Etomidate and other non-barbiturates. Handb Exp Pharmacol. 2008;182:267–282. doi: 10.1007/978-3-540-74806-9_13. [DOI] [PubMed] [Google Scholar]
- Veerawatananan B, Surakul P, Chutabhakdikul N. Maternal restraint stress delays maturation of cation-chloride cotransporters and GABAA receptor subunits in the hippocampus of rat pups at puberty. Neurobiol Stress. 2015;3:1–7. doi: 10.1016/j.ynstr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol. Teratol. 2016:pii. doi: 10.1016/j.ntt.2016.11.005. S0892-0362(16)30138-6. [DOI] [PubMed] [Google Scholar]
- Xu C, Tan S, Zhang J, Seubert CN, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE. Neonatal anesthesia with sevoflurane: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl− importer antagonists. Psychoneuroendocrinology. 2015;60:173–181. doi: 10.1016/j.psyneuen.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kuo CC, Moghadam SH, Monte L, Campbell SN, Rice KC, Sawchenko PE, Masliah E, Rissman RA. Corticotropin-releasing factor receptor-1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer’s disease. Alzheimers Dement. 2016;12:527–537. doi: 10.1016/j.jalz.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


