Abstract
Cocaine dependence is one of the most difficult substance use disorders to treat. While the powerful effects of chronic cocaine use on behavior were documented in the 19th century, it was not until the late 20th century that we realized chronic cocaine use was affecting brain tissue and function. Following a brief introduction (Part 1), this chapter will summarize our current knowledge regarding alterations in neural circuit function typically observed in chronic cocaine users (Part 2) and highlight an emerging body of literature which suggests that baseline limbic circuit activity may be a reliable predictor of clinical outcomes among individuals seeking treatment for cocaine (Part 3). Finally, as the field of addiction research strives to translate this neuroimaging data into something clinically meaningful, we will highlight several new brain stimulation approaches which utilizing functional brain imaging data to design non-invasive brain stimulation interventions for individuals seeking treatment for substance dependence disorders (Part 4).
Keywords: cocaine, treatment, functional MRI, brain stimulation, TMS, connectivity, executive, limbic, striatum, prefrontal
Dr. Watson admiring Sherlock Holmes “But consider! Count the cost! Your brain may, as you say, be roused and excited, but it is a pathological and morbid process, which involves increased tissue change and may at least leave permanent weakness….Why should you, for a mere passing pleasure, risk the loss of those great powers with which you have been endowed?”
– Sign of Four, Sherlock Holmes (Arthur Conan Doyle, 1894).
SECTION 1: INTRODUCTION
This quote from Dr. Watson comes as a plea to Sherlock Holmes who has taken up the bad habit of using cocaine in a “7% solution” when he is feeling bored. Whether Arthur Conan Doyle realized it or not this may be one of the first suggestions that regular use of cocaine could change the structure of the brain.
Over one hundred years later the recreational use of cocaine, which was present in Sir Arthur Conan Doyle’s time, has evolved to become a massive public health concern. In the 2013 National Survey of Drug Use and Health, approximately 1.5 million people used cocaine in the previous month (2014). The overall costs associated with cocaine and other illicit drug use was estimated at $180 billion, with an annual rate of increase of 5.34% over the preceding decade. In addition to causing psychosocial and economic hardship on the dependent individuals and their families, chronic cocaine use also leads to higher rates of myocardial infarction, arrhythmias, heart failure, stroke, rhabdomyolysis, intestinal ischemia, aortic dissection, seizures, bronchospasm, pulmonary edema, hypertensive emergency, and bruxism (sometimes leading to severe dental damage), as well as exposure to infectious diseases such as HIV and hepatitis.
Just after the surge of cocaine use that occurred in the United States in the 1980s, the United States Congress dubbed the 1990s the “Decade of the Brain.” At this point scientific research regarding the effects of chronic cocaine use on the brain grew exponentially. This fictional assertion from 221 Baker Street, London regarding cocaine’s effects on the brain and on tissue, can now be supported by shelves of brain imaging journals in the non-fiction section of your local University. Specifically, through structural magnetic resonance imaging (MRI) we now know that chronic cocaine use is associated with a loss of gray matter volume/density. Through diffusion tensor imaging we now know that this gray matter disruption is accompanied by white matter decay. Positron emission tomography (PET) has reliably demonstrated that acute cocaine exposure leads to a change in dopamine binding in the striatum, and that chronic exposure leads to a cascade of changes in dopamine binding which extend from the ventral, reward based areas of the brain to dorsal, habit based areas. These data have also been supported by non-human primate studies of acute and chronic cocaine use (Hanlon, Beveridge, & Porrino, 2013).
We now know that the neuropathology present in cocaine-dependent individuals is not restricted to a single brain region, a single cell type, or a single neurotransmitter system. Through advances in functional neuroimaging, it is apparent that the temporal progression many patients experience from initial exposure to cocaine, to cocaine dependence, to abstinence, and often on to relapse involves dysregulation in at least three major frontal-striatal systems that contribute to behavior - limbic processing, cognition, and basic motor control. These systems span both cortical and subcortical regions of the brain and therefore are vulnerable not only to pathology in a local population of cells, but also in the white matter tracts that connect these regions. Through functional MRI we are now able to investigate alterations in these neural circuits that are present at baseline in chronic cocaine users (e.g. though resting state functional connectivity), as well as alterations in these neural circuits that occur when treatment-seeking individuals are challenged with cocaine cues or engaged in a behavioral control task.
The abundance of functional neuroimaging data and structural neuroimaging data however, have given us a new puzzle to solve—how can we use this knowledge to improve patient-care and deliver a more effective treatment to these cocaine dependent individuals? Moving into the next decade our task is to distill something clinically meaningful from the 100s of neuroimaging papers that now exist on the topic of brain imaging in cocaine users. The goal of this chapter is to introduce the reader to the frontal-striatal neural circuits that are often implicated in addiction, and to review the recent evidence which suggests that some of these circuits may be useful predictors of relapse in treatment-engaged individuals. For this chapter we have chosen to focus on alterations in neural function (rather than structure), though we acknowledge that these domains are inextricably linked and potentially co-dependent. Specifically, for this chapter we will review 3 primary topics: 1) alterations in the executive control and limbic arousal neural networks that are most often cited as dysfunctional cocaine dependent individuals, 2) data from treatment-seeking individuals which demonstrates that baseline activity in these networks may predict relapse, and finally 3) emerging brain stimulation treatment data which suggests that increasing activity in the executive control network or decreasing activity in the limbic arousal network may be a fruitful new approach for addiction treatment. We intend to educate the reader and plant several seeds in his/her mind regarding the potential utility brain imaging has to predict treatment outcome or guide new neural circuit based treatment interventions for addiction.
SECTION 2: EXECUTIVE AND LIMBIC SYSTEM IRREGULARITIES IN COCAINE DEPENDENT INDIVIDUALS
The primary mechanism through which acute cocaine acts on the brain is through binding to the dopamine transporter which is highly concentrated in the striatum. The ventral aspect of the striatum, specifically the nucleus accumbens (NAcc), is the target of much of the afferent dopaminergic projections from the ventral tegmental area (VTA). The high dopaminergic innervations within this region give rise to its role as the primary location for the pharmacological effects of cocaine. Dopamine is not a particularly abundant neurotransmitter (relative to glutamate or GABA for example), however, it plays critical roles in reward, prediction and learning. Cocaine induces euphoria and feelings of reward by blocking the reuptake of dopamine in the synaptic cleft, thereby vastly increasing the concentration within the synapse.
From a clinical neuroimaging perspective, the NAcc is very small and very difficult to image given its size and proximity to cerebral sinuses and pulsating arterioles. Specifically, the NAcc is approximately 1–2cm in humans relative to a whole brain volume of 1100 + cubic centimeters. The relatively poor spatial resolution of PET (6mm) and BOLD fMRI (2 – 3mm) and the sensitivity of the latter to air/fluid interfaces and cardiac pulsations has limited its inclusion in many investigations. Despite these challenges many researchers have made substantial progress in investigating changes within this small region, both functionally and structurally, due to the use of cocaine.
Functional MRI and PET studies have found NAcc activation during cue-elicited and self-reported craving and during cocaine administration (Breiter et al., 1997; Kilts, Gross, Ely, & Drexler, 2004; Kufahl et al., 2005). In functional MRI studies in which cocaine was administered to current cocaine users while in the scanner, activity in the NAcc has been negatively correlated with reports of cocaine high and positively correlated with craving (Breiter et al., 1997; Risinger et al., 2005). Thus, activity in this component of reward circuitry of the brain may be suppressed with the acute effects of cocaine, but increased when the craving reinstates.
The effects of cocaine on the brain however are certainly not limited to the dopamine-rich ventral and dorsal striatum. Many of these striatal areas (including the NAcc, caudate, and putamen), receive direct input from the prefrontal cortex (Figure 1). Although the exact mechanism through which chronic cocaine use effects function and structure of these afferent projections is still an active area of research, lower prefrontal cortex activity (“hypofrontality”) among cocaine dependent individuals versus matched controls was one of the earliest, and most robust findings in clinical neuroimaging of cocaine use. Lower prefrontal cortex activity is present across multiple imaging modalities, including measures of glucose metabolism (Volkow et al., 1991), dopamine binding (Volkow et al., 1993), BOLD related activity and measures of neural integrity (Fein, Di Sclafani, & Meyerhoff, 2002; Franklin et al., 2002; O’Neill, Cardenas, & Meyerhoff, 2001).
Figure 1. Illustration of the structural connectivity between various prefrontal cortical areas to various dorsal and ventral striatal areas (from Haber & Knutson, 2010).
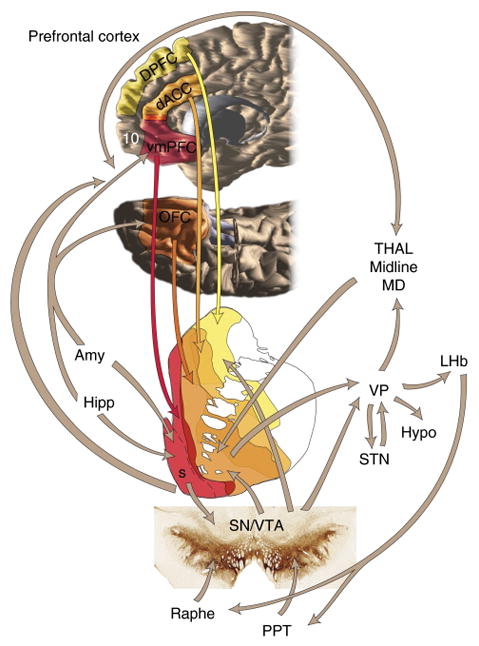
The other major inputs and outputs of the striatum are also shown. vmPFC: ventromedial prefrontal cortex; OFC: orbitofrontal cortex; dACC: dorsal anterior cingulate cortex; DPFC: dorsolateral prefrontal cortex; Amy: amygdala; Hipp: hippocampus; Hypo: hypothalamus; BNST: bed nucleus of the stria terminalis; VP: ventral pallidum; PPT: pedunculopontine nucleus; VTA/STN: ventral tegmental area, subthalamic nucleus; Thal/MD: medio-dorsal nucleus of the thalamus.
Located anterior to the precentral gyrus, the prefrontal cortex is often divided into 6 discrete areas (known as Brodmann Areas 9,10,11,12,46,47), which were defined in 1909 by German scientist Korbinian Brodmann. Although these regions were originally subdivided based on their cytoarchitectural heterogeneity, through electrophysiology and functional MRI we now know that these Brodmann Regions also have functional heterogeneity. For example, Brodmann Areas 9 and 46, often referred to as the dorsolateral prefrontal cortex, contribute to executive processing tasks including planning future events, decision making, the coordination of purposeful behavior (Miller & Cohen, 2001). Brodmann Areas 10, 11, 12 and 47, often referred to collectively as the orbitofrontal cortex, are involved in rewards, assigning value, limbic arousal and emotional processing. Together, these prefrontal regions all play key roles in addiction disorders.
The connectivity between these prefrontal cortical areas and their targets in the striatum very active area of research in both basic science and in clinical neuroscience of addiction. Of the four parallel frontal-striatal dopamine systems in the brain the mesocortical and mesolimbic circuits have been the primary focus of addiction science. These circuits are involved in the modulation of cognitive control, impulsivity, and habit formation experienced by cocaine users (Goldstein & Volkow, 2002). They mediate the volitional seeking of cocaine and subsequent learning, which eventually becomes an automatic response. Animal studies have demonstrated that the mesolimbic dopamine system regulates the rewarding, reinforcing properties of cocaine. As drug use progresses to abuse, the rewarding properties of the drug become less salient, and drug taking becomes guided by conditioned responses and habit formation, which is mediated by the mesocortical system (Everitt & Robbins, 2005; Porrino, Lyons, Smith, Daunais, & Nader, 2004). Once an individual has transitioned into dependence and is seeking treatment, engagement of these limbic arousal systems and cognitive control systems appear to play a large role in the likelihood that a given individual will relapse to cocaine use following drug cue exposure. We will first consider those regions that are anatomically connected to the ventral striatum and contribute to limbic arousal and cocaine cue-induced craving. This is followed by a description of brain regions connected to the dorsal striatum which contribute to executive control and withholding responses to drug cues.
Ventromedial and Dorsolateral Prefrontal Cortices
Medial Prefrontal cortex (mPFC)
The mPFC extends throughout the medial section of the frontal cortex and into the OFC (BA 10). Studies using PET and fMRI have shown that cocaine users have increases in mPFC activity when exposed to cocaine versus neutral cues (Garavan et al., 2000; Grant et al., 1996). Gray matter volume in the mPFC is lower in cocaine users than controls (Ersche et al., 2011; Matochik, London, Eldreth, Cadet, & Bolla, 2003). Additionally cross-sectional studies of gray matter integrity in cocaine users and abstainers have revealed that mPFC and OFC gray matter is higher in individuals able to remain abstinent from cocaine for 6 months compared to their drug-using peers (Hanlon, Dufault, Wesley, & Porrino, 2011), that lower mPFC gray matter is associated with longer duration of cocaine use (Ide et al., 2014), and that extended abstinence may lead to recovery in frontal cortical gray matter regions (Connolly, Bell, Foxe, & Garavan, 2013). Recent longitudinal work supports these findings, showing increases in prefrontal gray matter volume over 6 months of abstinence (Parvaz et al., 2016).
Although the ventral aspects of the mPFC typically are involved in mediating limbic arousal, dorsal aspects of the mPFC play an important role in the cognitive decrements seen in cocaine users. Functional imaging studies have shown general hypoactivation in the dorsal mPFC among cocaine users, relative to controls, during cognitive and attention tasks.(Bolla et al., 2003; Kaufman, Ross, Stein, & Garavan, 2003; Kubler, Murphy, & Garavan, 2005) Moreover, hypoactivation of the mPFC during a cognitive task (Stroop) in cocaine users was associated with faster relapse rates (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008).
Orbitofrontal cortex (OFC)
The OFC is located on the ventral section of the PFC, and contributes to reinforcement of cocaine (e.g., with NAcc, thalamus, ventral striatum and indirectly with amygdala, cingulate, hippocampus). Acute cocaine administration, for example, leads to elevated activity in the OFC which may act as an information integrator, leading to reinforcement of drug-taking (Volkow & Fowler, 2000). The OFC has been implicated in response inhibition and its dysregulation and may contribute to the continued use of cocaine after the rewarding effects no longer are experienced. Disruption of the OFC leads to risky decision-making and an inability to anticipate outcomes (see (Krawczyk, 2002)). It has also been associated with perceiving the value of a stimulus, such as reward; and learning the association between a stimulus and outcome (Kringelbach & Rolls, 2004).
As with other frontal areas, gray matter volume in the OFC is lower among cocaine-dependent people compared to controls (Matochik et al., 2003), and this decreased volume has been linked with greater cocaine-related compulsivity and longer duration of use (Ersche et al., 2011; Franklin et al., 2002; Sim et al., 2007). Functional neuroimaging has shown that the OFC is active during self-reported craving, and has been negatively correlated with reports of feeling high after administration.(Bonson et al., 2002; Risinger et al., 2005) The OFC is active in response to both implicit and explicit cocaine cues.(Bonson et al., 2002; Childress et al., 2008). Among abstinent cocaine users, hypoactivity in the OFC during a reward-related task was linked with increased amount of drug use prior to abstinence (Bolla et al., 2003). In a study where cocaine users were asked to inhibit their craving in response to cocaine cues, Volkow and colleagues (2010) showed that instructions to inhibit craving led to lower glucose metabolism in the medial OFC and nucleus accumbens (NAcc) compared to viewing the cues without inhibitory instructions. Self-reported craving was lower after inhibition, indicating that cocaine users can successfully deactivate two areas associated with drug craving, which shows promise for treatment.
Dorsolateral Prefrontal Cortex (DLPFC)
The DLPFC sends afferent projections to the dorsal striatum, and is involved in higher order processes, such as conscious decision making, reasoning, working memory, inhibition, as well as outcome prediction (see (Krawczyk, 2002)). Cocaine users have a significant reduction in cortical thickness in the DLPFC compared to controls (Makris et al., 2008). In a PET study, abstinent cocaine users have been found to have less activation in the DLPFC when engaging in reward-related decision-making tasks compared to non-users.(Bolla et al., 2003) However, less BOLD signal in the DLPFC during a cognitive task (Stroop) in cocaine users prior to entering treatment predicted longer stays in treatment. This perhaps indicated more efficient processing during the task indicative of greater decision-making abilities.(Brewer et al., 2008)
Ventral and Dorsal Striatum
As described above, addiction is a temporally dynamic process in which drug-seeking starts as a reward driven process but then progresses to a habit driven process over time. This behavioral transition is done in tandem with a neurobiological transition, wherein the initial effects of cocaine on striatal neurochemistry begin in the ventromedial aspects of the striatum (involved in reward seeking), but then follow an orderly progression towards the dorsal lateral aspects of the striatum (involved in habitual responding). This neurobiological cascade has been shown in rodent models (Everitt &Robbins, 2005), and non-human primate models of chronic cocaine use (Porrino et al., 2004). Although it is very difficult to perform a longitudinal study of the effects of accumulated cocaine dose on the human brain, we do know that that acute cocaine use effects the ventral striatum (Risinger et al., 2005) in humans, and that the functional dissociation between the ventral and dorsal striatum is degraded in chronic cocaine users (12 or more years of use) (Hanlon, Wesley, & Porrino, 2009).
The exact demarcation of the ventral versus dorsal striatum is difficult, as the divisions of the striatum are continuous and overlapping. Despite these difficulties, combined PET and DTI tractography studies (Tziortzi et al., 2014), resting state functional connectivity (Choi, Yeo, & Buckner, 2012) and large scale meta-analyses (Pauli, O’Reilly, Yarkoni, & Wager, 2016) reveal the ventral/dorsal divisions within the striatum, reliably identifying the caudate and the putamen as two structures making up the dorsal striatum. These midline brain structures are involved in decision making, reward related learning, movement, and the processing of sensorimotor, cognitive, and emotional information (see (Balleine, Delgado, & Hikosaka, 2007)).
Caudate Nucleus
The caudate nucleus in humans is fairly large and functionally heterotopic. That is the ventral aspects of the caudate are most likely involved in limbic arousal and have high connectivity to the medial prefrontal and orbital prefrontal cortex, whereas the dorsal lateral aspects of the caudate are involved in cognitive processing and habit formation. These dorsal lateral aspects of the caudate have higher connectivity with the dorsolateral prefrontal cortex (Haber & Knutson, 2010).
From the perspective of addiction, the caudate is generally involved with goal directed and motivated behavior (Balleine et al., 2007) and is active during cue-elicited craving (Garavan et al., 2000; Kilts et al., 2004). The caudate has increased BOLD signal when users report feeling the rush and high from cocaine administration.(Breiter et al., 1997; Risinger et al., 2005) Caudate volume may be larger in cocaine users compared to controls, and Ersche et al found that this increase in volume was positively correlated to problems with general attention.(Ersche et al., 2011; Jacobsen, Giedd, Gottschalk, Kosten, & Krystal, 2001) In a study with patients in treatment for cocaine abuse, Sinha and colleagues found that when cocaine users were instructed to imagine stressful situations while in the scanner, they had increased BOLD signal in the caudate. Activation in the caudate during this stress task was also associated with an increase in cocaine craving (Sinha et al., 2005).
Putamen
The putamen is generally involved in movement and learning (Balleine et al., 2007) and its volume has also been found to be larger among cocaine users.(Ide et al., 2014; Jacobsen et al., 2001) The putamen has a role in cocaine craving, with increased BOLD activity during craving following cocaine administration (Breiter et al., 1997). Using PET, Wong and colleagues found that when cocaine users were experiencing cue-induced craving, the putamen had increased levels of dopamine receptor occupancy (Wong et al., 2006). The putamen has also been implicated in cognitive performance, showing decreased BOLD signal compared to controls in a working memory task.(F. G. Moeller et al., 2010) In addition, Brewer and colleagues had cocaine users complete a Stroop color-naming task prior to starting treatment. They found that hypoactivation in the putamen during this cognitive task predicted shorter length of abstinence.(Brewer et al., 2008)
Beyond the Frontal-Striatal Connections
Anterior Cingulate Cortex (ACC)
The ACC is part of the limbic system and is involved regulation of attention and emotion, inhibitory control, error monitoring, and motivation (see (Bush, Luu, & Posner, 2000)). ACC dysregulation in cocaine users may underlie their inability to control their cravings. Cocaine users have decreased gray matter density in the ACC compared to controls (Matochik et al., 2003), and this reduction has been found to be greater for those with a longer history of abuse.(Ersche et al., 2011; Franklin et al., 2002) In addition, numerous reports have demonstrated that cocaine users have lower rates of glucose usage as measured with fluorodeoxyglucose (FDG) and PET, particularly in frontal regions including the orbitofrontal cortex and the cingulate gyri.(Goldstein et al., 2004; Goldstein & Volkow, 2002; Volkow et al., 1991; Volkow et al., 1992; Volkow et al., 2005) These depressed rates of functional activity have been reported to persist for up to three (Volkow et al., 1993) to five months (Hanlon, Beveridge, et al., 2013) of abstinence.
The ACC has increased BOLD signal during cocaine craving following administration (Garavan et al., 2000; Maas et al., 1998; Risinger et al., 2005) and activity is negatively correlated with reports of cocaine high (though see a positive correlation with rush (Breiter et al., 1997; Risinger et al., 2005) Cocaine users have decreased BOLD signal relative to controls in the ACC when completing a task requiring cognitive inhibition (Hester & Garavan, 2004; Kaufman et al., 2003) and visual attention. (Kubler et al., 2005) For instance, abstinent cocaine users in treatment, compared to controls, had less ACC activation when experiencing a stressful memory indicating a lack of ability to control or inhibit the stress.
Insula
The insula is a cortical structure located deep in the brain between the frontal and the temporal lobes, largely divided into the anterior and posterior insula. The insula has structural connections with many of the areas involved in addiction, including the amygdala, basal ganglia, thalamus, OFC, and PFC, giving it an important role in the neural circuitry of cocaine abuse (see (Singer, Critchley, & Preuschoff, 2009)). The insula is involved in emotion processing and arousal including awareness of one’s own bodily states, as well as decision-making and other executive processes. The insula shows increased activation when experiencing cocaine craving and high as measured by PET and fMRI.(Bonson et al., 2002; Kilts et al., 2001; Risinger et al., 2005) Insula volume is reduced among cocaine users, and longer use of cocaine is correlated to smaller volume in the insula.(Ersche et al., 2011) In addition smaller insula volume among cocaine users and abstainers is associated with decrements in attentional control.(Ersche et al., 2011; Hanlon et al., 2011)
Thalamus
The thalamus is one of the most highly interconnected brain regions, serving as a hub for nearly all incoming sensory information, and a relay between the striatum, pallidum, and prefrontal cortical areas. Although we have spent time discussing the importance of frontal-striatal connections in this chapter thus far, it is important to mention that these circuits are part of larger parallel, integrated frontal-striato-thalamic loops (Alexander, DeLong, & Strick, 1986; Middleton & Strick, 2000). From the perspective of cocaine addiction, it appears that thalamic gray matter volume is lower among cocaine-dependent individuals than controls (Sim et al., 2007). BOLD activity in the thalamus has been associated with the high and rush from acute administration (Breiter et al., 1997; Risinger et al., 2005), and during cocaine cue exposure (Garavan et al., 2000). Compared to controls, cocaine users have decreased BOLD signal in the thalamus during a visual attention and memory tasks.(F. G. Moeller et al., 2010; Tomasi et al., 2007a) Furthermore, deactivation of the thalamus during a working memory task has been associated with less effectiveness of treatment as measured by urine screens.(F. G. Moeller et al., 2010)
Ventral Tegmental Area (VTA)
The VTA is a structure in the midbrain where the dopaminergic projections to limbic and cortical areas originate making it a key component of the reward circuitry implicated in addiction. While the ventral striatum encodes positive and negative reward prediction errors in healthy controls, the VTA appears to encode only positive reward prediction errors (D’Ardenne, McClure, Nystrom, & Cohen, 2008). The VTA is a small structure and difficult to image, so activity related to this region is often not reported in studies on cocaine. During a mental fatigue task healthy controls show enhanced activity in midbrain dopaminergic structures, including the VTA; however, this is lost in cocaine users – who show a decrease in activity (S. J. Moeller, Tomasi, Honorio, Volkow, & Goldstein, 2012). Following cocaine administration in the MR scanner, activation in the VTA has been positively correlated with the rush or euphoria reported by cocaine users (Breiter et al., 1997). However, BOLD signal decreases have also been found in the VTA following administration (Kufahl et al., 2005), in line with prior work finding wide ranging reductions in glucose metabolism, including the VTA (London et al., 1990). In considering cue responses without delivery of the drug, one group found positive activity within the VTA, in addition to areas commonly associated with cues (e. g. MPFC, ACC) (Goudriaan, Veltman, van den Brink, Dom, & Schmaal, 2013). Consistent with these findings is increased BOLD response to drug vs neutral words (Goldstein et al., 2009) which take on similarly rewarding properties.
Amygdala
While not explicitly part of the well-defined frontal-striatal-thalamic loops, the amygdala is functionally very well aligned with limbic arousal and also sends projections to the ventral striatum. The amygdala is a subcortical structure receiving inputs from the thalamus and hippocampus, and is important in interpretation of the salience of sensory information and reinforcement.(Davis & Whalen, 2001) The amygdala directs attention and emotional response, memory formation, and instrumental behavior. For instance, increased amygdala activation in response to implicit cocaine cues correlated with positive affective response ratings to the cues—demonstrating its importance in identification of salience of drug-related stimuli even outside of awareness.(Childress et al., 2008) Amygdala volume has been reported as smaller among cocaine-dependent people compared to controls (Makris et al., 2004) however more recent studies with larger sample sizes have found divergent results (Mei, Xu, Carroll, & Potenza, 2015; Xu et al., 2014). Despite no significant differences in volume, Mei et al did find a negative relationship in cocaine users between amygdala volume and measures of impulsivity (Mei et al., 2015). Activation in the amygdala is increased during cue elicited craving.(Bonson et al., 2002; Childress et al., 1999; Kilts et al., 2001; Kufahl et al., 2005) Using PET, Childress and colleagues found increases in CBF in both the amygdala and ACC in cocaine users versus controls while viewing cocaine-related videos.(Childress et al., 1999) and recent work shows increased DA binding within the amygdala in response to cue videos, assessed via [18F] Fallypride (Fotros et al., 2013). As a whole these data support the proposal that increased connectivity between the hippocampal/amygdala regions and the VTA and NAcc plays a role in drug seeking behavior (Volkow, Wang, Fowler, Tomasi, & Telang, 2011).
Hippocampus
The hippocampus has a critical role in encoding and retrieving memory, imagining the future, familiarity and learning. It is one of very few brain regions that exhibits neurogenesis in the adult human (Eriksson et al., 1998). Blocking this neurogenesis in rodents leads to increased cocaine self-administration (Noonan, Bulin, Fuller, & Eisch, 2010). Extensive preclinical work has shown that the hippocampus, along with the amygdala, are involved in stress and cocaine cue induced reinstatement (Atkins, Mashhoon, & Kantak, 2008; Belujon & Grace, 2011; Fuchs, Eaddy, Su, & Bell, 2007; Fuchs et al., 2005; Rogers & See, 2007; See, 2005). Unlike the prefrontal cortex, the hippocampus is not typically smaller in cocaine users than controls (Makris et al., 2004; Mei et al., 2015; Xu et al., 2014), and one group has found that hippocampal volume in individuals entering treatment was prospectively associated with more drug use as measured by urine drug screens and self-report (Xu et al., 2014). Additionally, there is greater cerebral metabolism in the right hippocampus when individuals view cocaine videos compared to neutral videos (Fotros et al., 2013). As with the bulk of cross-sectional neuroimaging studies in substance dependence literature, it is unclear if these neurobiological differences are the product of accumulated cocaine use history (suggesting causality) or if these neurobiological patterns were existed before the individual started using cocaine (suggesting vulnerability to drug use) - the classic “correlation does not demonstrate causation” problem.
Although there are new analytical techniques such as dynamic causal modeling that aim to decode patterns of causality between brain executive, limbic, cortical and subcortical brain regions involved in cocaine addiction (Ray, Haney, Hanson, Biswal, & Hanson, 2015) longitudinal studies are required to test causality directly. In the next section we will discuss a growing body of literature that has performed longitudinal imaging studies of individuals entering drug treatment programs – following them through successful treatment and through relapse. One thing we will not cover however is the emerging body of literature on young adults at risk for initiating and escalation drug use. In 2015 the National Institute of Health initiated the longitudinal Adolescent Brain and Cognitive Development Study, which will follow 10,000 adolescents (all 9 or 10 years old) for 5–10 years. One of the primary aims of this initiative is to determine how drug use patterns effect typical brain development. Another key aim is to determine if there are brain based biomarkers that are associated with vulnerability to illicit drug use and escalation. As data from these adolescents at risk for developing cocaine dependence emerges and is combined with data from individuals entering cocaine treatment programs over the next decade we will develop a more comprehensive taxonomy of the effects of cocaine on the human brain.
SECTION 3: PREDICTING RELAPSE
Watson describing how he weaned Sherlock Holmes off of cocaine: “I was well aware that the fiend was not dead but sleeping; and I have known that the sleep was a light one and the waking near in periods of idleness.”
-The Adventure of the Missing Three Quarter (1904, written 1896)
Thus far, this chapter has focused on structural and functional irregularities that are present in current, typically chronic, cocaine users. For multiple pragmatic reasons, it is very difficult for human clinical studies to determine the extent to which these irregularities were present before the chronic cocaine use began (rendering the individual vulnerable) or are a result of accumulated cocaine burden. It is clear however, that some of these neural circuit irregularities are related to higher rates of relapse among individuals involved in substance abuse treatment programs. In this section of the chapter, we will focus to the utility of using neuroimaging data as a predictor of relapse and exploring the idea that neuroplasticity within these circuit may occur in individuals undergoing outpatient therapy.
Just as the structural and functional irregularities in the brains of chronic cocaine users are not limited to one spatially distinct circuit, there is also an important temporal component to the addiction process. That is, addiction exists on a continuum that likely extends from a vulnerable, drug-naïve individual that casually uses a drug, to an individual that becomes dependent, attempts abstinence and, typically, relapses. While several research groups have isolated traits that predict better than average treatment outcomes in cocaine users (Kampman et al., 2002; Poling, Kosten, & Sofuoglu, 2007; Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006) there are still no FDA approved medications for cocaine dependence and relapse rates remain very high.
Longitudinal studies of neural activity during this continuum are very difficult to perform in substance-dependent individuals for pragmatic reasons (e.g. identifying vulnerable individuals, loss to follow-up due to frequent changes in phone numbers, living arrangements, lack of transportation). There is, however, a growing body of research that has tried to address these questions. In considering only longitudinal assessments with fMRI, the applied methods fall into three overarching categories: 1) resting state fMRI, 2) limbic tasks and 3) executive tasks.
Resting State
Resting state fMRI involves acquiring data while the participant is not performing a task, such that spontaneous fluctuations in the BOLD signal that make up baseline brain activity can be examined (Gusnard, Raichle, & Raichle, 2001). The time courses of activity in one neural region can be correlated with the time course of other brain regions. This cross correlation technique can be applied to a network of preidentified regions of interest (as in dynamic causal modeling and graph theory), between one region of interest and every other voxel of brain imaging data collected (as in seed-based functional connectivity), or in a completely data driven manner (as in independent component analysis).
Previous studies have used resting state functional connectivity in a cross sectional manner, often exploring how cocaine users compare to healthy controls. Resting state studies of substance dependent individuals have found alterations in the normal resting state circuitry (e.g., (Gu et al., 2010; Kelly et al., 2011; Ma et al., 2010). For instance, connectivity, or correlated activity, between regions known to be important in cocaine addiction (VTA, hippocampus, ACC, amygdala, thalamus) was decreased at resting state in cocaine users relative to controls; and less connectivity strength among the VTA to the thalamus and NAcc was correlated with more years of cocaine use. (Gu et al., 2010). Li et al demonstrated that, at rest, acute cocaine administration decreases the functional correlation of voxels within isolated brain regions (S. J. Li et al., 2000). Interestingly, the nucleus accumbens did not show significantly different connectivity, despite its key role in addiction (Gu et al., 2010). Other groups have found reductions in interhemispheric connectivity, localized to frontal regions associated with executive control, motor planning and inhibition (Kelly et al., 2011). These findings suggest that disruption of the reward system in cocaine users can be detected even in the absence of relevant cues.
Recently resting state functional connectivity has been evaluated as a predictor of addiction treatment outcomes. One study examined 18 cocaine users while they were in an intermediate residential program (Camchong et al., 2014). These individuals were scanned at 5 weeks and 13 weeks of abstinence and then contacted for follow-up 6 months later to determine those who had relapsed (n=6, defined as any use). Using seed-based analysis techniques, the investigators measured connectivity with the nucleus accumbens (NAcc) and subgenual anterior cingulate cortex (sgACC; key role in cognitive evaluation and decision making). At the early abstinence phase (5 weeks) individuals that eventually relapsed had higher functional connectivity between the nucleus accumbens and the left prefrontal cortex, and the bilateral posterior cingulate cortex. These individuals also had greater functional connectivity, compared to abstainers, between the subgenual anterior cingulate cortex seed and the left prefrontal cortex. Seven weeks following the first scan, those same regions showed decreased functional connectivity compared to those who abstained. These data indicate that higher levels of nucleus accumbens and sgACC connectivity may contribute to relapse among treatment-seeking individuals.
Another group using seed-based analysis measured connectivity with the dorsal and ventral striatum in treatment-engaged cocaine users. Of the 20 individuals that enrolled in their study, the 9 individuals that relapsed to cocaine use had greater functional connectivity between the NAcc and sgACC. They did not find other significant connectivity differences (Contreras-Rodriguez et al., 2015). These results should be interpreted with caution as another study of 45 individuals placed seeds in similar regions but failed to find significant connectivity differences between the 24 individuals that relapsed and the 21 that remained abstinent (McHugh et al., 2013). In this larger study, the investigators also placed seeds in the basolateral amygdala and corticomedial amygdala. They demonstrated that elevated connectivity between the basolateral amygdala and the lingual gyrus, precuneus and parahippocampal gyrus was related to relapse. They also found that decreased connectivity between the corticomedial amygdala and the vmPFC was a powerful predictor of relapse (79.2% specificity and 66.7% sensitivity) (McHugh et al., 2014).
Given the a priori nature of seed selection, it is an ideal tool to explore findings derived from other imaging modalities. One group studied 40 individuals, using pseudo continuous arterial spin labeling (pCASL), which provides a measure of regional blood flow, interpreted as an indirect marker of synaptic activity. The only difference in cerebral blood flow between the 18 individuals that relapsed and the 22 that remained abstinent was the posterior hippocampus (Adinoff et al., 2015). By using this region as a seed in resting state data collected at the same time point, they discovered that the 18 relapsing individuals had increased functional connectivity between the posterior hippocampus and the posterior cingulate gyrus and precuneus. Connectivity measures were then used to construct a predictive model with 75% accuracy. The same group was able to replicate these predictive findings in an independent sample using the seed derived from their initial study and a measure of cerebral blood flow done with Single-photon emission computed tomography (SPECT, rather than pCASL) (Adinoff, Harris, Gu, & Stein, 2016). This proposed hippocampal hyperactivity supports other work in which hippocampal volume was positively associated with a return to drug use (Xu et al., 2014).
Across all of these studies, which included a variety of demographic measures, cognitive assessments, craving questionnaires it may be surprising that only years of education (Adinoff et al., 2015; McHugh et al., 2014) and years of smoking (McHugh et al., 2014) differentiated between groups. This is difficult to reconcile with findings from other groups that find demographic or behavioral measures to be predictive of poor outcomes, such as impulsivity (F. Gerard Moeller et al., 2001), stress induced craving (Sinha et al., 2006), but could be due to the increase in sensitivity provided by neuroimaging thereby allowing a detection of differences with smaller sample sizes.
Limbic Tasks
Whereas resting state data is able to investigate the baseline connectivity of limbic structures, task based fMRI enables more specific investigations, such as determining the neural correlates of receiving rewards or viewing images containing drug cues. Previously discussed cross sectional analyses have implicated drug induced limbic alterations in continued addiction (Volkow et al., 1991; Volkow et al., 1992; Volkow et al., 2005). These alterations can be easily appreciated by examining the brain responses to cue images or videos that depict a drug or drug use. For an individual who does not use the drug, these images will reliably activate visual areas of the brain, but in a current or former cocaine user there is increased activation across the brain, particularly in areas associated with reward (Wilcox, Teshiba, Merideth, Ling, & Mayer, 2011).
In 2006 a group showed a cocaine cue video to 17 cocaine dependent individuals prior to their inclusion in a clinical trial involving the administration of a selective serotonin reuptake inhibitor. Neural activity during the first 30 seconds of this video was compared to a neutral video determine its validity as an indicator of treatment outcomes (Kosten et al., 2006). These individuals were followed throughout outpatient treatment and the sum of all cocaine negative urine drug screens (UDS) was calculated (Treatment Effectiveness Score: TES (Ling et al., 1997)) Diverse regions of the brain correlated with the TES, including the left precentral gyrus, posterior cingulate, the right lingual gyrus, inferior occipital gyrus and the superior temporal gyrus. The 17 individuals were partitioned into relapse vs non relapse on the basis of their TES, revealing that increased activity in the posterior cingulate cortex, extending up to the boundary with the anterior cingulate and the left premotor cortex significantly differentiated those that relapsed from those that did not. No measures derived from demographics or craving significantly different between the groups, though interpretation is limited as this sample consisted of cocaine users diagnosed with depression.
One method frequently used to examine reward processing in addiction is the Monetary Incentive Delay Task (MIDT) (Knutson, Westdorp, Kaiser, & Hommer, 2000). During this task participants view a screen and first see an indicator of the type of upcoming event (potential gain, loss or no change). This is followed by a simple reaction time task, another delay, and then feedback about their gains or losses on that trial. When performed in a neuroimaging context the primary dependent measures in this task are brain activity in response to the anticipation of a reward (or loss), and the activation in response to receiving the reward (or in response to losing). Each of these can be reliable dissociated and measured independently to reveal the functioning of reward circuitry (Knutson, Fong, Adams, Varner, & Hommer, 2001). This task was first used as a predictor of relapse in 20 cocaine users, scanned prior to their first treatment session (2 separate clinical trials, random assignment) (Jia et al., 2011). These individuals were followed for 8 weeks to determine drug use. Using a priori regions of interest they discovered a negative correlation between activation in the bilateral thalamus and right putamen and cocaine negative urine toxicology. Additionally, activation of the left amygdala and parahippocampal gyrus was inversely associated with treatment retention. During the outcome phase they found that right thalamus, ventral striatum and left culmen were inversely correlated with negative urine toxicology. Whole-brain analysis further revealed areas of cognitive control with positive relationships with positive urine drug screens, such as the right middle frontal gyrus and posterior cingulate cortex during anticipation, and the right anterior cingulate cortex during outcome. These correlations of poor outcomes with non-drug reward brain activity reinforce the heavy involvement and alteration of natural reward systems in cocaine addiction.
These ideas were extended by further work in the same laboratory, in which individuals were scanned before and after treatment (Balodis et al., 2016). They found that elevated activity in the nucleus accumbens in response to a loss (post > pre) was negatively correlated with the number of negative urines. Individuals with a higher nucleus accumbens response to a monetary loss, were more likely to have positive urine drug screens for cocaine.
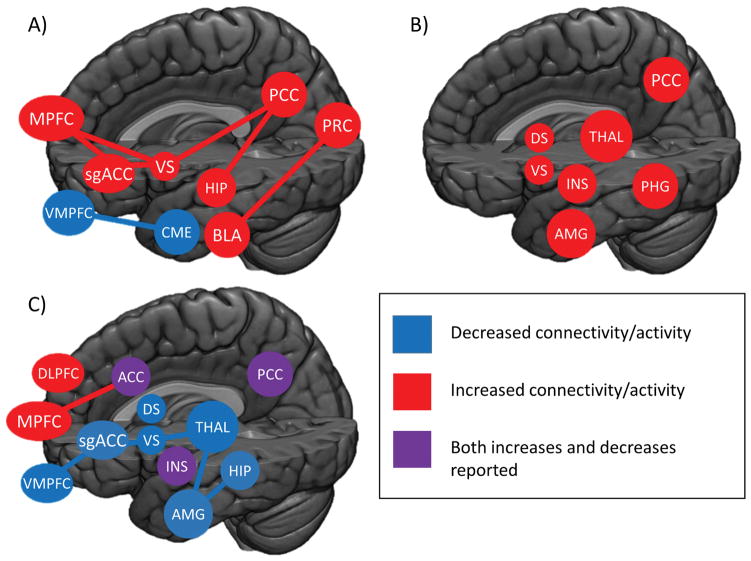
Taken together, nearly all of these data suggest that disrupted engagement of limbic (e.g. striatum, thalamus) regions during tasks that probe reward processing is associated with poorer outcomes. Given that these heightened responses appear to be predictive in response to both positive (cues, anticipation of reward) and negative (being informed of a loss) valence this may point towards higher gain within these circuits, leading to maladaptive behaviors. Methods that are able to attenuate activity in these regions may hold promise in reducing returns to drug use in these populations. In context with the resting state data, these studies of reward task engagement add to the evidence that elevated activity and connectivity with the limbic reward system (particularly the nucleus accumbens) is associated with poor outcomes in treatment. (Figure 2)
Figure 2. Regional brain connectivity or activity that was associated with poor treatment outcomes.
A) Results from seed-based connectivity during resting state. B) Activation related to poor outcomes during limbic-associated tasks is consistently elevated. C) Reduced activation during tasks measuring executive function was associated with worse outcomes, though cortical regions associated with internal state and self-reflection show mixed responses. Abbreviations. ACC: Anterior Cingulate Cortex; AMG: Amygdala; BLA: Basolateral Amygdala; CME: Corticomedial Amygdala; DS: Dorsal Striatum; HIP: Hippocampus; INS: Insula; MPFC: Medial Prefrontal Cortex; PCC: Posterior Cingulate Cortex; PHG: Parahippocampal gyrus; PRC: Precuneus; sgACC: subgenual Anterior Cingulate Cortex; THAL: Thalamus; vmPFC: ventromedial prefrontal cortex; VS: Ventral Striatum
Executive Tasks
Drug use is also associated with altered executive function, exemplified by poorer working memory (Tomasi et al., 2007b), difficulty in attentional shifting (Kubler et al., 2005) or measures of inhibition and mental flexibility (Verdejo-Garcia, Bechara, Recknor, & PÉRez-GarcÍA, 2006). While behavioral measures of such impairments can be valuable in predicting treatment completion (Streeter et al., 2008), the combination of brain imaging allows a determination of the anatomical substrates of these changes.
The Stroop color-word task is a classic and often modified tool to examine various aspects of cognitive control, including attentional bias. This involves the identifying the color in which a color name is written. In some cases, the color name and the color of the text match (congruent stimuli) and in others they do not (incongruent stimuli). Streeter and colleagues (2008) investigated Stroop performance in 74 individuals enrolled in a cocaine treatment trial. Individuals that completed the trial (n=50) took significantly less time on the Stroop task overall, particularly when there was interference. In their study, Stroop performance was a very robust predictor of trial completion, more so than Hamilton Depression Inventory scores, which were also significant. Yet another study using a version of the Stroop task that contains drug cue words failed to find a relationship between Stroop performance and cocaine relapse (Kennedy, Gross, Ely, Drexler, & Kilts, 2014). Brewer and colleagues (2008) also collected neuroimaging and behavioral data from 20 treatment-engaged cocaine users performing the Stroop task. Individuals with longer reaction times when responding to congruent and incongruent images remained enrolled in treatment significantly longer, however there was no relationship between behavioral performance and urine drugs screens (Brewer et al., 2008).
From a neuroimaging perspective, Brewer and colleagues (2008) demonstrated that decreased activity in the right putamen during the Stroop task was associated with more cocaine positive urine drug screens. Exploratory analysis at a lower statistical threshold (p <0.01 compared to p < 0.005) revealed a negative correlation between left DLPFC and weeks in treatment, such that the less activated this region was, the better the outcomes (Brewer et al., 2008). These data were further analyzed using Independent Component Analysis (ICA) (Worhunsky et al., 2013). This involves separating the whole brain BOLD signal timecourse into spatially distinct networks (made up of multiple brain regions), with each network being associated with a unique pattern of activity over time. In healthy controls at rest, this has yielded a set of canonical networks with high reliability (Damoiseaux et al., 2006). For this population, 24 networks were extracted from the data, with a subset of 5 that were engaged during incongruent stimuli selected for further analysis. These were the cingulo-opercular, fronto-parietal, fronto-cingular, subcortical and fronto-striatal networks. Activation in each network during the task was examined for relationships with treatment outcomes. Of the 5 networks, only 3 had further correlations with treatment. First, engagement of the fronto-cingular network (which includes the ACC, the medial and middle frontal regions and the dorsal insula) had a negative relationship with the number of weeks completed, in that more activation during incongruent stimuli yielded worse performance. Within the subcortical network, which consisted of the thalamus, striatum, amygdala, hippocampus as well as left inferior frontal cortex, lower engagement of this component was associated with more cocaine positive urine drug screens. Within the ventral fronto-striatal network (which included deactivations during incongruent stimuli in the vmPFC and subgenual and rostral ACC), deactivation was associated with more cocaine positive drug screens.
Similar to ICA, intrinsic connectivity (ICD) attempts to discover underlying network relationships. This method assigns each voxel a value based on the number of high correlations it has with other gray matter voxels in the brain. Mitchell et al employed this during the Stroop word-color task (Mitchell et al., 2013). A total of 15 cocaine users, selected from outpatient groups and 15 healthy controls were imaged, and the cocaine users were followed for a total of 12 weeks, with outcomes being determined via self-report (TLFB) and positive UDS (Percentage of positive screens). Similar to previous findings (Brewer et al., 2008) more interference was associated with more cocaine free urines. Globally the cocaine users showed considerably less intrinsic functional connectivity when compared to the healthy controls. When considering urine toxicology reports, ICD within the thalamus, ventral striatum and substantia nigra, right insula and left hippocampus were positively correlated with the percentage of positive screens. Together these data poor outcomes in cocaine treatment programs are associated with dopaminergic and limbic function even when investigating neural engagement in an executive control task.
The Stroop task can be modified to explicitly investigate how cocaine related language has modified attention. Rather than using colors with color names, the modified Stroop drug-word task uses cocaine related words and neutral words to measure cocaine related interference. Individuals continue to identify the color of a word, but cognitive processing is thought to be disrupted by the increased salience that cocaine related words have acquired (Hester, Dixon, & Garavan, 2006). These disruptions were examined in a group of 26 cocaine users who were followed for 3 months. They restricted their analysis to cocaine minus neutral words in regions previously implicated in attentional bias, specifically the dorsal anterior cingulate, insula, DLPFC, NAcc, amygdala and insula. Using a stepwise regression model to predict use, only craving in the week prior to treatment and right dorsal ACC were predictive of drug use in follow-up. No Stroop performance measures or demographic variables (years of use, route of administration) reached criteria to be included into the model. (Marhe, Luijten, van de Wetering, Smits, & Franken, 2013)
Other tasks to measure cognitive control, explicitly inhibition, include the Oddball or Stop-signal task. These tasks involve monitoring stimuli, and responding only when a target, but not distractor or stop signal is shown. The stop-signal task differs in that the go signal will be replaced (very quickly) with a stop signal, whereas in the oddball task the image presentation is either a target, or the oddball (the rare stimuli). Much of the work done with the oddball task involved measuring EEG event related potentials (ERPs) to a rare target or distractor stimuli. In response to the stimuli that the subjects have been instructed to attend to, the brain reliably produces a P300 ERP. This task has been expanded into the fMRI to take advantage of anatomical specificity (Clark, Fannon, Lai, Benson, & Bauer, 2000).
Clark and colleagues (2012) used an oddball task to capture selective attention and examined BOLD signal in 45 recently abstinent individuals who were then followed for 6 months to determine abstinence (22 individuals) vs relapse (23). All individuals showed reliable activation to the target stimuli, though no differences were found between BOLD response in those who relapsed, versus those who abstained. On the other hand, the BOLD response to distracting stimuli was significantly higher in the posterior cingulate cortex, right insula and anterior cingulate cortex in the relapsing group vs the abstinent group. No other demographic or behavioral measures reached statistical significance, though this group used an incredible variety of measures (over 20 in total). Poorer results on the Wisconsin Card Sorting Task, self-reported frequency and quantity of drugs of abuse used recently as well as CDIS-IV scores of PTSD and Mania were all associated with relapse but failed to meet the more stringent criteria required by multiple comparison correction. No significant differences were found in reaction time or the false alarm rate.
Though there were few significant demographic and behavioral differences as measured by a two sample t-test, the inclusion of prior drug dependence, years of education, Trail B (from Trails Making Test (Bowie & Harvey, 2006)) and the WCST “failure to maintain set” measure in a model led to obtaining a classification accuracy of 75.6%. With a similar logistic regression model the imaging data alone, as amplitude measures from the PCC and right insula obtained greater accuracy (77.8%) with higher statistical significance. The best performance was obtained using a feed forward neural network model (multilayer perceptron) that included on amplitude measures from the right PCC, insula, and precentral cortex and a diagnosis of mania. This model was able to obtain overall accuracy of 88.9% (sensitivity 87%, specificity 90.9%). Cross-validation (10-fold) verified the robustness of this model, with a final accuracy of 84.4%, which was equivalent to Bayesian logistic regression. This work is an extension of early work in the prediction of cocaine relapse that used an oddball task with EEG (Bauer, 1997) with similar findings.
Although the lifetime prevalence of cocaine use among men and women is similar, women tend to have higher rates of relapse and experience an accelerated transition to addiction, commonly called telescoping (Becker & Hu, 2008; Brady & Randall, 1999). Luo and colleagues (2013) sought to explore the potential contributions of cognitive control processes in these gender differences. To this end, they recruited 97 treatment seeking individuals (37 female) who performed a common stop-signal task during short term abstinence and were followed for 90 days after discharge from inpatient treatment. They chose to examine when stop error was greater than stop success, which is thought to reflect attentional monitoring and inhibition. While no behavioral measures from the stop signal task were predictive, using logistic regression and Cox regression for relapse and time in treatment respectively, they had robust imaging findings. Within females, decreased activation of the thalamus and ACC in stop error versus stop success was predictive of relapse. Furthermore, decreased activation of the thalamus was associated with an earlier time to relapse. In males, decreased anterior cingulate and left insula activity were predictive of relapse. When combined, the dorsal anterior cingulate remained predictive of relapse, suggesting that the thalamus and insula may be gender specific correlates of relapse, as measured by this task. Accuracy measures of their predictive model tended to exceed 70%, though when this sampled was split divided into training versus test sets, area under the curve (AUC; a measure of model accuracy) was reduced to approximately 60%.
Other work using a Go, no-go task only found a difference in activity within the precentral gyrus differentiated those who used prior to a one week follow up, with no differences found in behavioral measures (Prisciandaro, Myrick, Henderson, McRae-Clark, & Brady, 2013). In considering working memory, a group examined 19 cocaine users and 14 controls with fMRI while they performed a delayed memory task, requiring them to keep either 3, 5 or 7 digits in visual working memory. Outcomes were measured by TES and the total weeks of retention in the treatment protocol. They first extracted the mean activation from each of clusters in which controls (n=14) showed greater activation, which included regions within the prefrontal cortex, striatum and thalamus. Only the thalamic cluster showed a significant positive relationship with TES (r = 0.642), in that increased activation of the thalamus during working memory load was associated with better TES. No relationship between activation and weeks of retention was found (F. G. Moeller et al., 2010). Overall, decreased activity in response to executive tasks is associated with poor treatment outcomes.
These studies have revealed a variety of neural substrates across multiple functional MRI measures have associations with treatment outcomes. We first consider that when examining networks at rest, or examining intrinsic network connectivity we find that greater connectivity within typical limbic or reward related circuitry is associated with poorer outcomes – the network tone is too high. The inclusion of the posterior cingulate cortex across multiple measures is of interest, given its negative functional connectivity with the DLPFC and other regions of the cognitive network (Yu et al., 2011) and limbic regions, including the subgenual cingulate cortex (Vogt, Vogt, & Laureys, 2006) and insula (Cauda et al., 2011).
The second finding is that reward task related activity in limbic structures is associated with relapse, but this appears to be an issue of gain, as we see that heightened reward processing is predictive of a return to drug use. We must also consider the inverse patterns seen during executive processing, in which poor treatment outcomes are associated with less activity in similar brain regions, suggesting that these brain regions are insufficiently engaged. This lends itself to the conclusion that better measures of these inappropriate activations and deactivation in these regions may lead to robust predictors.
Moving Forward
As a whole these findings suggest that altered baseline and task-evoked activity in subcortical structures involved in limbic processing are fairly effective predictors of relapse. This pattern appears to be true when individuals are engaged in tasks which typically tax their reward circuitry as well as during tasks that typically tax their executive control circuitry. While the predictive validity of the limbic system as a biomarker for relapse seems high and several of these studies are very well powered, it is important not to interpret the lack of results from the executive control system as evidence that this system is NOT a predictor of relapse. Rather, we are unable to form strong conclusions regarding the role of the executive control system in relapse because the published studies to date have not explicitly placed seeds in areas that are more often associate with cognitive control, such as the dorsolateral prefrontal cortex. Within the resting state data for example, the selection of seeded regions (striatum, amygdala, hippocampus) biases our observations towards finding limbic regions. The use of ICA to analyze resting state data would be one approach to reveal contributions of more frontal and lateral regions. The application of different network analysis methods to these datasets is only limited by the availability of the data.
Beyond new analyses on existing data sets, there is a need to test predictors on independent data. Among these examples the collection of resting state data is an obvious choice. While concerns remain regarding inter-laboratory variance in instructions given to the participants while they lay in the MRI scanner (e.g. instructing them to keep their eyes closed versus eyes open), the lack of task constraints makes these existing data sets ideal for investigating alternative seeds.
A larger challenge that we face as a field trying to reconcile all of these studies is dealing with the variability of treatment that groups experience as well as the criteria by which treatment outcomes are determined. This is not meant to disparage opportunistic research, which has been critical in examining this field, but rather further highlight the value of studies that explicitly study more selective treatments. More standardized agreement on the best dependent measures to use, the crucial periods of sampling individuals during their drug use and treatment histories, and consensus on the types of comorbidities that are acceptable for enrollment in cocaine treatment trials would improve our assessments of the predictive validity of neuroimaging data – a step in the transition of functional and structural MRI to something that is clinically useful.
SECTION 4: BRAIN TO BEDSIDE-developing neural circuit based brain stimulation treatments for substance dependence
The theories which I have expressed there, and which appear to you to be so chimerical, are really extremely practical — so practical that I depend upon them for my bread and cheese.”
A Study in Scarlet, Sherlock Holmes (Arthur Conan Doyle,1887)
Now, with accumulating knowledge that limbic circuit abnormalities are related to relapse and poor outcomes in outpatient cocaine treatment programs, we, as a scientific community, have the burden of using this knowledge to help the prognosis and eventual outcomes of these treatment-seeking individuals. How will we do it? Are these circuits malleable and amenable to long term change? If we can change the resting state functional connectivity of the limbic circuit, will this in turn result in a clinically meaningful change in drug use, craving, and daily living for these individuals? Through the development of sophisticated molecular and optogenetic imaging techniques in the last 10 years we know that modifying activity in these frontal-striatal circuits can attenuate, and perhaps abolish, cocaine seeking in animals with extended cocaine use histories. Although this would potentially be a very fruitful strategy for treatment of cocaine use disorders, until recently, it has been impossible to alter activity in these frontal-striatal circuits in humans in a non-invasive manner. In this section we will discuss a growing new domain of research in addiction which is utilizing non-invasive transcranial magnetic stimulation (TMS) as a tool to decrease drug use and associated behaviors (e.g. craving) through long term potentiation and/or depression of the frontal-striatal circuits which have been discussed in the previous sections. Although this line of research is still in its infancy, the development of innovative, biologically-based brain stimulation therapies for substance dependence is among the best examples of translating decades of functional neuroimaging research into a clinically meaningful treatment.
The earliest treatments for addiction sought to address the underlying maladaptive behaviors, including compulsive use despite negative consequences. The behavioral interventions still used frequently include contingency management (in which patients are rewarded for maintaining sobriety) and cognitive behavioral therapy (in which patients learn to control and adjust behaviors that lead to drug taking). Although the behavioral therapies can play an important role in supporting abstinence, studies indicate that their efficacy is low to moderate depending on the substance of abuse (Dutra et al., 2008). As knowledge grew about the biological mechanisms for addiction, pharmacotherapies were explored based on the implicated receptors systems. To date, there are no FDA-approved pharmacologic treatments for either cocaine or methamphetamine, however some studies have shown modest reductions in substance use.
In the last 10 years, we have seen rapid advancement of a novel form on non-invasive human brain stimulation, repetitive transcranial magnetic stimulation. Through non-invasive brain stimulation techniques such as repetitive transcranial magnetic stimulation (rTMS), it is possible to induce circuit-specific long term depression (LTD) or potentiation (LTP) by applying different frequencies of stimulation to different cortical regions (reviewed in (Fitzgerald, Fountain, & Daskalakis, 2006; Thickbroom, 2007).
An FDA-approved treatment for major depressive disorder since 2008, rTMS is currently being evaluated as a treatment for craving among multiple substance dependent individuals (Table 2). The potential of rTMS as a new tool for modulating craving among substance dependent populations has garnered significant attention from both the National Institute of Health (NIH) and in the literature [see reviews: (Barr et al., 2011; Bellamoli et al., 2014; Feil & Zangen, 2010; Gorelick, Zangen, & George, 2014; Wing et al., 2013)].
WHAT IS TMS?
Transcranial magnetic stimulation is a non-invasive form of brain stimulation which induces a depolarization of neurons through electromagnetic induction. When applied over the hand knob of the primary motor cortex, a single, transient pulse of current through the TMS coil induces a reliable contraction of the contralateral hand which is proportional to the amplitude of the induced electrical field (Barker, Freeston, Jabinous, & Jarratt, 1986). Recently Mueller et al (2014) developed a technique which allowed them to simultaneously apply a real or sham TMS pulse to the scalp of an awake behaving non-human primate and measure induced action potentials and electric field strengths in the area below the coil (Mueller et al., 2014). When integrated into a magnetic resonance imaging environment, a single pulse of TMS induces an elevation in the blood oxygen level dependent (BOLD) signal in the area under the coil and in monosynaptic target regions (D. E. Bohning et al., 1998). The amplitude of the BOLD signal induced by a single pulse of TMS to the primary motor cortex is not significantly different than the amplitude of the BOLD signal induced by an individual that is instructed to squeeze their hand in a manner than mimics the TMS evoked muscle contraction (Denslow, Lomarev, George, & Bohning, 2005). This correspondence between the TMS-evoked (‘external’) BOLD signal and the internally evoked BOLD signal produced by a participant is important when considering how a TMS-evoked BOLD signal in the prefrontal cortex may mimic the BOLD signal evoked by an internal craving state or external cue exposure. Through the use of TMS in the MRI scanner we recently demonstrated that it is possible to differentially activate fronto-striatal circuits involved in limbic control from those involved in executive control through stimulating the MPFC and DLPFC respectively (Hanlon, Canterberry, et al., 2013).
Whereas single pulses of TMS coupled with neuroimaging provide a controlled method to probe function in a neural circuit, repetitive pulses of TMS can be used to induce long term potentiation (LTP) or long term depression (LTD) in a given neural region as well as its monosynaptic afferents (Bestmann, Baudewig, Siebner, Rothwell, & Frahm, 2004; Daryl E. Bohning et al., 2000; Denslow et al., 2005; Nahas et al., 2001). The effects of TMS on brain activity however are dependent upon several factors including the spatial location of stimulation, and stimulation protocol itself (which can be varied by frequency, intensity and duration).
An LTD-like effect can be achieved through TMS by either using a low frequency stimulation (typically 1 Hz) or through a bursting frequency, such as continuous theta burst (Huang et al 2005). In preclinical literature theta burst stimulation is a well-known form of electrical stimulation which, can induce long term potentiation (LTP) or depression (LTD) of synaptic activity in a given brain region (Bear & Malenka, 1994; Malenka & Bear, 2004). Human theta burst stimulation protocols use rTMS to induce similar forms of LTP and LTD by using intermittent or continuous bursts respectively (Di Lazzaro et al., 2005; Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005). With continuous theta burst stimulation (cTBS), bursts of 3 pulses at 50 Hz are applied at a frequency of 5 Hz at amplitude that is typically determined by the active motor threshold. When performed over the primary motor cortex, a lower amplitude of cTBS for 40 seconds leads to an attenuation of motor evoked potentials that is comparable to a higher amplitude to 1Hz single frequency stimulation for 20 minutes. Stagg and colleagues have demonstrated that this attenuating effect of cTBS is likely due to an increase in γ-aminobutyric acid (GABA) at the area of stimulation (Stagg et al., 2009) rather than a change in glutamate.
What are the potential targets for addiction?
As discussed above, among cocaine users attempting abstinence, high rates of relapse may be due to dysfunction in at least 2 neural circuits: 1) elevated functional activity within limbic neural circuitry, in the presence of a salient cue, (Ersche et al., 2011; F. Gerard Moeller et al., 2001) (including the medial prefrontal cortex and ventral striatum) or 2) depressed activity in the executive control loop (Goldstein et al., 2004; Kubler et al., 2005; F. G. Moeller et al., 2010) which is likely required to resist the limbic drive for the drug (including the dorsolateral prefrontal cortex and dorsal striatum). Selective modulation of these frontal-striatal circuits, specifically increasing the strength of the executive control network or decreasing the engagement of limbic reward circuit when an individual is presented with cocaine cues. (Figure 3).
Figure 3. Potential strategies for developing novel brain stimulation treatment strategies for addiction.
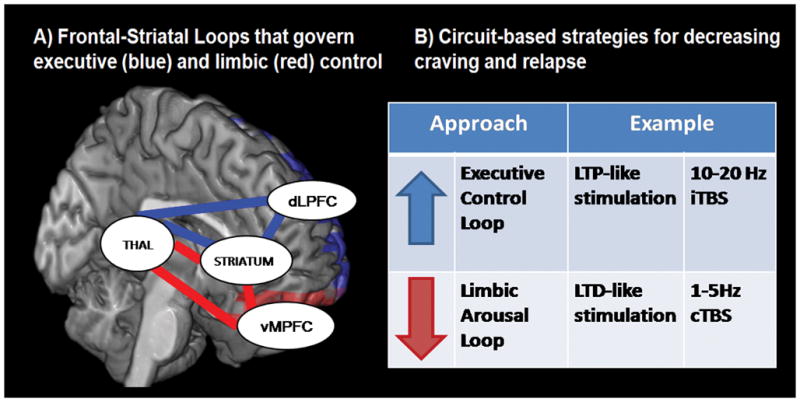
which leverage our knowledge of neural circuit irregularities that contribute to relapse among treatment-seeking cocaine users.
Increase executive control network
To date nearly all of the rTMS studies in addiction have targeted the same neural region – the dorsolateral prefrontal cortex (Camprodon, Martinez-Raga, Alonso-Alonso, Shih, & Pascual-Leone, 2007; Eichhammer et al., 2003; Herremans et al., 2012; Hoppner, Broese, Wendler, Berger, & Thome, 2011; X. Li et al., 2013; Mishra, Nizamie, Das, & Praharaj, 2010; Politi, Fauci, Santoro, & Smeraldi, 2008; Pripfl, Tomova, Riecansky, & Lamm, 2014). While many of these studies demonstrated that high frequency (LTP-like) rTMS stimulation to the DLPFC can result in a significant reduction of craving, the neurobiological mechanism through which this might happen is not clear. In a comprehensive review of the literature on the efficacy of rTMS as a treatment tool for smoking, Wing and colleagues (2013) present a model in which the beneficial effects of LTP-like TMS on the DLPFC are associated with a release of dopamine in the nucleus accumbens. This model is supported by important work from Strafella and colleagues which used positron emission tomography to demonstrate that DLPFC stimulation was associated with an increase in dopamine binding in the caudate (Strafella, Paus, Barrett, & Dagher, 2001).
Decrease craving network
The primary cortical input to the nucleus accumbens however is not the dorsolateral prefrontal cortex, but rather the medial and orbital prefrontal cortex. Given that the nucleus accumbens is one of the primary brain regions involved in craving, it seems that targeting the medial prefrontal cortex would be a more direct method to modulate nucleus accumbens activity among substance dependent populations. Given that craving for cocaine is associated with an increase in dopamine in the striatum, it is reasonable to pursue an LTD-like rTMS protocol over the medial prefrontal cortex to attenuate activity in this neural circuit. Prior data from our laboratory demonstrates that a single pulse of transcranial magnetic stimulation (TMS) to the medial prefrontal cortex in healthy individuals leads to an increase in BOLD signal in the ventral striatum (Hanlon, Canterberry, et al., 2013).
Recent extension of that work to cocaine dependent population (n = 15) has demonstrated that the cocaine users have a hyperactive BOLD response in the dorsal and ventral striatum relative to controls (n=15) (Hanlon et al 2016, in press). This elevated ventral striatal sensitivity following medial prefrontal cortex stimulation, a fronto-striatal circuit involved in the limbic aspects of craving, may be a prime circuit to attenuate in order to may these individuals less vulnerable to drug related cues.
In 2015, our group demonstrated that continuous theta burst stimulation to the vmPFC can decrease BOLD signal in the vmPFC and the nucleus accumbens of chronic cocaine users (Hanlon et al., 2015) (Figure 4). An extension of this study, examining the effects of continuous theta burst stimulation on cocaine cue-evoked brain activity, demonstrated that there was a significant amount of individual variability in treatment response within the group which was related to baseline variance in striatal network engagement when an individual viewed cocaine cues. Specifically, individuals that had stronger striatal activity when viewing cocaine cues, experienced a stronger attenuation of striatal activity to cues following theta burst stimulation. On the other hand, individuals with low striatal engagement to cocaine cues, had an elevated response to cues following cTBS. (Kearney-Ramos et al, under review). These data highlight the importance of considering individual variability in future clinical trials of rTMS as a treatment adjuvant to behavioral therapy for cocaine dependence. They will also need to be extended to determine if a positive treatment response can be predicted in a prospective manner.
Figure 4. The effects of continuous Theta Burst Stimulation (cTBS) to the left vmPFC/OFC in cocaine users (.
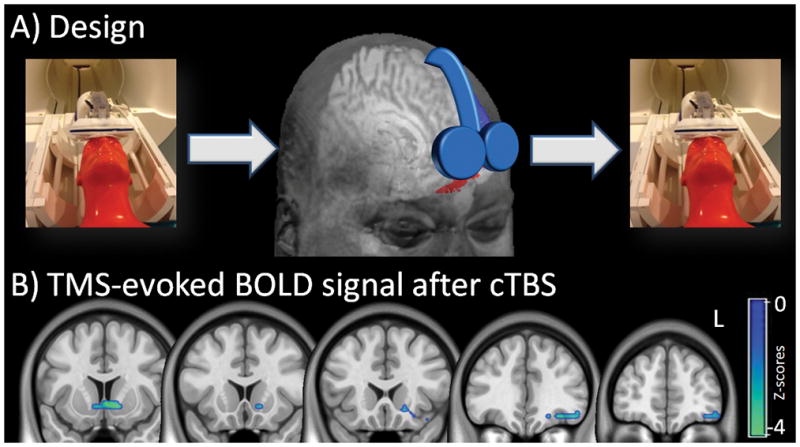
adapted from Hanlon et al 2015). interleaved TMS/BOLD imaging was used to measure TMS-evoked BOLD signal immediately before and after cocaine users were given a dose of cTBS to the left frontal pole (A). The TMS coil was placed over FP1 (EEG:10–20 system) for both the Interleaved TMS/BOLD scan (left & right panel) and the cTBS session (center panel). The red area represents the region of interest to which the coil is targeted (AAL: left superior and middle orbital prefrontal cortex inferior to the anterior commissure). Real cTBS (LTD-like) led to a significant decrease in BOLD signal in the left orbital/medial prefrontal cortex and ventral striatum (SPM8, p<0.05 Family Wise Error correction, negative Z-scores shown). The cTBS protocol was 2 trains of 1800 pulses, 110% RMT, 60 second intertrain interval., intensity ramped from 80–110% over first 30 sec. L = left hemisphere.
Moving Forward
Before pursuing large expensive clinical trials of dorsolateral prefrontal cortex stimulation or medial prefrontal cortex stimulation as a potential treatment adjuvant to cognitive behavioral therapy among substance dependent individuals however, it is critical to determine whether it is tolerable, feasible, effects craving, and improves outcomes to treatment-engaged individuals.
Here, we have presented a collection of findings (by no means intended to be exhaustive) from the perspectives of clinical neuroimaging in an attempt to answer two questions about whether there are markers or neural signatures that can predict recovery. While this chapter has focused on the alterations in brain structure and functional activity in the brains of cocaine users and abstainers, we have not addressed the critical behavioral hallmarks of the addiction and recovery process. From impulsivity to craving, cognitive set-shifting to measures of self-control, there is a very large body of research which has characterized behavioral phenotypes of addiction in both human users and in non-human primates following exposure to cocaine (Beveridge, Gill, Hanlon, & Porrino, 2008; Garavan & Hester, 2007; Stalnaker, Takahashi, Roesch, & Schoenbaum, 2009). As we attempt to bridge the neurobiological findings from clinical and preclinical studies of cocaine use and abstinence, it will be important to integrate the literature on behavioral patterns that predict successful recovery. Through the integration of these components we will be much more likely to generate individually-tailored therapies, both pharmacologic and behavioral, for those that find themselves on this continuum of addiction, from vulnerable adolescents to treatment-seeking individuals that recurrently relapse.
With regard to biomarkers that can predict successful recovery from cocaine abuse, we propose here that it is the functional engagement of the limbic system (specifically the vmPFC, OFC, ventral striatum, amygdala and insula) is perhaps the most important predictor. Longitudinal studies will be necessary to confirm this hypothesis, but an increasing numbers of studies that have been focused on the biology of successful recovery support this idea. While exploratory analysis decoding the differences between those who relapse and those do not is important, the use of predictive models employing training and test sets is crucial to reduce overfitting and improve the replicability of these findings. The next step that we all face is how to maximize the utility of this knowledge for our patients.
Table 1.
Studies that have examined the predictive validity of neuroimaging markers
| Study | Task | User Sample | Abstainer/Relapse or Continuous Variable | Controls | Domain | When was data collected? | Follow Up Time Frame |
|---|---|---|---|---|---|---|---|
| Kosten et al 2006 | Cue and Neutral Videos | 17 | 8/9 | n/a | Limbic | After two weeks of cocaine abstinence before any treatment | 10 weeks |
| Brewer et al 2008 | Stroop (Color Word) | 20 | CV (% UDS, self-report, treatment retention) | n/a | Executive | After an average of 5.35 days abstinence | 8 weeks |
| Moeller et al 2010 | Delayed Memory Task | 14 | CV (TES, treatment retention) | 19 | Executive | Prior to entering treatment | 16 weeks |
| Jia et al 2011 | Monetary Incentive Delay | 20 | CV (UDS, self-report, treatment retention) | 20 | Limbic | Before first treatment session | 8 weeks |
| Moeller et al 2012 | Stroop (Drug-Word) | 15 | 8/7 | 13 | Limbic | After 3 week detox, and follow-up | 6 months |
| Luo et al 2013 | Stop Signal | 97 | 17/80 | n/a | Executive | After 2–4 weeks abs in a monitoring unit | 90 days |
| Marhe et al 2013 | Stroop (Drug-Word) | 26 | CV (UDS, self-report) | n/a | Executive | 4 days after entering detox treatment | 3 months |
| Mitchell et al 2013 | Stroop (Color-Word) | 15 | CV (UDS, self-report abs.) | 15 | Executive | Prior to beginning treatment | 6/12 weeks |
| Prisciandaro et al 2013 | Cues, Go no-go task | 30 | 24/6 | n/a | Limbic | Within 1 week of entry into trial | 1 week |
| Worhunsky et al 2013 | Stroop (Color-Word) | 20 | CV (UDS, self–report, treatment retention) | 20 | Executive | After an average of 5.35 days abstinence | 8 weeks |
| Camchong et al 2014 | Resting State | 18 | 12/6 | 15 | Resting | After 5 and 13 weeks residential treatment | 6 months |
| Clark et al 2014 | Oddball Task | 45 | 22/23 | n/a | Executive | After 1 weeks abstinence | 6 months |
| McHugh et al 2014 | Resting State | 45 | 21/24 | 22 | Resting | Final Week of Treatment | 30 days |
| Adinoff et al 2015 | Resting State | 40 | 22/18 | 21 | Resting | Final Week of Treatment | 30 days |
| Contreras-Rodriguez et al 2015 | Resting State | 20 | 11/9 | 21 | Resting | After 15 days abstinence | 12 weeks |
| Balodis et al 2016 | Monetary Incentive Delay | 29 | CV (UDS) | 28 | Limbic | Pre- and post- treatment | up to 1 year |
References
- Adinoff B, Gu H, Merrick C, McHugh M, Jeon-Slaughter H, Lu H, … Stein EA. Basal Hippocampal Activity and Its Functional Connectivity Predicts Cocaine Relapse. Biol Psychiatry. 2015;78(7):496–504. doi: 10.1016/j.biopsych.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Harris TS, Gu H, Stein EA. Posterior hippocampal regional cerebral blood flow predicts abstinence: a replication study. Addict Biol. 2016 doi: 10.1111/adb.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90(3):481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. 27/31/8161 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, Potenza MN. Neurofunctional Reward Processing Changes in Cocaine Dependence During Recovery. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT, Freeston IL, Jabinous R, Jarratt JA. Clinical evaluation of conduction time measurements in central motor pathways using magnetic stimulation of human brain. Lancet. 1986;1(8493):1325–1326. doi: 10.1016/s0140-6736(86)91243-2. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry. 2011;23(5):454–466. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug Alcohol Depend. 1997;44(1):1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4(3):389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol. 2014;2014:815215. doi: 10.1155/2014/815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19(7):1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3257–3266. doi: 10.1098/rstb.2008.0102. U41J2132723M34N4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, … George MS. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest Radiol. 1998;33(6):336–340. doi: 10.1097/00004424-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Wassermann EM, Ziemann U, Lorberbaum JP, Nahas Z, … George MS. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS) Journal of Magnetic Resonance Imaging. 2000;11(6):569–574. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, … Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19(3):1085–1094. doi: 10.1016/s1053-8119(03)00113-7. S1053811903001137 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, … London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protocols. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. The Psychiatric Clinics of North America. 1999;22(2) doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, … Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. S0896-6273(00)80374-8 [pii] [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Camchong J, Macdonald AW, 3rd, Mueller BA, Nelson B, Specker S, Slaymaker V, Lim KO. Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend. 2014;139:145–151. doi: 10.1016/j.drugalcdep.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86(1):91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, … O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3(1):e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Beatty GK, Anderson RE, Kodituwakku P, Phillips JP, Lane TD, … Calhoun VD. Reduced fMRI activity predicts relapse in patients recovering from stimulant dependence. Hum Brain Mapp. 2012;35(2):414–428. doi: 10.1002/hbm.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol. 2000;83(5):3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One. 2013;8(3):e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Albein-Urios N, Perales JC, Martinez-Gonzalez JM, Vilar-Lopez R, Fernandez-Serrano MJ, … Verdejo-Garcia A. Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction. 2015;110(12):1953–1962. doi: 10.1111/add.13076. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Denslow S, Lomarev M, George MS, Bohning DE. Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)-induced movement: an interleaved TMS/functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(7):752–760. doi: 10.1016/j.biopsych.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, … Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565(Pt 3):945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry. 2003;64(8):951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134(Pt 7):2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. nn1579 [pii] [DOI] [PubMed] [Google Scholar]
- Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34(4):559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68(1):87–93. doi: 10.1016/s0376-8716(02)00110-2. S0376871602001102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fotros A, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Gravel P, … Leyton M. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [(1)(8)F]fallypride study in cocaine dependent participants. Neuropsychopharmacology. 2013;38(9):1780–1788. doi: 10.1038/npp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, … Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26(2):487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17(3):337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, … Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, … Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Honorio Carrillo J, Maloney T, Woicik PA, … Volkow ND. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009;29(18):6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: A randomized placebo-controlled cross-over study using pharmacological fMRI. Addictive Behaviors. 2013;38(2):1509–1517. doi: 10.1016/j.addbeh.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, … Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Beveridge TJ, Porrino LJ. Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci Biobehav Rev. 2013;37(9 Pt A):2037–2046. doi: 10.1016/j.neubiorev.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Canterberry M, Taylor JJ, DeVries W, Li X, Brown TR, George MS. Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One. 2013;8(7):e67917. doi: 10.1371/journal.pone.0067917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628(Pt A):199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218(4):681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102(1–3):88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, De Raedt R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 2012;120(1–3):209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81(3):251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry. 2011;12(Suppl 1):57–62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CS. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158(3):486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70(6):553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, … O’Brien CP. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27(2):251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. 23/21/7839 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, … Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69(7):684–692. doi: 10.1016/j.biopsych.2010.11.022. S0006-3223(10)01249-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Gross RE, Ely T, Drexler KP, Kilts CD. Clinical correlates of attentional bias to drug cues associated with cocaine dependence. Am J Addict. 2014;23(5):478–484. doi: 10.1111/j.1521-0391.2014.12134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, … Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, … Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. 2002;26(6):631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21(7):1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28(4):904–914. doi: 10.1016/j.neuroimage.2005.06.039. S1053-8119(05)00475-1 [pii] [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, … Stein Ea. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2000;43(1):45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. doi:10.1002. [DOI] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, … George MS. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73(8):714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Res Monogr. 1997;175:208–220. [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, … Wagner HN., Jr Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47(6):567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, … Li CS. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136(Pt 4):1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, … Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49(1):738–744. doi: 10.1016/j.neuroimage.2009.08.037. S1053-8119(09)00942-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, … Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155(1):124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, … Breiter HC. Cortical thickness abnormalities in cocaine addiction--a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008;60(1):174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, … Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44(4):729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJ, Smits M, Franken IH. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology. 2013;38(6):1085–1093. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19(3):1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39(6):424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Sr, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S, Xu J, Carroll KM, Potenza MN. Self-reported impulsivity is negatively correlated with amygdalar volumes in cocaine dependence. Psychiatry Res. 2015;233(2):212–217. doi: 10.1016/j.pscychresns.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2–3):236–250. doi: 10.1016/S0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105(1):49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Balodis IM, Devito EE, Lacadie CM, Yeston J, Scheinost D, … Potenza MN. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am J Drug Alcohol Abuse. 2013;39(6):392–402. doi: 10.3109/00952990.2013.841711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21(4):193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, … Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181(3):174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JK, Grigsby EM, Prevosto V, Petraglia FW, 3rd, Rao H, Deng ZD, … Grill WM. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci. 2014;17(8):1130–1136. doi: 10.1038/nn.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas Z, Lomarev M, Roberts DR, Shastri A, Lorberbaum JP, Teneback C, … Bohning DE. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biological Psychiatry. 2001;50(9):712–720. doi: 10.1016/s0006-3223(01)01199-4. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30(1):304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6(4):347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, Goldstein RZ. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2016 doi: 10.1111/adb.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, Wager TD. Regional specialization within the human striatum for diverse psychological functions. Proc Natl Acad Sci U S A. 2016;113(7):1907–1912. doi: 10.1073/pnas.1507610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. doi: 10.1080/00952990701199416. 778594497 [pii] [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17(4):345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24(14):3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7(2):226–233. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131(1–2):44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Haney M, Hanson C, Biswal B, Hanson SJ. Modeling Causal Relationship Between Brain Regions Within the Drug-Cue Processing Network in Chronic Cocaine Smokers. Neuropsychopharmacology. 2015;40(13):2960–2968. doi: 10.1038/npp.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, … Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87(4):688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. European Journal of Pharmacology. 2005;526(1–3):140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, … Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32(10):2229–2237. doi: 10.1038/sj.npp.1301346. 1301346 [pii] [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183(2):171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101(6):2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. S0028-3908(08)00272-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, … Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33(4):827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180(4):583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155(3):189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. S0006-8993(07)01391-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, … Gunn RN. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex. 2014;24(5):1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, PÉRez-GarcÍA M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12(03) doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, … Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, … Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, … Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148(5):621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, … Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. 25/15/3932 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115(1–2):137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, George TP. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6(3):221–230. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, … London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27(2):477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kober H, Wang X, DeVito EE, Carroll KM, Potenza MN. Hippocampal volume mediates the relationship between measures of pre-treatment cocaine use and within-treatment cocaine abstinence. Drug Alcohol Depend. 2014;143:74–80. doi: 10.1016/j.drugalcdep.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Zhou Y, Liu Y, Jiang T, Dong H, Zhang Y, Walter M. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 2011;54(4):2571–2581. doi: 10.1016/j.neuroimage.2010.11.018. [DOI] [PubMed] [Google Scholar]