Abstract
The immunogenicity and efficacy of a hybrid recombinant protein derived from the N-terminal end of the glutamate-rich protein (GLURP) and the C-terminal portion of the merozoite surface protein 3 (MSP3) of Plasmodium falciparum was evaluated in Saimiri sciureus monkeys. The GLURP/MSP3 hybrid protein, expressed in Lactococcus lactis, was administered in association with alum, Montanide ISA720, or complete or incomplete Freund adjuvant (CFA/IFA) in groups of five animals each. The three formulations were shown to be immunogenic, but the one with alum was shown to be weak compared to the other two, particularly CFA/IFA, which provided very high antibody titers (enzyme-linked immunosorbent assay titers of >3,000,000 and immunofluorescence antibody test titers of 6,400). After a challenge infection with P. falciparum FUP strain, all five monkeys from the GLURP/MSP3-alum group showed a rapid increase in parasitemia, reaching 10% and were treated early. The two monkeys with the highest antibody titers in group GLURP/MSP3-Montanide ISA720 had a delay in the course of parasitemia and were treated late due to a low hematocrit. In the GLURP/MSP3-CFA/IFA group, parasitemia remained below this threshold in four of the five animals and, after it reached a peak, parasitemia started to decrease and monkeys were treated late. When all animals were grouped according to the outcome, a statistically significant association between high antibody titers and partial protection was observed. The challenge infection boosted the antibody titers, and the importance of this event for vaccine efficacy in areas where this parasite is endemic is discussed. In conclusion, these data suggest that GLURP and MSP3 can induce protection against malaria infection if antibodies are induced at properly high titers.
Malaria remains one of the most serious public health problems in the world, particularly affecting tropical developing countries and killing mainly young children (1). Control strategies have not significantly decreased the incidence of the disease in most places where it is prevalent. Moreover, problems such as the spread of drug-resistant parasites bring even more concern for the populations living in areas of endemicity. A malaria vaccine is expected to be the most effective tool for changing this situation, and dozens of antigens derived from different stages of the parasite's life cycle have been described and are in preclinical or clinical vaccine trials (4). Among the blood-stage candidate antigens, the glutamate-rich protein (GLURP) and the merozoite surface protein 3 (MSP3) proteins of Plasmodium falciparum offer good perspectives for a vaccine since epidemiological and laboratory data suggest that immune responses targeting these antigens are associated with protection (2, 10, 14). We have previously tested seven different formulations containing MSP3-derived or GLURP-derived constructs (either recombinant proteins or synthetic peptides) in association with different adjuvants in Saimiri sciureus monkeys and have found that an MSP3 C-terminal recombinant protein in association with the AS02 adjuvant and a GLURP N-terminal recombinant protein in association with alum were immunogenic and able to induce partial protection in S. sciureus monkeys (5). Saimiri, along with Aotus (3, 7), monkeys are the World Health Organization-recommended primate models for malaria research, especially for vaccine preclinical trials (15). In the present study, a hybrid MSP3/GLURP recombinant protein, produced by using the Lactococcus lactis expression system (12), was tested with different adjuvants in S. sciureus monkeys in an attempt to optimize the immunogenicity and efficacy of the formulations containing these proteins.
MATERIALS AND METHODS
Animals.
S. sciureus monkeys were housed at the National Primate Center/SVS, Belém, Brazil. Detailed description of the animals' background is provided in Carvalho et al. (5). Animals were splenectomized at least 2 months before first immunization injection. Sixteen male (weighing 705 to 820 g) and four female (weighing 530 to 600 g) monkeys were distributed into three immunization groups and one control group (five monkeys per group, one female in each group). The Fiocruz Ethical Committee for Animal Experimentation (CEUA) approved the described protocols (CEUA protocol number P-0047-00).
GLURP/MSP3 hybrid recombinant protein production.
The hybrid molecule is a fusion protein containing the regions GLURP27-500 (GLURP-R0) and MSP3212-380 (MSP3 C-terminal). The production and purification of the GLURP/MSP3 hybrid molecule has been described in detail elsewhere (13), with the difference that in that study the protein was subjected to a three-step purification protocol (HiTrap Q Sepharose, followed by HiTrap SP Sepharose and then by Phenyl Sepharose High-Performance columns), whereas the intermediate step (HiTrap SP Sepharose) was not performed with the present preparation. For the control preparation, culture supernatant from cells transformed with the cloning plasmid (without an insert) was obtained, applied on to a HiTrap Q Sepharose High-Performance column, and the bound material was eluted and used for immunizing the control monkeys. Analysis of the proteins was performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Formulations and immunization protocol.
The GLURP/MSP3 hybrid protein was formulated with three different adjuvants: alum (Superfos Biosector, Vaerloese, Denmark), Montanide ISA720 (Seppic, Paris, France), and Freund's (Sigma Chemical Co., St. Louis, Mo.). A control formulation was prepared with culture supernatant proteins from wild-type L. lactis in association with Freund's adjuvant. In all cases, a final volume of 500 μl containing 50 μg of the protein thoroughly mixed with the respective adjuvant was administered subcutaneously at four different points on the shaved backs of the animals. Three doses were administered, on days 0, 30, and 90. In the case of Freund's adjuvant, the animals received complete Freund's adjuvant (CFA) in the first dose and the incomplete Freund's adjuvant (IFA) in the other two. Blood was collected on days 0, 30, 60, 90, and 135 for immunological assays. In each of these manipulations, hematological parameters were evaluated, monkeys were weighed, and the sites of injection were examined to check for local adverse reactions. Monkeys were then challenged with P. falciparum 45 days after the third dose.
Challenge infections.
Monkeys were challenged with 50,000 P. falciparum (FUP-SP strain) parasitized red blood cells, with a predominance of parasites in the ring and young trophozoite stages, obtained from a donor Saimiri monkey. Parasites were administered intravenously, and parasitemia was followed up daily by examination of Giemsa-stained thick and thin smears of blood obtained from the footpad. As a masking procedure for determining parasitemia, animals were assigned a different number each day, according to a random order at which they were taken from the cages for preparing the blood films, of which the microscopist was unaware. Rectal temperatures were daily evaluated and hematocrit levels were checked every 4 days. Monkeys were treated with chloroquine (three daily doses of 10 mg/kg) whenever parasitemia reached 10% or the hematocrit went below 30%. Three different outcomes were characterized as follows: (i) no protection (monkeys with parasitemia reaching 10%), (ii) full protection (monkeys able to clear parasitemia without treatment), and (iii) partial protection (monkeys able to keep parasitemia under control [<10%] but without spontaneous parasite clearance [being treated for low hematocrit, for instance]).
Enzyme-linked immunosorbent assay (ELISA).
Plates (96 well; Maxisorp, Nunc, Denmark) were coated with a 1-μg/ml concentration of (i) GLURP/MSP3 protein preparation produced in L. lactis; (ii) the L. lactis culture supernatant; or (iii) the GLURP27-500 (GLURP-R0) or MSP3212-380 (MSP3 C-term) recombinant proteins produced either in L. lactis or in Escherichia coli (100 μl/well) in carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. Uncoated sites were blocked with 200 μl of phosphate-buffered saline-0.05% Tween 20 (PBS-T) containing 3% nonfat milk for 1 h at room temperature, and the wells were washed three times with PBS-T. Serial dilutions of each serum sample were prepared in each plate with PBS-T-1% nonfat milk and incubated for 1 h at room temperature. Preimmunization serum samples and sera from nonimmunized animals were used as controls. Plates were washed three times with PBS-T, a rabbit anti-Saimiri immunoglobulin G (IgG) at 1:8,000 was added, and the plates were incubated for 1 h at room temperature. After a washing step, goat anti-rabbit IgG conjugated to peroxidase (Sigma A-9169; 1:5,000) was added, followed by incubation for 1 h at room temperature. After another washing step, 100 μl of a solution of orthophenylenediamine in citrate-phosphate buffer (10 mg/25 ml plus 10 μl of H2O2; pH 5.0) was added to each well, followed by incubation for 30 min at room temperature in the dark, and then 50 μl of 2 N H2SO4/well was used to stop the reaction. Plates were read at 492 nm in a spectrophotometer (Spectramax 250; Molecular Devices). Cutoff values were defined as the mean optical density of control wells plus three standard deviations (blank values subtracted). The results were expressed as endpoint titers.
ELISA for GLURP epitope mapping.
Plates were coated with 2.5 μg of streptavidin/ml (Sigma) in citrate-phosphate buffer (pH 5.0) overnight at 4°C. After being washed with 0.5 M NaCl-PBS-0.1% Tween 20, the biotin-conjugated peptides P3, P4, P5, P8, P9, P10, P11, and S3, covering known B-cell epitopes within the GLURP27-500 region (11), were added at 1 μg/ml in 0.37 M NaCl-PBS- 0.1% Tween 20 buffer for 1 h at room temperature. After being washed, serum samples diluted 1:200 in 0.7 M NaCl-PBS-0.1% Tween 20 were added for 1 h at room temperature. Plates were washed and a rabbit anti-Saimiri IgG (1:8,000) was added, and the plates were then incubated for 1 h at room temperature. After a washing step, a goat anti-rabbit IgG conjugated to peroxidase (Sigma A-9169; 1:5,000) was added, followed by incubation for 1 h at room temperature. Plates were washed, and development was performed as described above. The results were expressed as the ratio of the optical density of each test sample to the cutoff values (mean optical density plus three standard deviations of nonimmunized, noninfected Saimiri serum samples) for each peptide. The reactivity of each serum against GLURP27-500 was assessed in parallel at a 1:200 dilution.
IFAT.
Immunofluorescence antibody test (IFAT) was performed by using P. falciparum (FCR3 strain, schizont stage) obtained from in vitro cultures synchronized with sorbitol. Serum samples were diluted in PBS, added to the slides, and incubated at 37°C 40 min. After extensive washings in PBS, a goat anti-human IgG conjugated to fluorescein (Sigma) diluted 1:250 in PBS-Evans Blue was added, and the slides were incubated at 37°C 40 min and then washed, dried, mounted with a coverslip by using buffered glycerin solution, and read in a fluorescence microscope (Zeiss). Endpoint titers were determined on the wells giving fluorescence over the background of preimmunization serum samples.
Statistical analysis.
Two types of analysis were performed. First, exploratory analyses compared each test group with the control group, taking as parameters the day of treatment and the cumulative parasitemia (from the day of challenge infection until the day the first animal was treated). However, the amplitude of the variance of the parameters under analysis vis-à-vis the small number of animals in each subgroup precluded any valid inference. Second, all of the animals were divided into two groups according to the outcome (i.e., no protection versus partial or full protection [according to the criteria established above]) and, after assessment of the comparability of variances by using the Levene test, an independent-samples t test was used to analyze the relationship between antibody titers and outcome of infection.
RESULTS
Production and purification of recombinant MSP3/GLURP and of control L. lactis culture supernatant proteins.
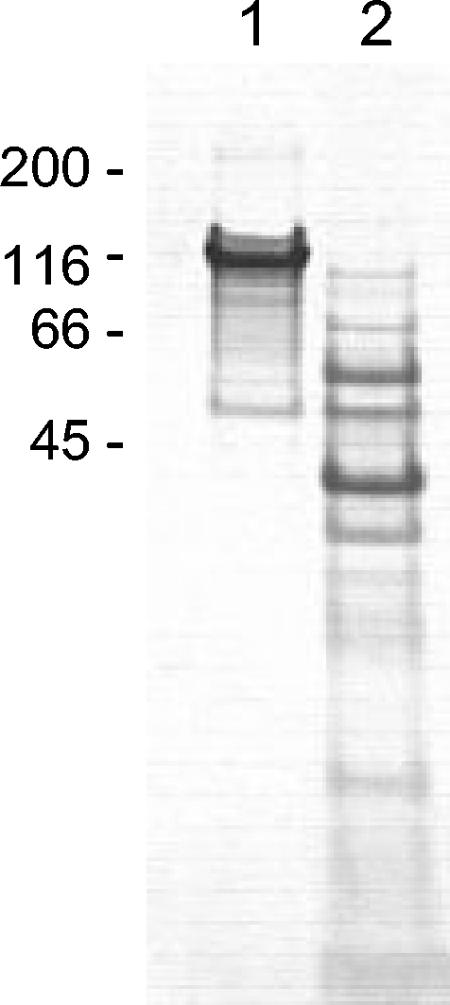
A GLURP27-500-MSP3212 fusion protein was produced as a secreted recombinant protein in L. lactis. This hybrid protein was purified by ion-exchange chromatography, followed by hydrophobic-interaction chromatography. Subsequent sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed a major band corresponding to the GLURP-MSP3 hybrid protein and a few contaminating lower-molecular-weight bands (Fig. 1). When analyzed by immunoblotting, the major band but not the others was specifically recognized by anti-GLURP and anti-MSP3 antibodies. As a control preparation for immunization, culture supernatant from cells transformed with the cloning plasmid without an insert was purified by ion-exchange chromatography. Bound proteins were eluted by the same procedure used for the GLURP/MSP3 protein and visualized (Fig. 1).
FIG. 1.
Coomassie blue-stained 12% polyacrylamide gel of purified GLURP/MSP3 hybrid protein (lane 1) and of L. lactis supernatant proteins (lane 2) used in the immunization experiments.
Immunogenicity.
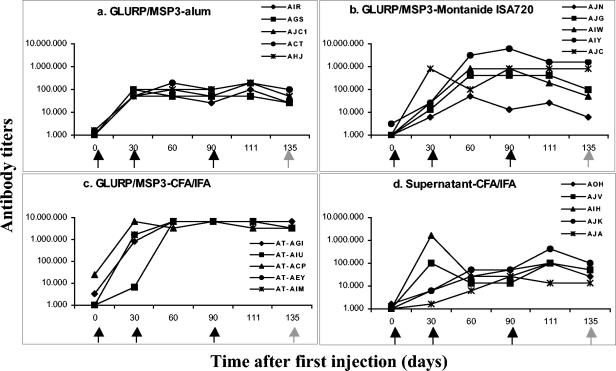
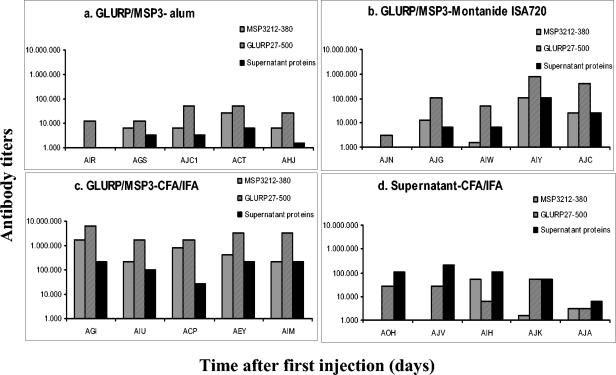
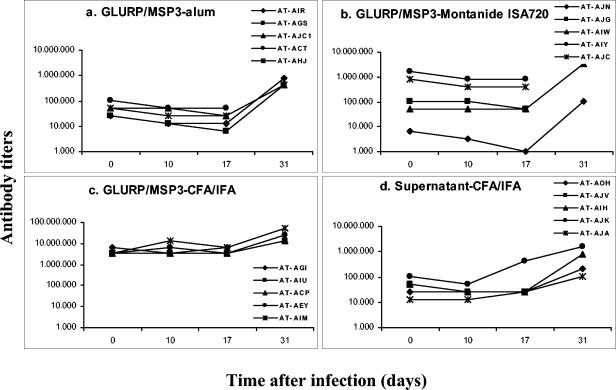
The three formulations tested were shown to be immunogenic in Saimiri monkeys (Fig. 2a to c). In most cases, two doses sufficed to elicit the highest levels of antibodies for a given formulation, and the third dose did not increase the titers further. As expected, the highest antibody titers were observed in the GLURP/MSP3-CFA/IFA group (Fig. 2c), and the lowest was seen in the GLURP/MSP3-alum (Fig. 2a). In the GLURP/MSP3-Montanide ISA720 group, titers were variable, with three monkeys showing low titers as in the alum group and two animals showing titers of >800,000 (Fig. 2b). In general, antibody titers against the individual proteins were higher for GLURP27-500 than for MSP3212-380 in all groups (Fig. 3a to c), and in all cases the titers of antibodies to the individual proteins were lower than titers of antibodies to the GLURP/MSP3 hybrid protein. Sera from animals receiving the GLURP-MSP3 fusion protein also showed antibody reactivity against culture supernatant proteins. This reactivity may be explained by antibodies raised against contaminating host cell proteins in the GLURP/MSP3 hybrid preparation. The presence of host cell protein in the GLURP/MSP3 preparation would also explain why sera from the control group tested positive in the GLURP/MSP3 ELISA.
FIG. 2.
Follow-up of antibody titers (ELISA) against the hybrid GLURP/MSP3 protein during immunization of S. sciureus monkeys with different antigen-adjuvant formulations. Black arrows indicate immunization injection points; gray arrows indicate the challenge infection point. The code names and associate symbols for each monkey are shown.
FIG. 3.
Titers of antibody (as determined by ELISA) to GLURP27-500 (GLURP-R0) and MSP3212-380 (MSP3-C-terminal) proteins after three injections of the hybrid GLURP/MSP3-derived or control formulations in S. sciureus monkeys.
The IFAT assays showed that the recognition of the native proteins was in most cases associated with high ELISA titers against the recombinant GLURP/MSP3 protein (Table). Animals from the GLURP/MSP3-CFA/IFA group showed the highest titers. In the Montanide ISA720 group, IFAT titers were variable and correlated with ELISA titers and in the alum group the IFAT titers were very low. Finally, sera from the L. lactis-CFA/IFA group were negative or presented a slight fluorescence over background at the first dilution (1:50).
Epitope mapping with GLURP27-500-derived peptides.
Prechallenge (but after the third immunization) serum samples from the Saimiri monkeys immunized with the GLURP/MSP3-CFA/IFA formulation showed a strong reactivity with the peptide P11 (the five monkeys), P5 (four monkeys), and S3 (three monkeys). In monkeys immunized with Montanide ISA720, only the two monkeys with the highest anti-GLURP/MSP3 antibody titers showed reactivity against two peptides, P11 and P8 (data not shown). This low frequency of reactivity can be in part explained by the fact that, at a 1:200 dilution, there was a considerable variation in the optical density values among the control serum samples and, therefore, the cutoff values were quite high for most of the eight peptides tested.
Safety.
All monkeys that received the CFA/IFA (with the GLURP/MSP3 protein or with the L. lactis culture supernatant) developed local inflammatory reaction with swelling at the injection site. Reactions were observed mainly after the second injection and increased in size and severity with the third dose. Local reactions were not observed in the monkeys immunized with the GLURP/MSP3-alum or GLURP/MSP3-Montanide ISA720. After immunizations, no major changes in weight or hematological parameters were observed.
Challenge infections.
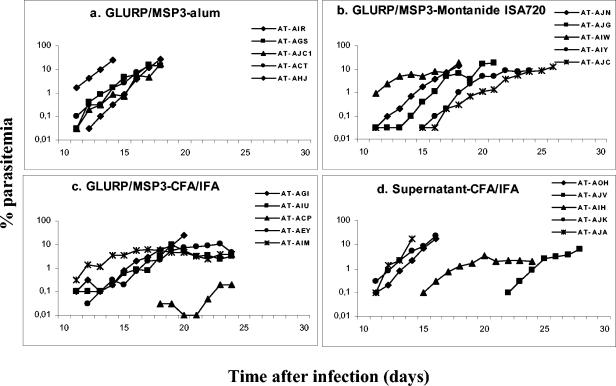
A summary of the antibody titers and outcome of infection is shown in Table 1. After 50,000 P. falciparum-parasitized red blood cells were inoculated intravenously, all 20 Saimiri monkeys showed parasitemia, with the earliest parasites appearing on day 11 (Fig. 4). Full protection was not observed in this experiment, i.e., no monkey was able to clear parasitemia without antimalarial treatment.
TABLE 1.
Individual antibody titers after immunization of S. sciureus with GLURP/MSP3-derived or control formulations and their relationship with the outcome of P. falciparum challenge infectiona
| Immunization group | Saimiri code | Prechallenge antibody titer
|
Outcome of infection (day) | |
|---|---|---|---|---|
| ELISA | IFAT | |||
| MSP3/GLURP-alum | AT-AIR | 25,600 | 50 | Ptmia > 10% (18) |
| AT-AGS | 25,600 | 50 | Ptmia > 10% (18) | |
| AT-AJC1 | 51,200 | 100 | Ptmia > 10% (18) | |
| AT-ACT | 102,400 | 200 | Ptmia > 10% (18) | |
| AT-AHJ | 51,200 | 200 | Ptmia > 10% (14) | |
| MSP3/GLURP- Montanide ISA720 | AT-AJN | 6,400 | 0 | Ptmia > 10% (18) |
| AT-AJG | 102,400 | 800 | Ptmia > 10% (21) | |
| AT-AIW | 51,200 | 200 | Ptmia > 10% (18) | |
| AT-AIY | 1,600,000 | 800 | TLH (26) | |
| AT-AJC | 800,000 | 1,600 | TLH (26) | |
| MSP3/GLURP- CFA/IFA | AT-AGI | 3,200,000 | 6,400 | Ptmia > 10% (20) |
| AT-AIU | 3,200,000 | 6,400 | TLH (24) | |
| AT-ACP | 3,200,000 | 6,400 | TLH (24) | |
| AT-AEY | 3,200,000 | 6,400 | TLH (24) | |
| AT-AIM | 3,200,000 | 6,400 | TLH (24) | |
| L. lactis supernatant- CFA/IFA | AT-AOH | 25,600 | 0 | Ptmia > 10% (16) |
| AT-AJV | 51,200 | 0 | TLH (28) | |
| AT-AIH | 102,400 | 50 | TLH (24) | |
| AT-AJK | 102,400 | 0 | Ptmia > 10% (16) | |
| AT-AJA | 12,800 | 50 | Ptmia > 10% (14) | |
Ptmia, parasitemia; TLH, treated for low (<30%) hematocrit.
FIG. 4.
Course of parasitemia of S. sciureus monkeys immunized with different formulations containing the different GLURP/MSP3 or control formulations, inoculated with 50,000 P. falciparum-parasitized red blood cells (FUP strain). The code names of each monkey and associated symbol are shown.
In the group of monkeys receiving L. lactis culture supernatant in CFA/IFA (i.e., the control group), three of the five animals showed a rapidly increasing parasitemia (as expected for naive, nonimmunized, monkeys), reaching 10% on days 14 to 16, and had to be treated. However, two other monkeys showed a delayed course of parasitemia, extending up to day 28 until being treated for low hematocrit (Fig. 4d).
The monkeys of the GLURP/MSP3-alum group had a course of parasitemia similar to that expected for naive, nonimmunized, animals, i.e., all of them showed a rapidly increasing parasitemia requiring treatment by day 18 (Fig. 4a).
In the GLURP/MSP3-Montanide ISA720 group, two animals were treated early and three presented a delayed course of parasitemia (Fig. 4b). The two animals with the highest antibody titers were the last to be treated, not because they reached the parasitemia threshold foreseen for treatment but because the longer-term infection led to a major decrease in hematocrit (<30%).
In the GLURP/MSP3-CFA/IFA group, only one of five animals had to be treated early because of a parasitemia level of >10%. One animal showed very low parasitemia, and the other three monkeys had relatively high parasitemias but were able to keep it below 10% for several days (Fig. 4c), reaching a peak on days 19 to 23 and then starting to decrease. They were treated late because of low hematocrit.
Two types of data analysis were conducted for the present study. First, to compare the outcomes in the test groups in relation to the control group, two parameters were analyzed: the day of treatment and the cumulative parasitemia (in this case, the endpoint was defined as the day the first animal was treated, i.e., day 14). The mean day of treatment in the GLURP/MSP3-CFA and GLURP/MSP3-Montanide groups (23.2 and 21.8 days, respectively) were longer than in the control and in the GLURP/MSP3-alum groups (19.6 and 17.2 days, respectively). The mean cumulative parasitemia until day 14 in the GLURP/MSP3-CFA and GLURP/MSP3-Montanide groups (1.62 and 3.15%, respectively) were lower than in the control and in the GLURP/MSP3-alum groups (6.64 and 9.78%, respectively). However, statistical analysis was precluded by the low number of animals in each group and the variance among animals within the same group. In addition, this analysis did not take into consideration the intra- and intergroup variations in antibody titers that might lead to different capacities to control infection. Thus, a second type of analysis was conducted by grouping all animals according to the outcome (no protection versus partial or full protection) and comparing the antibody titers between the two groups. Thus, partial protection was observed in 8 of the 20 monkeys, and this correlated with prechallenge antibody titers (Table). The mean antibody titers were much higher in the partially protected monkeys (3,506 [IFAT] versus 1,919,200 [ELISA]) than in the unprotected (671 [IFAT] versus 313,067 [ELISA]) (P = 0.043 and P = 0.018, respectively).
A decrease in the antibody titers was observed in most monkey sera during the first 3 weeks of infection (Fig. 5), probably reflecting a consumption of antibodies by the growing parasites. However, the titers were highly increased thereafter due to the booster effect of the infection, reaching titers much higher than those observed on day 0 of infection in all groups.
FIG. 5.
Follow-up of titers of antibody (as determined by ELISA) to the hybrid GLURP/MSP3 protein during challenge infection of S. sciureus monkeys with P. falciparum FUP strain.
DISCUSSION
We evaluated here the safety, immunogenicity, and efficacy of a hybrid GLURP/MSP3 recombinant protein in the S. sciureus-P. falciparum model. We used two adjuvants (alum and Montanide ISA720) that are used in human clinical trials, together with Freund's adjuvant as a “gold standard,” for protection in Saimiri monkeys.
As expected the alum-adjuvanted vaccine induced a much weaker humoral immune response than did the CFA/IFAT-adjuvanted vaccine, as measured by ELISA or by immunofluorescence assay against native parasite proteins, and animals in the alum group remained fully susceptible to challenge infection. In fact, the course of parasitemia in the alum group was similar to that observed in naive nonimmunized animals (5). In contrast, four of the five monkeys receiving the GLURP/MSP3-CFA/IFAT formulation were able to control parasite multiplication below the threshold for treatment (10%) during the follow-up and were treated late because their hematocrit reached the limit value of 30%. We have considered here treatment for low hematocrit as a partial protection because Saimiri monkeys, although not as much as Aotus monkeys (3), are vulnerable to severe anemia during P. falciparum infection (5). It is likely that an antiparasite immunity that acts to control parasite multiplication but is unable to eliminate it completely and rapidly might lead to a major decrease in hematocrit before parasite clearance can occur. This should not in principle be considered vaccine failure but rather a limitation of the model for long-term follow-ups. Using this criterion, 8 of the 20 animals in the present study are considered partially protected. The animals immunized with the GLURP/MSP3-Montanide ISA720 formulation showed more intragroup variation in the antibody titers than the other groups. The extended course of parasitemia seen in some of the animals was related to higher antibody titers against the GLURP-MSP3 hybrid protein. Thus, the two animals with the lowest ELISA-titers had the most rapidly increasing parasitemias, whereas the two monkeys with very high ELISA titers of >800,000 were able to control parasite multiplication to a level below 10%. The latter two animals also had the highest IFAT antibody titers and were the only animals that recognized individual GLURP peptides (P11 and P8). Although internal variation in a group with a limited number of animals does not allow inferring statistical significance for this relationship, these results point to the conclusion that antibody response against GLURP and MSP3 in terms of quantity (titers) and quality (ability to react with the native proteins and individual epitopes) are critical factors for inhibition of parasite growth in this model. This conclusion is further strengthened by the observation that anti-GLURP/MSP3 antibody titers are significantly higher in animals considered partially protected (n = 8) compared to animals that were susceptible to challenge infection (n = 12). These data are also in accordance with a previous study by Carvalho et al., in which recombinant proteins representing the C-terminal end of MSP3 adjuvanted in AS02 and the N-terminal end of GLURP adjuvanted in alum were found to be immunogenic and conferred partial protection upon challenge with the virulent P. falciparum FUP strain in S. sciureus monkeys (5). In both cases, control of parasitemia was related to the prechallenge specific antibody titers.
It is not clear why two of five animals in the L. lactis group were able to control parasite multiplication; however, this partial protection may be explained by nonspecific effects of the CFA/IFAT adjuvant. This adjuvant is known to be a potent stimulator of the immune system, and nonspecific protective effects of CFA/IFAT adjuvant against erythrocyte-stage malaria antigens have also been observed in two previous studies in Aotus monkeys (8, 9).
Another important point refers to the booster effect of infection. After an initial decrease in specific antibody titers, which can be explained by the consumption of antibodies through binding to the parasites, postinfection antibody titers at day 31 were much higher than those observed prior to challenge in all groups. Thus, an experimental P. falciparum infection can boost vaccine-induced antibody responses. It is therefore conceivable that repeated infections in areas of endemicity will boost human immune responses induced by vaccination with the GLURP/MSP3 hybrid protein. In the present study, we did not perform a second challenge to examine the effect of a natural boost on protection because Saimiri monkeys become largely resistant to homologous challenge after one infection (6).
In agreement with previous findings, GLURP-based vaccines induce a predominant antibody response to the P5 and P11 peptides in Saimiri monkeys (5) and in Aotus monkeys (F. A. Alves et al., unpublished data). Anti-GLURP antibodies from naturally exposed individuals do not recognize the P5 epitope, whereas the P11 epitope is frequently recognized by IgG antibodies from humans naturally exposed to malaria in Brazil (L. R. Pratt-Riccio et al., unpublished data) and in Liberia (13). These differences in epitope recognition are most likely related to genetic differences between humans and neotropical monkeys.
In conclusion, we have demonstrated that (i) the GLURP/MSP3 hybrid protein is safe and immunogenic in a nonhuman primate model and (ii) monkeys with high titers of specific antibodies that recognize parasite proteins are partially protected against an experimental challenge infection with P. falciparum.
Acknowledgments
This study was supported by the European Malaria Vaccine Initiative program of the Commission of the European Communities, the Brazilian National Research Council (CNPq), the Programa de Desenvolvimento Tecnológico em Insumos para Saúde (PDTIS/Fiocruz), and the Instituto Oswaldo Cruz/Fiocruz and with resources from the National Primate Center and Instituto Evandro Chagas/SVS. C.T.D.-R. and F.A.A are recipients of fellowships from CNPq. L.J.M.C and C.B. were recipient of fellowships from FAPERJ.
We thank Rodrigo del Rio do Valle for veterinary care of the animals enrolled in this work and Francisco Inacio Gastos for statistical analysis.
REFERENCES
- 1.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64:1-11. [DOI] [PubMed] [Google Scholar]
- 2.Borre, M. B., B. M. Dziegel, Hogh, E. Petersen, K. Rieneck, R. Riley, J. F. Meis, M. Aikawa, K. Nakamura, M. Harada, A. Wind, P. H. Jakobsen, J. Cowland, S. Jepsen, N. H. Axelsen, and J. Vuust. 1991. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate-rich protein (GLURP) expressed in both the pre-erythrocytic and erythrocytic stages of the vertebrate life cycle. Mol. Biochem. Parasitol. 49:119-132. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho, L. J. M., F. A. Alves, S. G. Oliveira, R. D. R. Valle, A. A. M. Fernandes, J. A. P. C. Muniz, and C. T. Daniel-Ribeiro. 2003. Severe anemia affects both splenectomized and non-splenectomized Plasmodium falciparum-infected Aotus infulatus monkeys. Mem. Inst. Oswaldo Cruz. 98:679-686. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho, L. J. M., C. T. Daniel-Ribeiro, and H. Goto. 2002. Malaria vaccine: candidate antigens, mechanisms, constraints, and prospects. Scand. J. Immunol. 56:327-343. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho, L. J., S. G. Oliveira, M. Theisen, F. A. Alves, M. C. Andrade, G. M. Zanini, M. C. Brigido, C. Oeuvray, M. M. Povoa, J. A. Muniz, P. Druilhe, and C. T. Daniel-Ribeiro. 2004. Immunization of Saimiri sciureus monkeys with Plasmodium falciparum merozoite surface protein-3 and glutamate-rich protein suggests that protection is related to antibody levels. Scand. J. Immunol. 59:363-372. [DOI] [PubMed] [Google Scholar]
- 6.Fandeur, T., and W. Chalvet. 1998. Variant- and strain-specific immunity in Saimiri infected with Plasmodium falciparum. Am. J. Trop. Med. Hyg. 58:225-231. [DOI] [PubMed] [Google Scholar]
- 7.Herrera, S., B. L. Perlaza, A. Bonelo, and M. Arevalo-Herrera. 2002. Aotus monkeys: their great value for antimalaria vaccines and drug testing. Int. J. Parasitol. 32:1625-1635. [DOI] [PubMed] [Google Scholar]
- 8.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein 3 and protection against malaria in Aotus monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 9.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. G. Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkey of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C-terminal of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oeuvray, C., H. Bouharoun-Tayoun, H. Grass-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. Filgueira, C. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by co-operation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 11.Theisen, M., D. Dodoo, A. Toure-Balde, S. Soe, G. Corradin, K. K. Koram, J. A. Kurtzhals, L. Hviid, T. Theander, B. Akanmori, M. Ndiaye, and P. Druilhe. 2001. Selection of glutamate-rich protein long synthetic peptides for vaccine development: antigenicity and relationship with clinical protection and immunogenicity. Infect. Immun. 69:5223-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theisen, M., S. Soe, K. Brunstedt, F. Follmann, L. Bredmose, H. Israelsen, S. M. Madsen, and P. Druilhe. 2004. A Plasmodium falciparum GLURP-MSP3 chimeric protein: expression in Lactococcus lactis, immunogenicity, and induction of biologically active antibodies. Vaccine 22:1188-1198. [DOI] [PubMed] [Google Scholar]
- 13.Theisen, M., S. Soe, S. G. Jessing, L. M. Okkels, S. Danielsen, C. Oeuvray, P. Druilhe, and S. Jepsen. 2001. Identification of a major B-cell epitope of the Plasmodium falciparum glutamate-rich protein (GLURP), targeted by human antibodies mediating parasite killing. Vaccine 19:204-212. [DOI] [PubMed] [Google Scholar]
- 14.Theisen, M., S. Soe, C. Oeuvray, A. W. Thomas, J. Vuust, S. Danielsen, S. Jepsen, and P. Druilhe. 1998. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect. Immun. 66:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 1988. Memorandum from a W.H.O. meeting: role of non-human primates in malaria vaccine development. Bull. W.H.O. 66:719-728 [PMC free article] [PubMed] [Google Scholar]