Abstract
The effects of long-term infection with Helicobacter pylori on the gastric mucosa of Mongolian gerbils were examined. Colonization by H. pylori was evaluated by both microaerobic cultivation and real-time reverse transcriptase PCR (RT-PCR). Persistent infection with H. pylori in gastric mucosa was detected by real-time RT-PCR during 6 months after infection, but no H. pylori was isolated 4 months after infection by cultivation. Infiltration with neutrophils and mononuclear cells was observed from 2 months after infection. Both intestinal metaplasia and gastric atrophy were also detected from 2 months after infection. The results by enzyme-linked immunosorbent assay indicated that antibody titers against whole H. pylori antigens, H. pylori heat shock protein 60 (HSP60), and Escherichia coli GroEL were significantly higher in the infected gerbils than in noninfected gerbils. After long-term infection with H. pylori for 18 months, marked atrophy of gastric mucosa and multiple cysts in the submucosa were observed in the glandular stomach of the infected gerbils. In addition, squamous cell papilloma with hyperkeratosis was observed in cardia of all the infected gerbils. These results indicate that evaluation of bacterial colonization during long-term infection can be done by real-time RT-PCR and that mucosal damage might be induced by host immune response against whole H. pylori antigen.
Warren and Marshall first isolated Helicobacter pylori in 1983 from the gastric mucosa of patients with chronic active gastritis (39), and since then the relation between this microorganism and gastric diseases has been widely studied (27, 28, 33, 37). A working group of the World Health Organization International Agency for Research on Cancer concluded in 1994 that H. pylori is a group I definite carcinogen in humans (13). However, the prevalence of H. pylori in patients with gastric carcinoma still remains considerably variable among studies, and there has been no direct demonstration that H. pylori is actually associated with gastric carcinogenesis.
Since Hirayama et al. (10) reported the establishment of persistent H. pylori infection in Mongolian gerbils as an animal model in 1996, many investigations on the association between H. pylori and gastric diseases have been carried out (12, 17, 21, 29, 30) and, recently, some reported that H. pylori was directly related to gastric carcinogenesis (9, 11, 32, 40). The purposes of almost all of the studies which have ever been carried out were predominantly focused on the measurement of the amount of gastric colonization, the serum antibody titer against H. pylori, and histopathological observation of the stomach. In the meantime, many investigators have reported that heat shock protein 60 (HSP60) or Lewis antigens of H. pylori are associated with the immune response (16, 20, 34, 35, 43). In this study, by using Mongolian gerbils infected with H. pylori we examined how H. pylori contributes to the autoimmune response by checking the relation between the histopathological changes in the stomach and the antibody titers against the various antigens, including HSP60 and Lewis antigens. In addition, we performed a basic examination on the difference in the detectable sensitivity of the number of H. pylori organisms between the routine culture method and the real-time reverse transcriptase PCR (RT-PCR) method.
MATERIALS AND METHODS
Animals.
Male 4-week-old Mongolian gerbils (MGS/Sea; specific pathogen free; body weight, 20 to 30 g) were purchased from Seac Yoshitomi (Fukuoka, Japan) and maintained in plastic cages under standard laboratory conditions (room temperature, 23 ± 2°C; relative humidity, 40 to 60%; 12-h light-dark cycle). A standard diet (CE-2; Clea Japan, Tokyo, Japan) and sterilized tap water were provided ad libitum.
Bacterial strain.
The bacterial strain used was H. pylori TK1402, isolated from gastric biopsy specimens of a patient with gastric ulcer and duodenal ulcer. It was determined by the PCR method that the strain has the vacuolating cytotoxin gene (vacA) and the cagA gene. That is, the primers used for the detection of cagA were F1 (5′-GATAACAGGCAAGCTTTTGAGG ) and B1 (5′-CTGCAAAAGATTGTTTGGCAGA ) (2). For the detection of the vacA gene, the primers used were VA3-F (5′-GGTCAAAATGCGGTCATGG ) and VA3-R (5′-CCATTGGTACCTGTAGAAAC ) and VA4-F (5′-GGAGCCCCAGGAAACATTG ) and VA4-R (5′-CATAACTAGCGCCTTGCA ) (2). Genomic DNA was extracted using MagExtractor (Toyobo). One microliter of extracted genomic DNA was mixed with each primer solution (5 pmol) and 0.5 U of Taq polymerase in a final volume of 20 μl. Reactions were performed for 30 cycles of 94°C for 1 min, 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min with a thermal cycler (Gene Amp PCR system 9600-R; Perkin-Elmer).
Furthermore, it was shown that the strain had the Lewisy antigen (LeY) on the cell surface. The bacteria were cultured in brain heart infusion agar (Difco, Detroit, Mich.) with 7% defibrinated horse blood (Wako Pure Chemical Industries Ltd., Osaka, Japan) under microaerobic conditions using GasPak jars (Mitsubishi Gas Chemical Company, Inc.) consisting of 85% N2, 10% CO2, and 5% O2 at 37°C for 2 days.
Experimental design.
Twenty-three Mongolian gerbils were inoculated with 1 ml of inoculum of 109 CFU of H. pylori TK1402/ml intragastrically for two consecutive days by using a feeding needle. The gerbils were fasted from 16 h before the first inoculation to completion of the second inoculation. Two to four gerbils were sacrificed under anesthesia with pentobarbital (50 mg/kg of body weight, intraperitoneally; Dainippon Pharmaceutical Co., Ltd.) at 0.5, 1, 2, 3, 4, 5, and 6 months after inoculation with H. pylori. After sacrifice, determination of the number of the microorganisms in the stomach, determination of serum antibody titers against various antigens, and both macroscopic and histopathological examinations of the stomach were carried out as described below. As a control group, the noninfected group was set in order to compare with the H. pylori-infected group, and one to two gerbils were sacrificed at the same measurable points as from the H. pylori-infected group.
Measurement of the number of microorganisms in the stomach by culture methods.
After inoculation with H. pylori, the stomachs were extirpated from the sacrificed gerbils at each point. The stomach was dissected along the greater curvature and washed with the 0.01 M phosphate-buffered saline (PBS, pH 7.4). Then, it was divided longitudinally into two halves, including the forestomach-to-pylorus part. The right half was used for the determination of the number of microorganisms. The left half was used for the histopathological examinations. The mucus layer was collected from the right half-stomach with Spartel in 1 ml of Hanks balanced salt solution and homogenized. Then, the suspended solution was inoculated onto M-BHM selective medium (Nikken Seibutsu, Kyoto, Japan) and incubated at 37°C for 7 days. After incubation, the number of gold colonies was counted and the number of viable organisms in the gastric mucosa was calculated.
Real-time RT-PCR assay.
A real-time RT-PCR assay was performed using the method reported by Lesley et al. (19) with some modifications. Tissue samples were lysed with lysozyme (400 μg/ml) in 100 μl of TE buffer (10 mM Tris-Cl, 1 mM EDTA; pH 8) by vortexing, followed by addition of guanidine isothiocyanate-containing lysis buffer (QIAGEN, Valencia, Calif.). Total RNA was isolated from the lysed sample according to the instructions using an RNeasy Mini kit. Quantification of RNA was done by measuring the A260/A280 ratio. Contaminating chromosomal DNA was digested with DNase I (DNA free; 1 U/μg of RNA; Ambion, Austin, Tex.) for 20 min at 37°C. One microgram of DNase I-treated total RNA was incubated with avian myeloblastosis virus reverse transcriptase with random primers in a commercial reaction mixture (20 μl; AMV reverse transcription system; Promega, Madison, Wis.). First-strand cDNA synthesis was performed.
cDNA was amplified using PCR primers for 16S rRNA of H. pylori, 16SB-F (5′-GCTAAGAGATCAGCCTATGTCC-3′) and 16SB-R (5′-TGGCAATCAGCGTCAGGTAATG-3′) (4). Amplification of the G3PDH gene on cDNA derived from Mongolian gerbils by using the primers G3PDH-F (5′-ACCACAGTCCATGCCATCAC-3′) and G3PDH-R (5′-TCCACCACCCTGTTGCTGTA-3′)was used for a noninfected control of total RNA extraction and for standardization of results of target gene transcriptional activity.
Quantitative analysis was performed using SYBR Green methods. The generation of quantitative data was based on the different PCR kinetics of samples with different levels of target gene expression. We used a relative quantification in which the expression levels of H. pylori target genes were compared to the data from a standard curve, which was generated by amplifying serial dilutions of a known quantity of amplicons. For each primer set, PCR was performed in parallel reactions using different amount of H. pylori strain TK1402 chromosomal DNA. Quantification data were analyzed using 5700 quantification software (Applied Biosystems). In this analysis, the background fluorescence was removed by manually setting a noise band. The long-linear portion of the standard's amplification curve was identified, and the crossing point was the intersection of the best-fit line through the long-linear region and the noise band. The standard curve was a plot of the crossing points versus the log bacterial number (in CFU per milliliter). The quantification software determined the unknown concentration by interpolating the noise band intercept of an unknown sample against the standard curve of known concentrations. The quantitative data were calculated from the standard curve of the PCR. For this approach, the identity and specificity of the PCR product were confirmed by dissociation curve analysis, which is part of the 5700 quantification program.
Histopathological examination.
After macroscopic observation of the stomach at necropsy, the remaining left halves of stomachs were fixed at 4°C for 2 h in cold Carnoy's solution (ethanol-chloroform-acetic acid, 6:3:1). After fixation, the stomachs were sliced longitudinally to include the forestomach to the glandular stomachs at intervals of 5-mm width. All specimens were then dehydrated in absolute alcohol and embedded in paraffin wax. Longitudinal sections with 5-μm thickness were processed from these paraffin blocks. The sections were stained with hematoxylin and eosin (H-E) and periodic acid-Schiff. The histopathological examinations were carried out, and the classification and grading of gastritis were based on the updated Sydney system (3).
ELISA.
Each antigen for ELISA was prepared by the following procedures. After H. pylori TK1402 was cultured on the blood agar medium at 37°C for 3 days, the harvested whole organisms were suspended in the 0.01 M PBS (pH 7.4) and sonicated by using an ultrasonic Sonifier 250 (Branson Ultrasonics Co., Danbury, Conn.) for 5 min at 20 kHz. Microtitration plates (Greiner Labortechnik Japan, Tokyo, Japan) were coated at 4°C for 18 h with the whole-sonicate antigens (3 μg/well) of H. pylori. The fixed H. pylori (whole) cells were used as the surface antigens of the organism. After this, H. pylori TK1402 (107 CFU) was poured in each well and fixed with 2% paraformaldehyde at 4°C for 18 h. Lipopolysaccharide (LPS) derived from H. pylori strain TK1313, which was extracted by the hot phenol-water technique (41), was used. Klebsiella pneumoniae LPS preparation (Sigma), Lewis Y tetrasaccharide (LeY; Sigma), human-derived recombinant HSP60 (Sigma), and E. coli-derived recombinant GroEL (Sigma) were also applied to the microtitration plates at 1 μg/well. In addition to them, H. pylori-derived HSP60 was prepared using the method of Yamaguchi et al. (43) and applied on the plates in the same way. Mucosal cells of the gastric body and pylorus, as antigens of 6-month-old Mongolian gerbils, were prepared by ultrasonication and applied to the plates at 2 μg/well. In addition, as human antigens, the AGS cell line derived from a gastric cancer and HEp2 cell line derived from a laryngeal cancer were sonicated by the method described above and applied on the plates at 2 μg/well. After coating the plates with each antigen at 4°C overnight, they were washed with PBS three times. After this, each antigen was blocked with PBS containing 1% skim milk (PBS-S; Yukijirushi Nyugyo Co., Tokyo, Japan) at 37°C for 1 h. After washing with PBS, each serum sample taken from the infected or noninfected gerbils with H. pylori was used after dilution with PBS by 20- or 300-fold. One hundred microliters of each diluted serum was added to the plates and reacted with each antigen at 37°C for 2 h. After washing with PBS three times, 100 μl of horseradish peroxidase-protein G (Sigma) at 25 μg/ml in PBS-S was added to the plates and reacted with the antigen-antibody complex at 37°C for 1 h. The plates were treated with 0.1% o-phenylenediamine in the developing buffer (0.1 M citric acid, 0.07 M sodium phosphate dibasic, 0.035% H2O2). After incubation at room temperature for 5 min, the reaction was stopped by adding 50 μl of 2 N H2SO4. The optical density was measured at 490 nm by using a microplate reader (model 550; Bio-Rad).
Statistic analysis.
The correlation between the number of microorganisms and serum antibody titer and the correlation between serum antibody titer and the inflammation scores based on the updated Sydney system were examined by the statistic analysis software StatView (HuLinks Co. Ltd.). Also, statistically significant differences in serum antibody titers between the infected and the noninfected gerbils with H. pylori were examined by using Welch's t test.
RESULTS
Measurement of the number of microorganisms in the stomach.
A change in the number of H. pylori organisms in the stomach by the culture method is shown in Table 1. The numbers of microorganisms were 102.60 ± 101.42, 101.90 ± 101.70, 101.41 ± 101.96, and 104.45 ± 102.75 CFU/g of tissue at 0.5, 1, 2, and 3 months after inoculation with H. pylori, respectively, but less than the detectable limit (20 CFU/g of tissue) later at 4 months.
TABLE 1.
Isolation of H. pylori from stomachs of infected Mongolian gerbils
| Time after infection (mos) | Infection statusa | Mean no. of H. pylori isolatedb (CFU/g of tissue) |
|---|---|---|
| 0.5 | 5/6 | 2.60 ± 1.42 |
| 1 | 5/8 | 1.90 ± 1.70 |
| 2 | 3/8 | 1.41 ± 1.96 |
| 3 | 6/8 | 4.45 ± 2.75 |
| 4 | 0/4 | —c |
| 5 | 0/4 | — |
| 6 | 0/4 | — |
Number of H. pylori-positive gerbils/number of gerbils examined.
Mean±standard deviation (log 10).
No H. pylori was isolated.
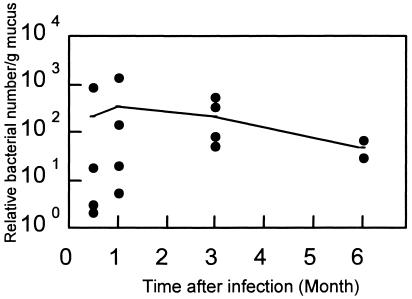
The change in the relative number of H. pylori strain TK1402 organisms in the stomach (estimated microorganism number per PCR mixture) by the real-time RT-PCR method is shown in Fig. 1. The melting point in the real-time RT-PCR method was 89.3°C. The number of microorganisms was found to be 10 to 103 at 1 month after infection with H. pylori and did not change by 3 months after infection.
FIG. 1.
Number of H. pylori in stomachs of Mongolian gerbils infected with H. pylori strain TK1402, calculated by the real-time RT-PCR method. •, individual relative bacterial number per gram of mucus from H. pylori-infected gerbils; -, average relative bacterial number per gram of mucus.
Histopathological findings of the stomach.
As shown in Table 2, two to four gerbils infected with H. pylori were sacrificed per time point for pathology. There were no differences in the histopathological findings between the infected gerbils (Fig. 2B) and the noninfected gerbils (Fig. 2A) 1 month after inoculation with H. pylori. However, 2 months after infection with H. pylori, inflammatory cell infiltration by neutrophils and lymphocytes, hyperplasia of regenerative epithelium in the lamina propria mucosa, and atrophic changes were observed (Fig. 2C). Some prominent lymphoid follicles were also observed in both the lamina propria mucosa and submucosa. In severe cases, the inflammatory cell infiltration expanded to the lamina muscularis mucosa or more deeply, reaching the serosa. In the histopathological examination at 3 and 6 months after infection, intestinal metaplasia was also observed in one out of three gerbils and one out of four gerbils, respectively (Fig. 2D). Judging from the severity of the inflammation, there were no histopathological differences between 2 and 6 months after infection.
TABLE 2.
Scoring of histopathological findings based on the updated Sydney system in stomachs of Mongolian gerbils infected with H. pylori
| Finding | Time after infection with H. pylori (mo)a
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5
|
1
|
2
|
3
|
4
|
5
|
6
|
||||||||
| N (0) | H (2) | N (1) | H (3) | N (1) | H (3) | N (2) | H (3) | N (2) | H (4) | N (2) | H (4) | N (2) | H (4) | |
| Neutrophil infiltration (activity) | — | 0 | 0 | 0 | 0 | 3.0 | 0 | 3.0 | 0 | 2.8 | 0 | 2.5 | 0 | 3.0 |
| Mononuclear cell infiltration | — | 0 | 0 | 0 | 0 | 3.0 | 0 | 3.0 | 0 | 2.8 | 0 | 2.5 | 0 | 3.0 |
| Intestinal metaplasia | — | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0.3 |
| Atrophy | — | 0 | 0 | 0 | 0 | 1.3 | 0 | 1.3 | 0 | 1.0 | 0 | 1.0 | 0 | 1.5 |
N, noninfected gerbils; H, H. pylori-infected gerbils; —, not examined. Values in parentheses are the number of gerbils in each group.
FIG. 2.
Histopathological findings in gastric mucosa of Mongolian gerbils at 1, 2, and 6 months after H. pylori infection (H-E stain). (A) Noninfected; (B) 1 month after infection; (C) 2 months after infection; (D) 6 months after infection. Magnification, ×90.
The histopathological findings observed in each Mongolian gerbil were scored based on the updated Sydney system (neutrophil infiltration [activity], mononuclear cell infiltration, intestinal metaplasia, and atrophy) and are summarized in Table 2. The severity of the findings was classified as mild (+ or 1), moderate (++ or 2), and marked (+++ or 3). The score for neutrophil infiltration or mononuclear cell infiltration was 2.5 to 3.0 at later than 2 months after infection with H. pylori. The score for intestinal metaplasia was 0.3 at 3 months and 6 months after infection with H. pylori. The score for atrophy was 1.0 to 1.5. Neutrophil infiltration and mononuclear cell infiltration were predominant, rather than intestinal metaplasia or atrophy.
ELISA results.
The results of the ELISA are shown in Table 3. Antibody titer against TK1402 whole sonicate of H. pylori-infected gerbils tended to increase at later than 2 months after infection with H. pylori, and it was significantly higher than that of the noninfected gerbils (P < 0.01) (Fig. 3A). Antibody titers against H. pylori HSP60 and GroEL in the infected gerbils at 3 to 6 months after infection also increased and were significantly higher than those of the noninfected gerbils (P < 0.01 and 0.05, respectively). The antibody titers against human HSP60 and gerbil tissues (pylorus and gastric body) in H. pylori-infected gerbils were higher than those in noninfected gerbils, but there were no statistically significant differences between groups because of their deviations. These antigens were considered to have no cross-reaction with anti-H. pylori antibody in the infected Mongolian gerbils.
TABLE 3.
Serum antibody titers to the various antigens in Mongolian gerbils with or without H. pylori infection
| Antigen tested | Antibody titer (mean ± SD)
|
|
|---|---|---|
| Noninfected gerbilsa | H. pylori-infected gerbilsa | |
| H. pylori TK1402 (sonicate) | 0.011 ± 0.052 | 1.355 ± 0.726** |
| H. pylori TK1402 (whole) | 0.000 ± 0.010 | 0.109 ± 0.091** |
| HEp2 | 0.013 ± 0.023 | 0.019 ± 0.042 |
| AGS | 0.037 ± 0.080 | 0.000 ± 0.050 |
| H. pylori HSP60 | 0.035 ± 0.018 | 0.386 ± 0.200** |
| Human HSP60 | 0.000 ± 0.047 | 0.187 ± 0.339 |
| E. coli GroEL | 0.021 ± 0.068 | 0.139 ± 0.167* |
| LeY | 0.003 ± 0.009 | 0.012 ± 0.030 |
| H. pylori TK1313 LPS | 0.547 ± 0.428 | 0.521 ± 0.399 |
| K. pneumoniae LPS | 0.010 ± 0.007 | 0.020 ± 0.054 |
| Tissue (pylorus) | 0.415 ± 0.700 | 0.811 ± 1.031 |
| Tissue (gastric body) | 0.205 ± 0.501 | 0.813 ± 0.943 |
For H. pylori TK1402 antigens (sonicate or whole), HEp2, and AGS, n = 10 noninfected gerbils or 23 H. pylori-infected gerbils and the ELISA was conducted 2 to 6 months after infection (serum was diluted 300-fold in PBS). For all other antigens, n = 6 noninfected gerbils or 17 H. pylori infected gerbils and the ELISA was conducted 3 to 6 months after infection (serum was diluted 20-fold in PBS). *, significantly different from noninfected gerbils (P < 0.05); **, significantly different from noninfected gerbils (P < 0.01).
FIG. 3.
Correlation between antibodies against H. pylori TK1402 sonicates and cell infiltration based on the updated Sydney system. (A) Antibody titer against H. pylori TK1402 sonicates after infection. (B) Correlation between antibody titer and score of neutrophil infiltration (activity).
There was no statistically significant difference in antibody titer against K. pneumoniae LPS, LeY, pylorus and gastric body of the Mongolian gerbils, AGS cell line, and HEp2 cell line between H. pylori-infected gerbils and the noninfected gerbils. A correlation between antibody titer against H. pylori TK1402 sonicates and neutrophil infiltration based on the updated Sydney system was seen (r = 0.7778, P < 0.05) (Fig. 3B). Antibody titer against TK1402 whole sonicate increased 2 months after H. pylori infection, and a positive correlation between its antibody titer and neutrophil infiltration was shown.
Effect of long-term infection with H. pylori on stomachs of Mongolian gerbils.
The relative number of H. pylori organisms estimated by real-time RT-PCR was below the detectable level 18 months after infection with H. pylori. At autopsy, macroscopically polyps in the glandular stomachs and thickening of the cardia in all three infected gerbils were detected. Histopathologically, marked atrophy of mucosa and multiple cysts in the submucosa were observed in the glandular stomachs of the three gerbils (Fig. 4A). In part of one glandular stomach, the disappearance of almost all gastric glands and fibrosis were observed in the lamina propria mucosa. This lesion was considered to be well-differentiated adenocarcinoma; however, the glandular epithelium was strongly atypical, and the nucleus/cytoplasm ratio was high (Fig. 4B). At the thick part of the cardia of three gerbils, squamous cell papillomas with hyperkeratosis were observed (Fig. 4C). In two noninfected gerbils sacrificed at the same time as the above-mentioned three infected gerbils, neither macroscopic nor histopathological changes were observed in either glandular stomach or cardia (Fig. 4D and E). The antibody titers against H. pylori TK1402 (sonicate) and H. pylori HSP60 were 1.868 ± 0.959 (n = 3) and 0.287 ± 0.120 (n = 3), respectively, 18 months after infection, and these were significantly higher than those of noninfected gerbils. There was no increase of antibody titers in the noninfected gerbils sacrificed at the same time.
FIG. 4.
Histopathological findings in gastric mucosa of Mongolian gerbils with (A, B, and C) or without (D and E) long-term H. pylori infection for 18 months (H-E stain). (A) Glandular stomach. Multiple cysts were seen in the submucosa. Magnification, ×50. (B) Glandular stomach. The submucosa was fibrous, and a well-differentiated adenocarcinoma was seen. Magnification, ×90. (C) Cardia. A squamous cell papilloma with hyperkeratosis was seen in the forestomach. Magnification, ×35. (D) Glandular stomach of a noninfected gerbil. No significant change was seen. Magnification, ×90. (E) Cardia of a noninfected gerbil. No significant change was seen. Magnification, ×90.
DISCUSSION
The infection model for H. pylori using Mongolian gerbils is considered to be a useful model, since gastric mucosal changes in stomachs of Mongolian gerbils were similar to those in human stomachs following H. pylori infection. Gastric colonization by H. pylori in Mongolian gerbils was reported to be related to several factors of the organism, such as urease productivity, motility, etc. Iwao et al. reported that the motility of H. pylori was an important factor for gastric colonization in Mongolian gerbils, and there were differences in its gastric colonization due to differences in phenotypic strains of the gerbils (14). Ogura et al. reported that there were differences in gastric colonization, suppression of body weight gain, and histopathology following H. pylori infection, depending on the H. pylori strains (24). Kurita et al. (18) and Ohkusa et al. (25) also recognized that different strains induced different severities of infection in Mongolian gerbils. Strain TK1402, isolated from a patient with duodenal ulcer and gastric ulcer, has both cagA and vacA. There are two reports on the virulence of this strain in gnotobiotic mice (26) and C57BL mice (42). In this study, we found that histopathologically severe inflammatory cell infiltration with neutrophils and mononuclear cells, lymphoid follicles, and intestinal metaplasia were induced after infection with H. pylori. Judging from the onset time and severity of the histopathological changes, these were almost the same changes as with the standard strain, ATCC 43504, which has been used in many infection experiments with Mongolian gerbils (10-12, 14, 25, 30). The pathogenicity of strain TK1402 is considered to be almost equal to that of strain ATCC 43504.
Causality between the presence of H. pylori and its carcinogenicity in the stomach has not been made clear yet. Recently, however, there were several papers suggesting the causality between H. pylori and its carcinogenicity, as adenocarcinoma or carcinoid were observed in a long-term infection experiment with H. pylori in Mongolian gerbil (9, 40) and H. pylori, as promoter, enhanced the adenocarcinoma induced by N-methyl-N-nitrosourea (22, 31). In addition, Touati et al. (38) examined the role of H. pylori infections in the induction of mutations in gastric epithelial cells by using Big Blue transgenic mice, and they reported that the gastric mutant frequency was fourfold higher in H. pylori-infected mice than in noninfected mice 6 months after inoculation and that this genotoxicity could be attributable to oxidative DNA damage involving the host inflammatory response. We did not find gastric cancer in the 6-month study. But in the long-term study for 18 months under the same conditions, well-differentiated adenocarcinoma was observed in one glandular stomach of three animals and squamous cell papillomas with hyperkeratosis were observed in the cardia of all three animals infected with H. pylori. No studies with squamous cell papillomas with hyperkeratosis have ever been reported for Mongolian gerbils infected with H. pylori.
In this study, we tried to quantify the number of colonized organisms in the stomach by the real-time RT-PCR method in addition to the routine culture method. With the culture method, many investigators have reported that they could detect the 104 to 105 CFU of H. pylori in the gastric mucosa when 107 to 109 CFU of H. pylori were inoculated (21, 29, 40). On the other hand, there was a report in which H. pylori was not detected by the culture method in spite of a high titer of anti-H. pylori antibody in the serum (9). The number of H. pylori organisms isolated was not always accordant among the reports using the culture method. In our case, we could detect viable H. pylori in the stomach by the routine culture method at 1 to 3 months after inoculation, but not 4 months later. On the other hand, we could detect H. pylori even 6 months after inoculation and obtained better sensitivity with the real-time RT-PCR method than the culture method. As we could calculate the number of H. pylori in all the infected Mongolian gerbils by using the real-time RT-PCR method at 6 months after inoculation, this PCR-based method is a useful tool for quantification of H. pylori.
Antibody titer against H. pylori whole-sonicate antigen or its surface antigen significantly increased from 2 months after inoculation. Many studies suggest that gastritis or gastric atrophy in humans is related to the production of autoantibodies (5, 15, 23, 35, 36). HSP60 is considered to be related to the induction of gastritis mediated by autoimmunity (16), and we have reported that the anti-HSP60 antibody titer increased in sera taken from patients with various gastric diseases (43). Gobert et al. (7) reported that H. pylori HSP60 mediated production of interleukin-6 and played a patho-physiological role in the autoimmune response of chronic gastritis. In addition, as LeX and LeY antigens, which are the components of the H. pylori LPS, are the same antigens as expressed on blood cells and parietal cells, the possibility that antibodies against these molecules react as an autoantibody has been pointed out (1, 8). We examined whether autoantibodies against these antigens were produced, and we confirmed the elevation of antibody titer against the HSP60 family. That is, autoantibody against GroEL derived from E. coli, which showed molecular homogeneity with H. pylori HSP60, also increased. Ferrero et al. (6) assessed H. pylori HSPs as potential protective antigens in mice with gastric H. pylori infection, and they reported that a protein belonging to the GroES class of HSPs induced protective immunity. Yamaguchi et al. (43) also pointed out the possibility of protection against H. pylori infection by an HSP60 homologue. The results of our present study may be related to theirs. On the contrary, there was no increase in antibody titers against LeY or LPS derived from H. pylori.
It is possible that the raised antibodies to these antigens following long-term infection with H. pylori might play an important role for induction of the mucosal damage in the stomach of the infected gerbils. However, the correlation between the production of these antibodies and the induction of gastric tumors, such as adenocarcinoma and squamous cell papilloma, remains to be determined.
REFERENCES
- 1.Appelmelk, B. J., I. Simoons-Smit, R. Negrini, A. P. Moran, G. O. Aspinall, J. G. Forte, T. de Vries, H. Quan, T. Verboom, J. J. Maaskant, P. Ghiara, E. J. Kuipers, E. Bloemena, T. M. Tadema, R. R. Townsend, K. Tyagarajan, J. M. Crothers, Jr., M. A. Monteiro, A. Savio, and J. de Graaff. 1996. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect. Immun. 64:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton, J. C., P. Cao, R. M. Peek, M. K. R. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 3.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 4.Engstrand, L., A. M. Nguyen, D. Y. Graham, and F. A. El-Saatari. 1992. Reverse transcription and polymerase chain reaction amplification of rRNA for detection of Helicobacter species. J. Clin. Microbiol. 30:2295-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faller, G., S. Ruff, N. Reiche, J. Hochberger, E. G. Hahn, and T. Kirchner. 2000. Mucosal production of antigastric autoantibodies in Helicobacter pylori gastritis. Helicobacter 5:129-134. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero, R. L., J.-M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobert, A. P., J. C. Bambou, C. Werts, V. Balloy, M. Chignard, A. P. Moran, and R. L. Ferrero. 2004. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J. Biol. Chem. 279:245-250. [DOI] [PubMed] [Google Scholar]
- 8.Heneghan, M. A., C. F. McCarthy, D. Janulaityte, and A. P. Moran. 2001. Relationship of anti-Lewis x and anti-Lewis y antibodies in serum samples from gastric cancer and chronic gastritis patients to Helicobacter pylori-mediated autoimmunity. Infect. Immun. 69:4774-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirayama, F., S. Takagi, E. Iwao, Y. Yokoyama, K. Haga, and S. Hanada. 1999. Development of poorly differentiated adenocarcinoma and carcinoid due to long-term Helicobacter pylori colonization in Mongolian gerbils. J. Gastroenterol. 34:450-454. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama, F., S. Takagi, Y. Yokoyama, E. Iwao, and Y. Ikeda. 1996. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J. Gastroenterol. 31:24-28. [PubMed] [Google Scholar]
- 11.Honda, S., T. Fujioka, M. Tokieda, R. Satoh, A. Nishizono, and M. Nasu. 1998. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 58:4255-4259. [PubMed] [Google Scholar]
- 12.Ikeno, T., H. Ota, A. Sugiyama, K. Ishida, T. Katsuyama, R. M. Genta, and S. Kawasaki. 1999. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am. J. Pathol. 154:951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer, World Health Organization. 1994. Schistosomes, liver flukes, and Helicobacter pylori. Monogr. Eval. Carcinog. Risks Hum. 61:218-220. [PMC free article] [PubMed] [Google Scholar]
- 14.Iwao, E., F. Hirayama, S. Takagi, Y. Yokoyama, and Y. Ikeda. 1999. Virulence factors of Helicobacter pylori affecting its gastric colonization in Mongolian gerbils. J. Gastroenterol. 34:47-54. [PubMed] [Google Scholar]
- 15.Kalabay, L., B. Fekete, L. Czirjak, L. Horvath, M. R. Daha, A. Veres, G. Fonyad, A. Horvath, A. Viczian, M. Singh, I. Hoffer, G. Füst, L. Romics, and Z. Prohaszka. 2002. Helicobacter pylori infection in connective tissue disorders is associated with high levels of antibodies to mycobacterial hsp65 but not to human hsp60. Helicobacter 7:250-256. [DOI] [PubMed] [Google Scholar]
- 16.Kamoshida, S., Y. Satoh, S. Kamiya, and Y. Tsutsumi. 1999. Heat shock protein 60 (HSP60) immunoreactivity in gastric epithelium associated with Helicobacter pylori infection: a pitfall in immunohistochemically interpreting HSP60-mediated autoimmune response. Pathol. Infect. 49:88-90. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai, T., J. Yan, D. Y. Graham, M. Tozuka, Y. Okimura, T. Ikeno, A. Sugiyama, T. Katsuyama, and H. Ota. 2001. Serum immunoglobulin G immune response to Helicobacter pylori antigens in Mongolian gerbils. J. Clin. Microbiol. 39:1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurita, M., T. Kouchiyama, K. Okita, and T. Nakazawa. 1991. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am. J. Gastroenterol. 86:1596-1603. [PubMed] [Google Scholar]
- 19.Lesley, E. S., J. Chen, J. R. Lindsey, P. Ghiara, P. D. Smith, and K. B. Waites. 2000. Quantitative analysis of Helicobacter pylori infection in a mouse model. J. Immunol. Methods 242:67-78. [DOI] [PubMed] [Google Scholar]
- 20.Mahdavi, J., T. Boren, C. Vandenbroucke-Grauls, and B. J. Appelmelk. 2003. Limited role of lipopolysaccharide Lewis antigens in adherence of Helicobacter pylori to the human gastric epithelium. Infect. Immun. 71:2876-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto, S., Y. Washizuka, Y. Matsumoto, S. Tawara, F. Ikeda, Y. Yokota, and M. Karita. 1997. Induction of ulceration and severe gastritis in Mongolian gerbil by Helicobacter pylori infection. J. Med. Microbiol. 46:391-397. [DOI] [PubMed] [Google Scholar]
- 22.Murata, F., A. Sugiyama, K. Ishida, T. Ikeno, M. Murakami, S. Kawasaki, H. Ota, M. Tatematsu, and T. Katsuyama. 2000. Timing of N-methyl-N-nitrosourea administration affects gastric carcinogenesis in Mongolian gerbils infected with Helicobacter pylori. Cancer Lett. 160:99-105. [DOI] [PubMed] [Google Scholar]
- 23.Negrini, R., A. Savio, and B. J. Appelmelk. 1997. Autoantibodies to gastric mucosa in Helicobacter pylori infection. Helicobacter 2:S-13-S-16. [DOI] [PubMed] [Google Scholar]
- 24.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 192:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkusa, T., I. Okayasu, H. Miwa, K. Ohtaka, S. Endo, and N. Sato. 2003. Helicobacter pylori infection induces duodenitis and superficial duodenal ulcer in Mongolian gerbils. Gut 52:797-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osaki, T., H. Taguchi, H. Yamaguchi, and S. Kamiya. 1998. Detection of Helicobacter pylori in fecal samples of gnotobiotic mice infected with H. pylori by an immunomagnetic-bead separation technique. J. Clin. Microbiol. 36:321-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsonnet, J., D. Vandersteen, J. Goates, R. K. Sibley, J. Pritikin, and Y. Chang. 1991. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J. Natl. Cancer Inst. 83:640-643. [DOI] [PubMed] [Google Scholar]
- 28.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 29.Sawada, Y., N. Yamamoto, T. Sakagami, Y. Fukuda, T. Shimoyama, T. Nishigami, K. Uematsu, and K. Nakagawa. 1999. Comparison of pathologic changes in Helicobacter pylori-induced Mongolian gerbils and humans. J. Gastroenterol. 34:55-60. [PubMed] [Google Scholar]
- 30.Sawada, Y., Y. Kuroda, H. Sashio, N. Yamamoto, Y. Tonokatsu, T. Sakagami, Y. Fukuda, T. Shimoyama, T. Nishigami, and K. Uematsu. 1998. Pathological changes in glandular stomach of Helicobacter pylori-induced Mongolian gerbil model. J. Gastroenterol. 33:22-25. [PubMed] [Google Scholar]
- 31.Sugiyama, A., F. Maruta, T. Ikeno, K. Ishida, S. Kawasaki, T. Katsuyama, N. Shimizu, and M. Tatematsu. 1998. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 58:2067-2069. [PubMed] [Google Scholar]
- 32.Sugiyama, T., S. Hige, and M. Asaka. 2002. Development of an H. pylori-induced animal model and gastric cancer: recent progress and issues. J. Gastroenterol. 37:6-9. [DOI] [PubMed] [Google Scholar]
- 33.Talley, N. J., A. R. Zinsmeister, A. Weaver, E. P. DiMagno, H. A. Carpenter, G. I. Perez-Perez, and M. J. Blaser. 1991. Gastric adenocarcinoma and Helicobacter pylori infection. J. Natl. Cancer Inst. 83:1734-1739. [DOI] [PubMed] [Google Scholar]
- 34.Taylor, D. E., D. A. Rasko, R. Sherburne, C. Ho, and L. D. Jewell. 1998. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterology 115:1113-1122. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, D. E., R. N. Fedorak, and R. Sherburne. 1999. Antigenic mimicry between Helicobacter pylori and gastric mucosa: failure to implicate heat-shock protein 60 using immunoelectron microscopy. Helicobacter 4:148-153. [DOI] [PubMed] [Google Scholar]
- 36.Telford, J. L., A. Covacci, R. Rappuoli, and P. Ghiara. 1997. Immunobiology of Helicobacter pylori infection. Curr. Opin. Immunol. 9:498-503. [DOI] [PubMed] [Google Scholar]
- 37.The Eurogast Study Group. 1993. An international association between Helicobacter pylori infection and cancer. Lancet 341:1359-1362. [PubMed] [Google Scholar]
- 38.Touati, E., V. Michel, J.-M. Thiberge, N. Wuscher, M. Huerre, and A. Labigne. 2003. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology 124:1408-1419. [DOI] [PubMed] [Google Scholar]
- 39.Warren, J. R., and B. J. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273-1275. [PubMed] [Google Scholar]
- 40.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]
- 41.Westphal, O., O. Lüderits, and F. Bister. 1952. Über die extraction von bakterien mit phenol/wasser. Z. Naturforsch. Teil B 7:148-155. [Google Scholar]
- 42.Yamaguchi, H., T. Osaki, H. Taguchi, N. Sato, A. Toyoda, M. Takahashi, M. Kai, N. Nakata, A. Komatsu, Y. Atomi, and S. Kamiya. 2003. Effect of bacterial flora on postimmunization gastritis following oral vaccination of mice with Helicobacter pylori heat shock protein 60. Clin. Diagn. Lab. Immunol. 10:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi, H., T. Osaki, M. Kai, H. Taguchi, and S. Kamiya. 2000. Immune response against a cross-reactive epitope on the heat shock protein 60 homologue of Helicobacter pylori. Infect. Immun. 68:3448-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]