Abstract
Schistosoma mansoni surface membrane components play a relevant role in the host-parasite interaction, and some are released in vivo as circulating antigens. n-Butanol extraction favors the release of membrane antigens like alkaline phosphatase, which has been shown to be specifically recognized by antibodies from S. mansoni-infected humans and animals. In the present study, components in the n-butanol extract (BE) of the adult S. mansoni worm membrane fraction were separated by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (1D SDS-PAGE [15%]) and further analyzed by immunoblotting (immunoglobulin G) using defined sera. S. mansoni-infected patient sera, but not sera of uninfected patients or sera obtained from patients infected with other parasite species, specifically and variably recognized up to 20 polypeptides in the molecular mass range of ∼8 to >80 kDa. There were some differences in the number, intensity, and frequency of recognition of the BE antigens among sera from Venezuelan sites of endemicity with a different status of schistosomiasis transmission. Antigens in the 28- to 24-kDa molecular mass range appeared as immunodominants and were recognized by S. mansoni-positive sera from all the sites, with recognition frequencies varying between 57.5 and 97.5%. Immunoblotting with BE membrane antigens resulted in a highly sensitive (98.1%), specific (96.1.0%), and confirmatory test for the immunodiagnosis of schistosomiasis in low-transmission areas.
In Venezuela, intestinal schistosomiasis caused by Schistosoma mansoni is distributed in the central-northern region of the country. Diagnosis of schistosomiasis relied for many years on the finding of parasite eggs in the feces. After decades of successful national programs to control the disease, the prevalence (as assessed by coprologic examination) decreased to 1.4% in 1996 (3) and the intensity of the infection, as assessed by the number of schistosome eggs per gram of feces, decreased to less than 100 eggs per gram, with most carriers becoming asymptomatic; disease transmission became low, but the disease was never eradicated. In this epidemiological situation, coprological analyses became insensitive to detect infections and serodiagnosis emerged as a valid diagnostic alternative to estimate infections (4). Based on serology, prevalence figures increased in low-transmission areas (4). Particular attention was thus given to identify and characterize sensitive and specific Schistosoma antigens to obtain better diagnoses in these areas. A few defined soluble antigens were reported to show high sensitivity and specificity in such areas (15, 25). Other studies provided evidence for the relevant antigenicity of some S. mansoni membrane components (22, 27). Adult S. mansoni membrane-bound enzymes, like alkaline phosphatase, acid phosphatase, type I phosphodiesterase, and Ca2+-ATPase, present in n-butanol extracts (BE) (8, 18, 19), were shown to be antigenic when tested in enzyme immunoassays (9). The antigenic property of the S. mansoni alkaline phosphatase was used for the development of a new diagnostic test called an alkaline phosphatase immunoassay (APIA), which takes direct advantage of the intrinsic catalytic activity of the antigen. This assay has been successfully used to detect exposure to S. mansoni in low-transmission areas (4, 8, 9, 17). Besides the above-mentioned enzymes, other adult S. mansoni worm membrane components are known to be present in the BE (10, 18). In the present work, we report the results of an immunoblot analysis performed with BE and sera of S. mansoni-infected patients, uninfected patients, or patients infected with other parasite species from Venezuelan sites endemicity with a different status of schistosomiasis transmission; results revealed that BE antigens proved very sensitive and specific for diagnosis and/or for diagnosis confirmation of other schistosomiasis tests in low-transmission areas.
MATERIALS AND METHODS
Patients and examinations.
Sera used in this study were selected from a universe of blood samples ethically collected from volunteers in field work performed between 1997 and 2000 by medical personnel from the Venezuelan Ministry of Health (Maracay, Venezuela), Instituto Venezolano de Investigaciones Científicas, and the Institute for Tropical Medicine (Universidad Central de Venezuela, Caracas, Venezuela) in four different communities of the Venezuelan area of endemicity (5). The sites of endemicity and their epidemiological characteristics were as follows: (i) “José Leonardo Chirinos” (hereafter referred to as J.L. Chirinos; Carabobo State), a relatively recently established marginal urban transmission site south of the city of Valencia with about 1% of captured snails eliminating S. mansoni cercariae and 2.0% of people (19 years old on average) excreting a low number of S. mansoni eggs in the feces (5). (ii) Caraballeda (Vargas State), an older marginal urban site where transmission ceased about 21 years before blood collection, with no vector snails in the area but with 9.2% of the examined population (35 years old on average) still excreting S. mansoni eggs in feces (5); these people probably remained undiagnosed and untreated until blood collection. (iii) Belén (Carabobo State), a traditional rural past-transmission site that became reemergent a few years before blood collection, with about 1% of the captured snails eliminating S. mansoni cercariae and 88.8% of the examined people (21 years old on average) excreting eggs in the feces (5). (iv) San Sebastián de los Reyes (Aragua State), a rural site which was endemic for schistosomiasis in the past, with no vector snails and without transmission for the last 30 years; all the people examined (23 years old on average) had no eggs in the feces and showed negative serology. Diagnosis for schistosomiasis was performed with four different tests per individual: Kato-Katz (KK) analysis (12), the circumoval precipitating test (COPT) (16), enzyme-linked immunosorbent assay (ELISA) (26) using standard soluble egg antigen (SEA), and APIA (17); all these techniques were previously used in field work reported by Alarcón de Noya et al. (4, 5) and Cesari et al. (10). For the aim of the present study, we selected 40 positive sera (by the above-mentioned tests) from the four different sites (see Table 1, below). We also selected and evaluated 36 sera from patients of the above endemic areas harboring other infectious agents (19 females and 17 males; mean age, 19.4 years; range, 2 to 56 years): Trichuris trichura, Ascaris lumbricoides, Strongyloides stercoraris, Enterobium vermicularis, Necator americanus, Ancylostoma sp., Hymenolepis nana, Entamoeba histolytica, Entamoeba coli, Iodoamoeba butschlii, Endolimax nana, Giardia lamblia, and/or Blastocystis hominis. Cysticercosis sera were also evaluated.
TABLE 1.
Diagnostic characteristics of patients with schistosomiasis mansoni from the different endemic sites used in the present study
| Site | Transmission status | n | Mean age (yrs) | No. of S. mansoni-positive sera by:
|
||||
|---|---|---|---|---|---|---|---|---|
| KK | COPT | ELISAa | APIA | IB | ||||
| Belén | Reemergent | 40 | 21.0 | 25 | 39 | 40 | 40 | 40 |
| Caraballeda | Old | 40 | 32.6 | 3 | 22 | 32 | 37 | 38 |
| J.L. Chirinos | Recent | 40 | 18.3 | 1 | 12 | 25 | 25 | 35 |
| S. Sebastián | Free | 40 | 19.8 | 0 | 0 | 0 | 0 | 0 |
ELISA was performed with SEA. IB, immunoblot assay.
Parasites.
Adult S. mansoni (Venezuelan JL strain) worms were obtained by perfusion (11) of hamsters infected with 400 cercariae 7 weeks before, washed in sterile saline, and frozen at −80°C until used.
Membrane antigen preparation.
Adult S. mansoni worms were homogenized in Potter-Elvejhem at 4°C with 50 mM Tris-HCl, pH 8.0, containing 1 mM MgCl2 (Tris-Mg buffer). The worm homogenate was centrifuged in a Beckman L8-M ultracentrifuge using an SW65 rotor for 2 h at 100,000 × g and 4°C; after withdrawing the supernatant, the particulated pellet was washed two times with the same buffer and recentrifuged as above. The pellet was resuspended in Tris-Mg buffer, extracted with 50% (vol/vol) water-saturated n-butanol, and mixed vigorously at room temperature, and the suspension was centrifuged for 15 min at 14,000 × g and 4°C. The aqueous phase was recovered and dialyzed overnight against Tris-Mg buffer, pH 8.0, and designated as BE (18). Since BE components could slowly aggregate and/or precipitate at 4°C, Triton X-100 (0.1% final concentration [wt/vol]) was added to the extract to keep them in solution. Sodium azide (0.02% [wt/vol]) was also added, the extract was centrifuged at low speed, and the supernatant was filtered through a 0.22-μm-pore-size Millipore membrane and stored at 4°C until used. Protein content in BE samples was determined according to the method of Bradford (6), using bovine serum albumin as the standard protein.
Electrophoresis and immunoblot assay.
Homogeneous nonreducing one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (1D SDS-PAGE) at 15% (wt/vol) was carried out as described by Laemmli (14) in minigels (6 by 9 cm) in a Mini-Protean II electrophoresis chamber (Bio-Rad Laboratories). BE components (250 μl; 0.58 mg/ml) were separated at 200 V (constant). Prestained molecular weight standards (GIBCO) were run simultaneously on the same gel. BE polypeptides were then electrophoretically transferred onto nitrocellulose (NC; 0.45 μm; Hybond ECL; Amersham) in a semidry blotter (Bio-Rad) at 90 mA for 90 min at 5°C in 25 mM Tris, 192 mM glycine, and 20% methanol (24). Free-reacting sites on NC were blocked with 5% skimmed milk in Tris-buffered saline (0.01 M; pH 8.2) containing 1% Tween 20 under constant agitation for 2 h at room temperature. NC strips were cut (2 mm wide), and individual strips were incubated at room temperature under constant agitation for 90 min with the respective serum sample diluted 1:100 in blocking solution. The strips were next washed with Tris-buffered saline containing 0.05% Tween 20 to remove unbound serum components and then incubated for 90 min at room temperature with a second antibody (anti-human immunoglobulin G [IgG] peroxidase conjugate; Sigma) diluted 1:5,000 in blocking solution. Immune reactions were detected by autoradiography on Hyperfilm (Amersham) and using a peroxidase chemiluminescent substrate (ECL detection system; Amersham). Films were registered with an EPSON Expression 1600 scanner, and molecular weights of antigens were analyzed with the LabWorks-UVP software. The criterion for immunoblot positivity was the presence of at least one specific Schistosoma band in the blot. Sensitivity was calculated by dividing the number of individuals positive in immunoblot assay that were also positive by other S. mansoni tests (combined results) by the total number of individuals testing positive by these tests, × 100. Specificity was calculated by dividing the number of individuals negative on immunoblotting that were also negative by other S. mansoni tests (combined results) by the total number of individuals testing negative by these tests, × 100.
RESULTS
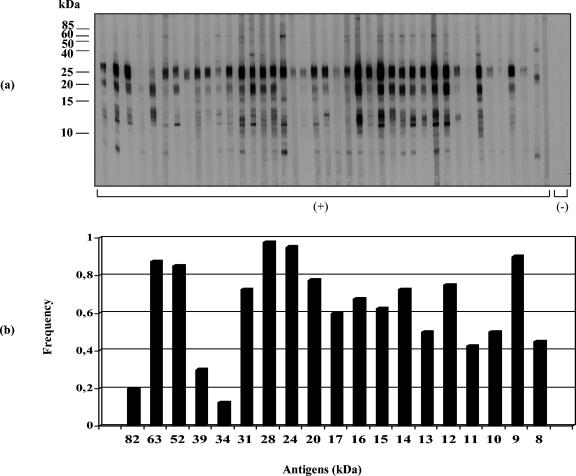
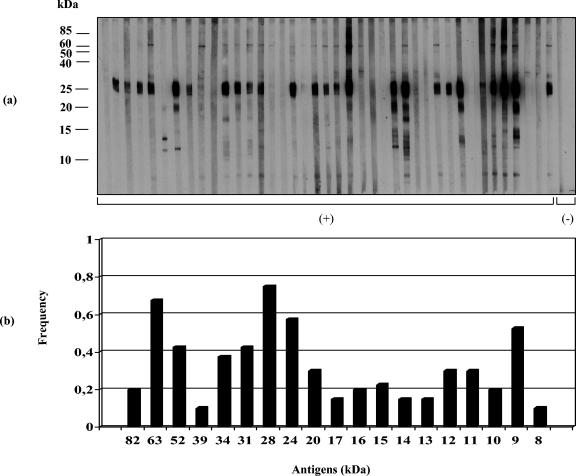
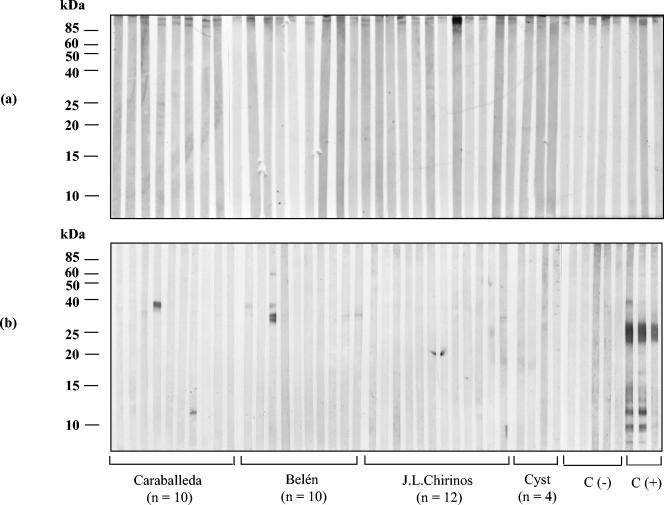
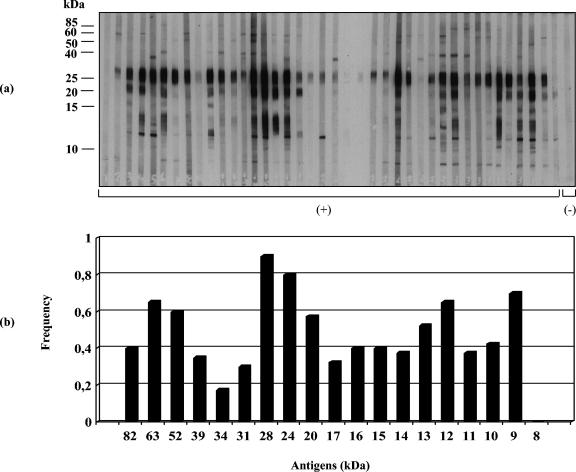
Immunoblot assays (IgG antibodies) were performed with sera from patients of different Venezuelan transmission sites. S. mansoni-positive sera reacted with about 19 to 20 BE polypeptides ranging from 8 to >80 kDa (Fig. 1 to 3). Sera from San Sebastián de los Reyes (Fig. 4a) and most sera from S. mansoni-negative individuals infected with other parasite species (Fig. 4b) were negative in immunoblot assays with BE antigens. Recognition of BE antigens by S. mansoni-positive sera showed individual and site-related differences regarding the number, intensity, and frequency of recognition of antigen bands (Fig. 1 to 3). Serial dilutions (1:50, 1:100, 1:250, and 1:500) mostly affected the intensity of recognition, with weaker bands disappearing at dilutions higher than 1:250; a 1:100 dilution was considered suitable for a comparative immunoblot analysis. Under these conditions, we obtained the following results: (i) all Belén patient sera tested (n = 40) selected as positive by KK, COPT, ELISA (SEA), and/or APIA were also positive by immunoblotting (Table 1); the recognition frequency of BE antigens was mainly as follows (antigen size in kilodaltons): 28 (97.5%) > 24 (95%) > 9 (90%) > 63 (87.5%) > 52 (85%) > 22/20 doublet (77.5%) > 12 (75%) > 31 and 14 (72.5%) > 16 (67.5%) > 15 (62.5%) > 17 (60%) > 13 and 10 (50%), with other antigens being recognized with frequencies lower than 50% (Fig. 1). On the other hand, the intensity of antigen recognition was as follows (antigen size in kilodaltons): 28 > 24 > 22/20 > 12 > 9 > 63 > 52 > 17 > 14 > other antigens (Fig. 1). (ii) Thirty-eight out of 40 Caraballeda patient sera, selected as positive by the above criteria, were also positive by immunoblotting (Table 1); recognition frequencies of BE antigens were as follows (in kilodaltons): 28 (90%) > 24 (80%) > 9 (70%) > 63 and 12 (65%) > 52 (60%) > 22/20 doublet (57.5%) > 13 (52.5%), with other antigens being recognized with frequencies lower than 50% (Fig. 2). Intensity of the antigens in Caraballeda was as follows (in kilodaltons): 28 > 24 > 22/20 > 12 > 39 > 9 > 63 > 17 > 52 > other antigens; apparently, the weak 8-kDa antigen was not seen in Caraballeda sera (Fig. 2). (iii) Thirty-five out of 40 selected positive sera from J.L. Chirinos were also positive by immunoblotting (Table 1); frequency recognition was the following (antigen size in kilodaltons): 28 (75%) > 63 (67.5%) > 24 (57.5%) > 9 (52.5%), with other antigens showing recognition frequencies lower than 50% (Fig. 3). Intensity of the antigens was as follows (in kilodaltons): 28 > 24 > 22/20 > 12 > 63 > 9 > 17 > 14 > other antigens (Fig. 3).
FIG. 1.
Immunoblot analysis of human antibody (IgG) response to S. mansoni antigens extracted with n-butanol from the adult worm membrane fraction. (a) Immunoblot profile of BE with sera (n = 40) from Belén (reemergent transmission site); (b) antigen recognition frequency. Molecular mass markers (in kilodaltons) are shown on the left. C(+), S. mansoni-positive sera; C(-), controls. The results shown are representative of three independent replicated experiments.
FIG. 3.
Immunoblot analysis of human antibody (IgG) response to S. mansoni antigens extracted with n-butanol from the adult worm membrane fraction. (a) Immunoblot profile of BE with sera (n = 40) from J.L. Chirinos (recently established transmission site); (b) antigen recognition frequency. Molecular mass markers (in kilodaltons) are shown on the left. C(+), S. mansoni-positive sera; C(-), controls. The results shown are representative of three independent replicated experiments.
FIG. 4.
Immunoblot analysis of human antibody (IgG) response to S. mansoni antigens extracted with n-butanol from the adult worm membrane fraction. (a) Immunoblot profile of BE with sera (n = 40) from San Sebastián de los Reyes (old endemic site with no transmission since 35 years ago); (b) immunoblot profile of BE with sera of individuals from J.L. Chirinos (n = 12), Caraballeda (n = 10), and Belén (n = 10) infected with the following: T. trichura, A. lumbricoides, S. stercoraris, E. vermicularis, N. americanus, Ancylostoma sp., H. nana, E. histolytica, Entamoeba coli, I. butschlii, E. nana, G. lamblia, and/or B. hominis. Cyst, cysticercosis sera (n = 4); C(-), sera from healthy people (n = 5); C(+), S. mansoni-positive sera (n = 3); CC, conjugate control. Molecular mass markers (in kilodaltons) are shown on the left.
FIG. 2.
Immunoblot analysis of human antibody (IgG) response to S. mansoni antigens extracted with n-butanol from the adult worm membrane fraction. (a) Immunoblot profile of BE with sera (n = 40) from Caraballeda (a site without transmission since 20 years ago but still having infected patients); (b) antigen recognition frequency. Molecular mass markers (in kilodaltons) are shown on the left. C(+), S. mansoni-positive sera; C(-), controls. The results shown are representative of three independent replicated experiments.
The 28-kDa component was the most strongly and the most frequently recognized (75.0 to 97.5%) BE antigen in all S. mansoni-positive sites (Fig. 1 to 3); recognition of this antigen persisted even after sera were diluted 1:500 (data not shown). However, the intensity and the frequency of recognition for a given antigen were not always coincident among sites, and this made the difference between sites. The 24-kDa antigen (strong intensity) and the 9-kDa antigen (moderate intensity) were second in recognition frequency after the 28-kDa antigen in Belén and Caraballeda but not in J.L. Chirinos, where the 63-kDa antigen (moderate intensity) was second in importance (Fig. 1 to 3). The immunodominant 22/20-kDa doublet and the 12-kDa antigens were frequently seen in Belén and Caraballeda but not in J.L. Chirinos sera (Fig. 1 to 3). Other differences involving antigens with masses lower than 20 kDa (Fig. 1 to 3) were seen between sites. A two-by-two table was created with the 160 sera data from the four sites under study (Fig. 1 to 4) to compare positive and negative results obtained by KK, COPT, ELISA, and/or APIA with the positive and negative results obtained by immunoblotting for the same universe of sera. The immunoblotting performed with BE antigens showed 98.1% sensitivity and 96.1% specificity (data not shown).
DISCUSSION
Many membrane-bound schistosome components are targets for the host immune response against the parasite and have been reported to be relevant to host-parasite relationships (21-23). Some of these components can be extracted into the aqueous phase obtained after n-butanol treatment of membranous parasite suspensions. In adult S. mansoni worms, several BE membrane proteins have been shown to be glycosylated and/or have phosphoesterase activities like alkaline phosphatase, type I phosphodiesterase, acid phosphatase, and Ca2+-ATPase (8, 18). Some of the BE components show antigenicity when confronted with infected mouse serum (18) and were studied in this work by nonreducing 1D SDS-PAGE (15%) and immunoblotting with sera of different Venezuelan infected human patients. Venezuelan sites of schistosomiasis endemicity are generally characterized by low disease transmission (3-5). Currently, about 70% of infected people in those sites show low intensity of infection (<100 eggs/g of feces) (5). All-or-nothing results were seen between S. mansoni-positive and -negative sera, and only S. mansoni-positive sera were found to be immunoreactive with about 19 to 20 BE polypeptide antigens, supporting an elevated specificity for the immunoblotting analysis with these antigens (Fig. 1 to 4). Indeed, when the presence of at least one specific BE antigenic band was taken as a criterion for positiveness, the immunoblot analysis, when compared to diagnostic results obtained by other schistosomiasis tests like KK, COPT, ELISA, and/or APIA, appeared as a highly sensitive (98.1%) and specific (96.1%) assay. In general, there was a similar pattern of recognition for most BE antigens between sites; nevertheless, immune recognition of some BE antigens was variable and apparently site related. The 28-kDa component was immunodominant and the most frequently recognized BE antigen by sera from all the sites (97.5% in Belén, 90.0% in Caraballeda, 75.0% in J.L. Chirinos). Second in importance was the 24-kDa antigen for Belén (95%) and Caraballeda (80%) sera, although J.L. Chirinos sera saw the 63-kDa antigen (72.5%) as the second most important antigen (Fig. 1 to 3). The 22/20-kDa doublet and the 12-kDa antigen were also immunodominant, and they were frequently seen in Belén and Caraballeda sera but not in J.L. Chirinos sera, which recognized other antigens (52 and 9 kDa) with higher frequency (Fig. 1 to 3). In general, sera from Belén (a reemergent transmission site) recognized a higher number of bands, and about 75% of the antigens were recognized with higher frequency than in the other two sites. The antigenic pattern recognized by Caraballeda sera followed in complexity that seen in sera from Belén. In Caraballeda, schistosomiasis transmission ceased about 21 years before blood collection, but coprology analysis indicated that 9.2% of the people were still harboring an S. mansoni infection at the moment of blood collection (5). Only adult patients were found positive (mean age, 35 years old) compared to the other two sites (ca. 20 years old), because no more transmission was present. Except for a few antigens, sera from J.L. Chirinos recognized antigens having masses of <24 kDa, with a lower frequency than in sera from Belén and Caraballeda (Fig. 1 to 3).
Most of the above antigen recognition differences between sites persisted after sera were diluted 1:250, although the less intense bands disappeared at higher dilutions (data not shown), suggesting that some qualitative and quantitative differences were intrinsic properties of sera. We do not know exactly why there are such differences, but they could be related to (i) the elapsed time of chronic infection within the host (an unknown factor to us); (ii) the actual parasite charge; (iii) differential response (could be a result of antigenic variability among S. mansoni local parasite strains); (iv) differential immune response from the local population; (v) an unbalanced schistosome sex ratio that might occur in low-transmission areas and with the presence of a sex-related immune response. The more complex response observed with sera from Belén (Fig. 2) may be related to reinfections and higher parasitic charges present in that site. However, when the number of bands was plotted against the number of eggs per gram of feces with the Belén data, no correlation was observed between the parasite load and the pattern of response (data not shown). Sera of patients from San Sebastián de los Reyes (mean age, 19.8 years old), an old and treated endemic site without S. mansoni transmission since 30 years ago, were totally negative (Fig. 4a). Sera of people that tested negative for S. mansoni but positive for other parasitosis (n = 36) did not recognize BE antigens except for three sera that recognized polypeptides of 71, 38, 34, and 12 kDa not associated with a particular parasite species, supporting the specificity of BE antigen recognition (Fig. 4b).
Preliminary analyses of sera from patients infected with S. haematobium, S. intercalatum, S. japonicum, or S. mekongi indicate a lack of cross-reaction with S. mansoni BE antigens, in particular with the 28- and 24-kDa antigen complex, supporting the view that they may be species specific. Recognition of these antigens by sera of S. mansoni-infected people from the French Caribbean island of Guadeloupe and from Senegal supports this assumption (unpublished data). It was difficult to relate the observed S. mansoni BE membrane antigens with those membrane antigens reported in the literature. Nonetheless, in 1974 Sher et al., using n-butanol, extracted glycoproteins of 21 and 33 kDa from adult worm membranes that were able to induce a lethal antibody response against schistosomula in vitro; however, this response did not play an important protective role in vivo (21). BE antigens seen in the 20- to 35-kDa range (Fig. 1 to 3) may correspond to the above-reported antigens. Butterworth et al. (7) described a 24.5-kDa tegumental membrane antigen that correlated to resistance in S. mansoni-infected humans. On the other hand, Smithers et al. (22), by using immunoblotting, reported that the principal antigens recognized by antibodies from mice protectively vaccinated with tegumental membranes showed molecular masses of 25, 15, and 13 kDa. The Sm13 tegumental antigen (1) and a calcium binding protein of 8 kDa known as Sm8 (2, 20) were specifically recognized in immunoblotting with sera of schistosomiasis patients. On the other hand, a 36-kDa antigen (15) and a highly immunogenic Sm31/32 protein fraction (homologous to S. mansoni cathepsin B and asparaginyl endoprotease, respectively) (25) were found to be useful serologic markers for diagnosing and for differentiating by immunoblotting between acute and chronic schistosomiasis infection in low-transmission areas. However, these antigens are soluble adult worm antigens (SAWA) and probably are not homologous to the antigens seen in our membrane preparation.
Although differences in molecular mass might result in some cases from different extraction procedures, most antigens tested in our present study were insoluble membrane-bound components and needed n-butanol to be released from the washed adult worm membrane fraction; most soluble proteins in a SAWA preparation are usually inactivated by this solvent. In addition, rabbit polyclonal antibodies to specific antifusion proteins MS2Sm31 and MS2Sm32 (kindly given to us by M.-Q. Klinkert) (13) did not see antigens in our BE preparation, although they were able to recognize their specific antigens in SAWA and in adult worm vomitus (data not shown). Characterization of the bands by using available monoclonal antibodies for known antigens might underline the relevance of BE antigens in diagnosis and in the host-parasite relationship. The immunological mechanisms responsible for the differential pattern observed will be difficult to explain until the structural nature of these bands has been identified. As a main conclusion, we can say that immunoblotting with extracted membrane antigens may represent an additional very helpful diagnostic and/or confirmatory immunological test for schistosomiasis mansoni in epidemiological surveys in low-transmission areas where sensitivities of most known tests tend to decrease as the intensity of the infection diminishes.
Acknowledgments
This work was partially supported by grant VEN 96-002 from the PCEE-UNDP/World Bank.
We thank all members of the communities of Belén, J.L. Chirinos, Caraballeda, and San Sebastián de los Reyes for their agreement and spontaneous cooperation in giving a small amount of blood for the present study.
REFERENCES
- 1.Abath, F. G., E. M. Xavier, R. Allen, Y. M. Gomes, N. Lucena-Silva, M. Baliza, and A. J. G. Simpson. 2000. Characterization of Sm13, a tegumental antigen of Schistosoma mansoni. Parasitol. Res. 86:745-752. [DOI] [PubMed] [Google Scholar]
- 2.Abath, F. G., E. M. Xavier, S. M. Montenegro, and R. P. Werkhauser. 2002. Partial molecular characterization of Sm8, a tegumental antigen of Schistosoma mansoni. Mem. Inst. Oswaldo Cruz 97:91-93. [DOI] [PubMed] [Google Scholar]
- 3.Alarcón de Noya, B., C. Balzán, C. Arteaga, I. Cesari, and O. Noya. 1999. The last fifteen years of schistosomiasis in Venezuela: features and evolution. Mem. Inst. Oswaldo Cruz 94:139-146. [DOI] [PubMed] [Google Scholar]
- 4.Alarcón de Noya, B., I. M. Cesari, S. Losada, C. Colmenares, C. Balzán, J. Hoebeke, and O. Noya. 1997. Evaluation of alkaline phosphatase immunoassay and comparison with other diagnostic methods in areas of low transmission of schistosomiasis. Acta Trop. 66:69-78. [DOI] [PubMed] [Google Scholar]
- 5.Alarcón de Noya, B., O. Noya, R. Ruiz, C. Colmenares, S. Losada, R. Contreras, A. Bruces, G. Certad, A. Hernan, C. Sierra, J. Toro, N. Chacón, and I. M. Cesari. 2003. Prevalencia de las parasitosis intestinales y esquistosomosis en comunidades del área centro norte de Venezuela. Bol. Malariol. San. Amb. XLIII:21-30. [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth, A. E., M. Capron, J. S. Cordingly, P. R. Dalton, D. W. Dunne, H. C. Kariuki, G. Kimani, D. Koech, M. Mugambi, J. H. Ouma, et al. 1985. Immunity after treatment of human schistosomiasis. II. Identification of resistant individuals, and analysis of their immune responses. Trans. R. Soc. Trop. Med. Hyg. 79:393-408. [DOI] [PubMed] [Google Scholar]
- 8.Cesari, I. M., F. H. Pujol, B. Alarcón de Noya, O. Noya, J. Hoebeke, and D. Bout. 1992. Immunodiagnosis based on enzyme markers, p. 83-101. In R. Bergquist (ed.), Diagnostic approaches in schistosomiasis. John Wiley & Sons, London, United Kingdom.
- 9.Cesari, I. M., F. H. Pujol, M. Rodríguez, and B. Alarcón de Noya. 1987. Possible use of Schistosoma mansoni enzymes as antigens for immunodiagnosis. Mem. Inst. Oswaldo Cruz 82(Suppl. IV):175-177. [DOI] [PubMed] [Google Scholar]
- 10.Cesari, I. M., L. Mendoza, D. E. Ballen, and B. Alarcón de Noya. 2002. Evaluación del “IEFA” como técnica de inmunodiagnóstico en el programa de lucha contra la bilharziosis. Bol. Malariol. San. Amb. XLII:29-32. [Google Scholar]
- 11.Duvall, R., and W. B. Dewitt 1967. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am. J. Trop. Med. Hyg. 16:483-486. [DOI] [PubMed] [Google Scholar]
- 12.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 14:397-400. [PubMed] [Google Scholar]
- 13.Klinkert, M.-Q., K. Bommert, R. Felleisen, D. Moser, G. Link, O. Doumbo, and E. Beck. 1992. Evaluation of recombinant Schistosoma mansoni antigens Sm31 and Sm32 for immunodiagnosis, p. 83-101. In R. Bergquist (ed.), Diagnostic approaches in schistosomiasis. John Wiley & Sons Publishers, London, United Kingdom.
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Noya, O., Z. Fermín, B. Alarcón de Noya, S. Losada, C. Colmenares, and T. Hermoso. 1995. Humoral immune response of children with chronic schistosomiasis. Isotype recognition of adult worm antigens. Parasite Immunol. 17:319-328. [DOI] [PubMed] [Google Scholar]
- 16.Oliver-González, J. 1954. Anti-egg precipitations in sera of human infected with Schistosoma mansoni. J. Infect. Dis. 95:86-91. [DOI] [PubMed] [Google Scholar]
- 17.Pujol, F. H., B. Alarcón de Noya, and I. M. Cesari. 1989. Immunodiagnosis of schistosomiasis mansoni with APIA (alkaline phosphatase). Immunol. Investig. 18:1071-1080. [DOI] [PubMed] [Google Scholar]
- 18.Pujol, F. H., and I. M. Cesari. 1990. Antigenicity of adult Schistosoma mansoni alkaline phosphatase. Parasite Immunol. 12:189-198. [DOI] [PubMed] [Google Scholar]
- 19.Pujol, F. H., and I. M. Cesari. 1993. Schistosoma mansoni: surface membrane isolation with lectin-coated beads. Mem. Biochem. 10:155-161. [DOI] [PubMed] [Google Scholar]
- 20.Ram, D., B. Romano, and I. Schechter. 1994. Immunochemical studies on the cercarial-specific calcium binding protein of Schistosoma mansoni. Parasitology 108:289-300. [DOI] [PubMed] [Google Scholar]
- 21.Sher, A., J. Kusel, H. Pérez, and J. Clegg. 1974. Partial isolation of a membrane antigen which includes formation of antibodies lethal to schistosomes cultured in vitro. Exp. Immunol. 18:357-369. [PMC free article] [PubMed] [Google Scholar]
- 22.Smithers, S. R., F. Hackett, P. O. Ali, and A. J. G. Simpson. 1989. Protective immunization of mice against Schistosoma mansoni with purified adult worm surface membranes. Parasite Immunol. 11:301-318. [DOI] [PubMed] [Google Scholar]
- 23.Smithers, S. R., F. Hackett, V. Braga, and A. J. G. Simpson. 1990. Immunoblotting identifies additional antigens recognized by mice protectively vaccinated with adult Schistosoma mansoni tegumental membranes. Parasitol. Res. 76:454-456. [DOI] [PubMed] [Google Scholar]
- 24.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology 24:145-149. [PubMed] [Google Scholar]
- 25.Valli, L. C. P., H. Kanamura, R. M. Da Silva, R. Ribeiro-Rodrigues, and R. Dietze. 1999. Schistosomiasis mansoni: immunoblot análisis to diagnose and differentiate recent and chronic infection. Am. J. Trop. Med. Hyg. 61:302-307. [DOI] [PubMed] [Google Scholar]
- 26.Voller, A., A. Bartlett, and D. Bidwell. 1976. Enzyme immunoassay for parasitic diseases. Trans. R. Soc. Trop. Med. Hyg. 70:98-105. [DOI] [PubMed] [Google Scholar]
- 27.Xavier, E. M., N. Lucena-Silva, R. P. Werkhauser, G. R. Franco, R. A. Santos, A. J. G. Simpson, and F. G. Abath. 1998. The tegument of Schistosoma mansoni: genes, antigens and the host-parasite relationship. Mem. Inst. Oswaldo Cruz 93(Suppl. I):85-86. [DOI] [PubMed] [Google Scholar]