Abstract
Localization of the site of the unknown primary tumor is critical for surgical treatment of patients presenting with neuroendocrine tumor (NET) with metastases. Methods: Forty patients with metastatic NET and unknown primary site underwent 68Ga-DOTATOC PET/CT in a single-site prospective study. The 68Ga-DOTATOC PET/CT was considered true-positive if the positive primary site was confirmed by histology or follow-up imaging. The scan was considered false-positive if no primary lesion was found corresponding to the 68Ga-DOTATOC–positive site. All negative scans for primary tumor were considered false-negative. A scan was classified unconfirmed if 68Ga-DOTATOC PET/CT suggested a primary, however, no histology was obtained and imaging follow-up was not confirmatory. Results: The true-positive, false-positive, false-negative, and unconfirmed rates for unknown primary tumor were 38%, 7%, 50%, and 5%, respectively. Conclusion: 68Ga-DOTATOC PET/CT is an effective modality in the localization of unknown primary in patients with metastatic NET.
Keywords: neuroendocrine, PET/CT, 68Ga-DOTATOC, neuroendocrine tumor, unknown primary
Between 9% and 19% of patients with neuroendocrine tumors (NETs) present with metastatic disease with an unknown primary tumor site (1). Localization of the primary tumor is highly relevant in the management of this patient population because complete resection of the primary tumor and metastases is the treatment goal for patients with well-differentiated NET metastases (2). Even if the metastases are not completely resectable, debulking surgery can improve symptom control in patients with endocrine symptoms and may improve survival (2–4).
The standard imaging for staging of NETs includes CT and MRI as well as somatostatin receptor scintigraphy. CT and MRI are limited for evaluation of primary small bowel NETs; somatostatin receptor imaging with 111In-octreotide (Octreoscan; Mallinckrodt Pharmaceuticals) also shows limited detectability, with only 37% of small bowel primary NETs detected preoperatively with 111In-octreotide (5). More recently, somatostatin receptor imaging with positron emitters has been developed using 68Ga (a generator product with a half-life of 68 min) and DOTA as chelator. The most widely studied 68Ga-DOTA-octreotide analogs for PET imaging are 68Ga-DOTATATE, 68Ga-DOTATOC, and 68Ga-DOTANOC. All of these radiopharmaceuticals have higher affinity than 111In-octreotide for the somatostatin receptor subtype 2, the primary target in NETs, and are more sensitive than 111In-octreotide in the detection of NET lesions (6–8).
The objective of this study was to evaluate the accuracy of 68Ga-DOTATOC PET/CT imaging in the localization of the site of the unknown primary tumor in patients with metastatic NET.
MATERIALS AND METHODS
Patients
A single-center prospective study was performed to evaluate the safety and efficacy of 68Ga-DOTATOC PET/CT in patients with a previous diagnosis of or suspected NET. A database of enrolled subjects was created and classified according to the scan indications, which included initial staging and restaging of NETs, diagnosis of NET in patients with suspected disease, and localization of unknown primary in patients with metastatic NET. Patients with histologically proven NET metastases and an unknown primary were included in this analysis. This study was performed under a physician-sponsored Investigational New Drug application from the U.S. Food and Drug Administration for 68Ga-DOTATOC (IND# 114,398). The study protocol was approved by the University of Iowa Institutional Review Board, and all subjects signed a written informed consent form. The clinical trial was listed in clinicaltrials.gov (NCT01619865).
68Ga-DOTATOC PET/CT Imaging
68Ga-DOTATOC was synthesized using an automated 68Ge/68Ga generator (IGG100; Eckert & Ziegler) coupled with a ModularLab PharmTracer synthesis module (Eckert & Ziegler) as previously described (9–12). Briefly, 68Ga was eluted from the generator with 0.1 M hydrochloric acid (6 mL) and passed through an in-line cation exchange resin (STRATA-XC; Phenomenex). Purified 68Ga was then eluted with 98% acetone/0.02 M HCl (0.8 mL) to a glass reaction vessel containing DOTATOC (39 nmol, 55 μg) in sodium acetate buffer, pH 4 (2 mL). Radiolabeling was performed at 95°C for 6 min, and acetone was removed by vent to waste during the radiolabeling step. After being radiolabeled, 68Ga-DOTATOC was transferred to an in-line SPE tC-18 cartridge (Waters); free 68Ga was removed by saline rinse. Pure 68Ga-DOTATOC was first eluted with 47.5% ethanol:water (1.2 mL) to the product vial via a 0.22-μm sterilizing filter followed by isotonic saline for injection (7 mL). Quality control parameters of radiochemical and radionuclidic purity, half-life, pH, endotoxin content, and sterility were measured by standard techniques.
Whole-body images from the top of the head to proximal thighs were obtained on a Siemens Biograph PET/CT scanner after a 60-min uptake period after the intravenous administration of 148–185 MBq of 68Ga-DOTATOC. A low-dose, noncontrast whole-body CT scan (30–60 mAs, 120 kV, 5-mm slice thickness, and pitch of 0.8) was acquired for attenuation correction. PET/CT images were iteratively reconstructed using 4 iterations and 8 subsets with a 7-mm gaussian postprocessing filter.
Data Analysis
PET/CT images were read qualitatively by an experienced nuclear medicine specialist, with focal uptake of 68Ga-DOTATOC above normal background considered positive for NET. The clinical indication of the scan and previous conventional imaging studies were available to the reading physicians. The scan was considered true-positive if the primary lesion suggested by 68Ga-DOTATOC PET/CT was confirmed by histology or follow-up imaging. A scan was considered false-positive if no primary lesion was found corresponding to a 68Ga-DOTATOC–positive site on surgery, endoscopy, or imaging follow-up. All 68Ga-DOTATOC PET/CT scans negative for primary tumor were considered false-negative. None of the scans was considered true-negative for primary tumor because all patients had histologically proven NET metastases (present or previously resected). A scan was classified as unconfirmed if a potential primary tumor site was identified on 68Ga-DOTATOC PET/CT, but no histology was obtained and imaging and clinical follow-up did not confirm the lesion. The effectiveness of 68Ga-DOTATOC PET/CT scans was measured with regard to identification of primary tumors.
RESULTS
Patients
Forty patients, 23 men and 17 women with an age range of 20–75 y (55 ± 12 y), with metastatic NET and unknown primary site, were imaged with 68Ga-DOTATOC PET/CT. Before 68Ga-DOTATOC PET/CT scanning, all patients underwent a CT or MRI of the abdomen–pelvis (conventional imaging) that failed to localize a primary tumor. Conventional imaging was obtained within a median of 3 mo before 68Ga-DOTATOC PET/CT (range, 1–27 mo; in 37 patients within 1 y). Seventeen patients also underwent an 111In-octreotide scan within 1 y of 68Ga-DOTATOC PET/CT scanning (median, 4 mo). The metastatic sites identified before 68Ga-DOTATOC PET/CT were the liver (n = 29); lymph nodes (n = 8); bones (n = 6); lungs and pleura (n = 5); peritoneum (n = 2); and skin, brain, or kidney lesions (n = 1 patient each). In 18 patients, the liver was the only known site of metastasis. The distribution of tumor grade for metastatic sites, available in 38 patients, was low-grade in 13 patients, intermediate-grade in 17 patients, and high-grade in 8 patients. The initial diagnosis of metastatic NET was made between 1 and 178 mo (median, 6.5 mo) before 68Ga-DOTATOC PET/CT scanning; 20 scans were obtained within 6 mo of diagnosis, 6 scans within 6–12 mo, 10 scans within 1–5 y, and 4 scans more than 5 y after initial diagnosis of NET.
Localization of Unknown Primary
68Ga-DOTATOC PET/CT was true-positive for primary tumor in 15 patients (38%; confidence interval, 23%–53%), unconfirmed positive in 2 patients (5%), false-positive in 3 patients (7%), and false-negative in 20 patients (50%). The true-positive sites for primary NET on 68Ga-DOTATOC were the pancreas in 6 patients, ileum in 8 patients, and retrorectal in 1 patient. The true-positive tumor sites were confirmed with histology in 11 of 15 patients and follow-up imaging in 4 of 15 patients. There was no significant difference in detection rate of the primary tumor between patients imaged within 1 y of diagnosis versus after 1 y (44% vs. 27%). The true-positive rate for primary tumor detection was 5 of 13 for patients with low-grade metastases, 7 of 17 for intermediate-grade metastases, and 2 of 8 for high-grade metastases, with no statistically significant difference between the groups. Among 11 primary tumors with histopathologic confirmation, 8 were low-grade and 3 were intermediate-grade. There were 3 false-positive scans, one in the ileum in an area of lymphoid hyperplasia found on surgical histopathology, one in the stomach with negative follow-up endoscopy, and another in the pancreatic head with negative conventional imaging and endoscopic ultrasound on follow-up. In 17 patients who underwent both 111In-octreoscan and 68Ga-DOTATOC within 1 y, there were 1 true-positive on 111In-octreoscan (also positive on 68Ga-DOTATOC) and 7 true-positive 68Ga-DOTATOC (P = 0.03). The performance characteristics of 68Ga-DOTATOC PET/CT in detection of unknown primary NETs are summarized in Table 1. Figures 1 and 2 show 2 true-positive scans of ileal and pancreatic primary NETs, and Figure 3 shows a false-positive scan in ileal lymphoid hyperplasia.
TABLE 1.
Performance Characteristics of 68Ga-DOTATOC PET/CT in Detection of Unknown Primary NET (n = 40)
| Characteristic | Percentage |
| True-positive | 38 |
| Unconfirmed | 5 |
| False-positive | 7 |
| False-negative | 50 |
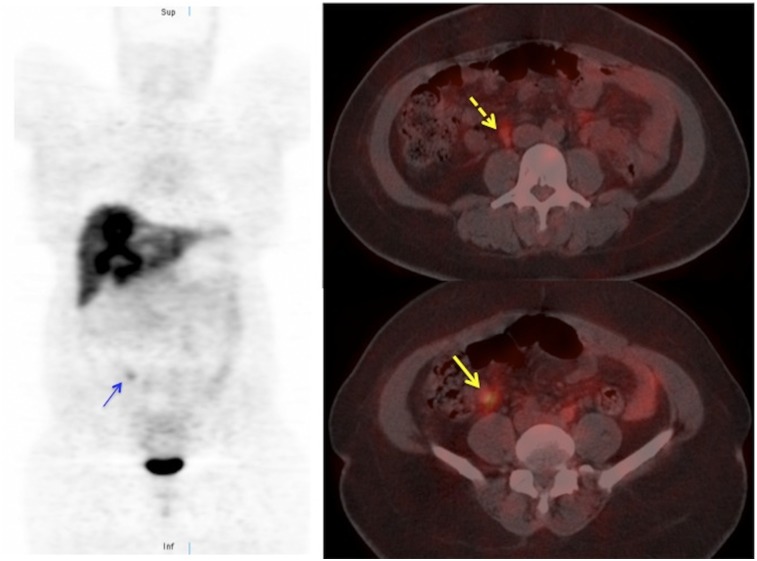
FIGURE 1.
68Ga-DOTATOC PET/CT images of patient with liver metastases on CT. In addition to multiple liver lesions, 68Ga-DOTATOC PET/CT shows focus of increased uptake in right lower quadrant (arrows), consistent with ileal NET. Subcentimeter paracaval node is also visible (dashed arrow). Patient underwent resection of primary ileal NET and radiofrequency ablation of liver metastases.
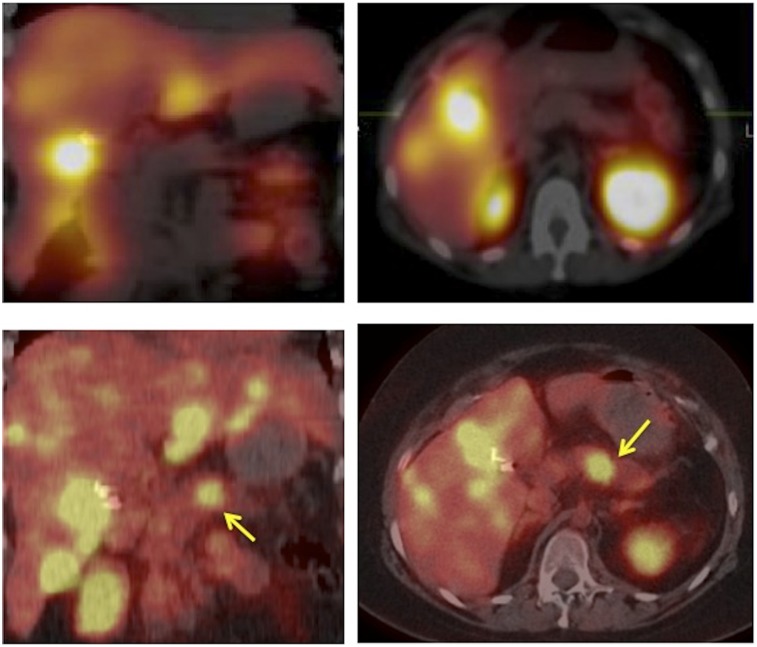
FIGURE 2.
111In-octreotide coronal and transaxial SPECT/CT images (top) and 68Ga-DOTATOC coronal and transaxial PET/CT images (bottom) of patient with liver metastases presenting with Cushing syndrome and unknown primary. Multiple liver lesions were seen on both scans although many more on 68Ga-DOTATOC PET/CT. Pancreatic body lesion is clearly identified on 68Ga-DOTATOC PET/CT images (arrows) but not visualized on the 111In-octreotide SPECT/CT scan obtained 2 mo before 68Ga-DOTATOC PET/CT scan. Patient underwent distal pancreatectomy, adrenalectomy, and radiofrequency ablation of liver metastases.
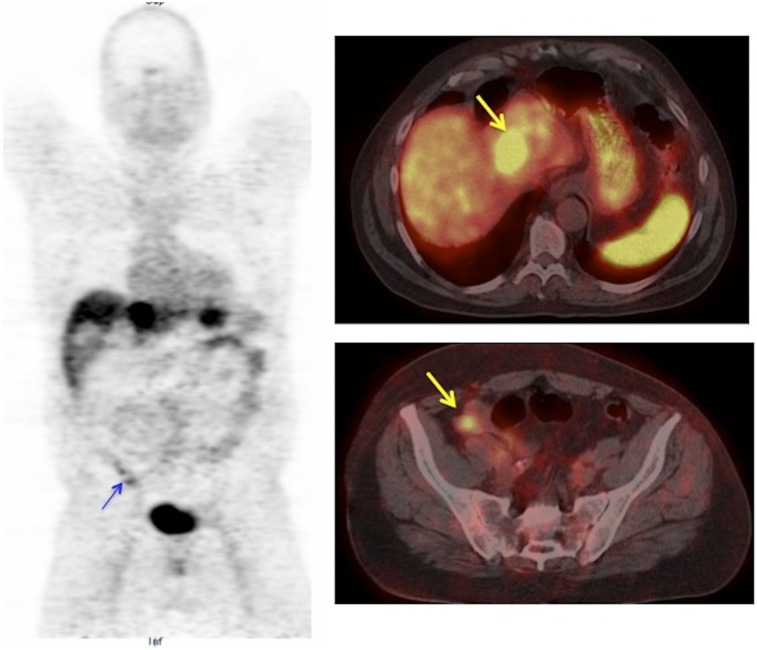
FIGURE 3.
False-positive 68Ga-DOTATOC PET/CT in patient with ileal lymphoid hyperplasia. Liver metastases seen on whole-body coronal and transaxial PET/CT images (arrow, right top). Mild focal uptake noted in right lower quadrant (arrow) suspicious for ileal NET. Surgical histopathology of ileal resection showed lymphoid hyperplasia.
DISCUSSION
NETs of unknown primary are the third most common site of origin for NETs after rectal and pulmonary carcinoids, surpassing pancreatic and ileal primary sites (13). Identification of primary tumor is critical if curative intent therapy is planned with complete surgical resection of metastases and the primary tumor. Even in patients with unresectable liver metastases, a systematic review of the literature has found a potential benefit of resection of primary NET (3). In a multiinstitutional retrospective analysis of patients with metastatic liver disease from midgut NET, resection of the primary tumor was an independent predictor of survival, with a median survival of 9.92 y after resection of the primary versus 4.68 y for patients with no resection of the primary tumor (14). Improved survival was also reported in patients after resection of primary pancreatic tumor in metastatic NET in 882 patients, with a median survival of 5.42 y for patients who had resection versus 0.83 y for patients who did not have surgery for primary tumor (4). It should be noted, however, that these studies reporting improved survival after resection were limited in their retrospective nature and did not prospectively randomize patients to systemic therapy versus surgery.
In our study, 68Ga-DOTATOC PET/CT identified the primary tumor in 38% of patients with metastases after conventional imaging failed to detect the primary lesion. 68Ga-DOTATOC PET/CT was equally effective in localization of unknown primary tumor in low-grade and intermediate-grade metastases. The 68Ga-DOTATOC PET/CT, however, led to unnecessary invasive procedures in 3 patients (8%). In 2 patients, variants of presumed normal uptake of 68Ga-DOTATOC were considered sufficiently suspicious in the pancreatic head (uncinate process) and gastric fundus to recommend endoscopic procedures, both of which failed to identify a primary tumor. In a third patient (Fig. 3), lymphoid hyperplasia in the ileum caused a false-positive scan that led to unnecessary small bowel resection. This likely reflected the high somatostatin receptor density in inflammatory bowel disease reported previously by somatostatin receptor autoradiography (15) and on 111In-octreotide scintigraphy (16).
There are few reports of case series in the literature on patients with unknown primary tumors imaged with 68Ga-DOTA-octreotide analogs (DOTATOC, DOTANOC, or DOTATATE). The largest study was reported by Prasad et al. with 68Ga-DOTANOC in 59 patients with metastatic NETs (17). In this latter study, the reported detectability of primary tumor with 68Ga-DOTANOC was higher at 59%, although 49% of the positive scans for primary tumor were unconfirmed and surgical histopathology was available in 17% of patients. There may be also differences in detectability of tumor between 68Ga-DOTANOC and 68Ga-DOTATOC PET/CT, although the receptor affinity of both agents is similar for somatostatin receptor subtype 2, the predominant somatostatin receptor on NETs, whereas 68Ga-DOTANOC has significantly higher affinity for somatostatin receptor subtype 3 and somatostatin receptor subtype 5 (18). In another study by Schreiter et al., patients with metastatic NET and unknown primary underwent either 68Ga-DOTATOC PET/CT (n = 33) or 111In-octreotide SPECT/CT (n = 50) (19). The detection rate of 46% for the unknown primary site with 68Ga DOTATOC was similar to our findings and significantly better than 8% obtained with 111In-octreotide. With 68Ga-DOTATATE PET/CT, Alonso et al. reported a detection rate of 59% of primary tumor in 29 patients with metastatic NETs, with a change in management in 24% of patients (20). Findings of our study along with these studies clearly demonstrate the value of PET/CT with 68Ga-DOTA-octreotide analogs in patients with unknown primary NETs and suggest that for this indication 68Ga-labeled DOTATOC, DOTANOC, and DOTATATE may be equivalent. At the time of writing of this article, only 68Ga-DOTATATE is approved by the U.S. Food and Drug Administration for clinical use and 68Ga-DOTANOC and 68Ga-DOTATOC are investigational.
Our study had some limitations. Not all primary tumor sites suggested by 68Ga-DOTATOC PET/CT could be verified by histology because some patients were not considered surgical candidates after workup. Although we were able to confirm or exclude primary tumor in 18 of 20 patients with positive 68Ga-DOTATOC PET/CT by histology or imaging, 2 patients remained unconfirmed. The workup before 68Ga DOTATOC PET/CT scanning was not homogeneous because conventional imaging studies were done at different institutions with potentially different protocols and read by different readers.
CONCLUSION
68Ga-DOTATOC PET/CT is an effective modality in localization of unknown primary tumor site in patients with metastatic NETs.
DISCLOSURE
This study was funded in part by NIH grants 1R01CA167632 and 1P50CA174521-01A1. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
This study was partly presented at the 2015 Society of Nuclear Medicine and Molecular Imaging Annual Meeting.
REFERENCES
- 1.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol. 2013;20:285–314. [DOI] [PubMed] [Google Scholar]
- 2.Pavel M, Baudin E, Couvelard A, et al. ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. [DOI] [PubMed] [Google Scholar]
- 3.Capurso G, Rinzivillo M, Bettini R, Boninsegna L, Delle Fave G, Falconi M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012;99:1480–1486. [DOI] [PubMed] [Google Scholar]
- 4.Keutgen XM, Nilubol N, Glanville J, et al. Resection of primary tumor site is associated with prolonged survival in metastatic nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2016;159:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell JE, Sherman SK, Menda Y, Wang D, O’Dorisio TM, Howe JR. Limitations of somatostatin scintigraphy in primary small bowel neuroendocrine tumors. J Surg Res. 2014;190:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. [DOI] [PubMed] [Google Scholar]
- 7.Kowalski J, Henze M, Schuhmacher J, Macke HR, Hofmann M, Haberkorn U. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe(1)-Tyr(3)-Octreotide in comparison to [111In]-DTPAOC SPECT: first results in patients with neuroendocrine tumors. Mol Imaging Biol. 2003;5:42–48. [DOI] [PubMed] [Google Scholar]
- 8.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. [DOI] [PubMed] [Google Scholar]
- 9.Menda Y, Ponto LL, Schultz MK, et al. Repeatability of gallium-68 DOTATOC positron emission tomographic imaging in neuroendocrine tumors. Pancreas. 2013;42:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhernosekov KP, Filosofov DV, Baum RP, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–1748. [DOI] [PubMed] [Google Scholar]
- 11.Mueller D, Klette I, Baum RP, Gottschaldt M, Schultz MK, Breeman WA. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem. 2012;23:1712–1717. [DOI] [PubMed] [Google Scholar]
- 12.Schultz MK, Mueller D, Baum RP, Leonard Watkins G, Breeman WA. A new automated NaCl based robust method for routine production of gallium-68 labeled peptides. Appl Radiat Isot. 2013;76:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–894. [DOI] [PubMed] [Google Scholar]
- 15.Reubi JC, Mazzucchelli L, Laissue JA. Intestinal vessels express a high density of somatostatin receptors in human inflammatory bowel disease. Gastroenterology. 1994;106:951–959. [DOI] [PubMed] [Google Scholar]
- 16.Marko J, Lamba R, Miller F, Buchman A, Spies S, Nikolaidis P. OctreoScan positive Crohn’s disease mimicking an ileal carcinoid tumor. J Clin Gastroenterol. 2008;42:66–68. [DOI] [PubMed] [Google Scholar]
- 17.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using 68Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging. 2010;37:67–77. [DOI] [PubMed] [Google Scholar]
- 18.Antunes P, Ginj M, Zhang H, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–993. [DOI] [PubMed] [Google Scholar]
- 19.Schreiter NF, Bartels AM, Froeling V, et al. Searching for primaries in patients with neuroendocrine tumors (NET) of unknown primary and clinically suspected NET: evaluation of Ga-68 DOTATOC PET/CT and In-111 DTPA octreotide SPECT/CT. Radiol Oncol. 2014;48:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso O, Rodriguez-Taroco M, Savio E, Bentancourt C, Gambini JP, Engler H. 68Ga-DOTATATE PET/CT in the evaluation of patients with neuroendocrine metastatic carcinoma of unknown origin. Ann Nucl Med. 2014;28:638–645. [DOI] [PubMed] [Google Scholar]