Abstract
Purpose
We assessed the safety and antitumor activity of avelumab, a fully human anti–programmed death-ligand 1 (PD-L1) IgG1 antibody, in patients with refractory metastatic urothelial carcinoma.
Methods
In this phase Ib, multicenter, expansion cohort, patients with urothelial carcinoma progressing after platinum-based chemotherapy and unselected for PD-L1 expression received avelumab 10 mg/kg intravenously every 2 weeks. The primary objectives were safety and tolerability. Secondary objectives included confirmed objective response rate (Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1), progression-free survival, overall survival (OS), and PD-L1–associated clinical activity. PD-L1 positivity was defined as expression by immunohistochemistry on ≥ 5% of tumor cells.
Results
Forty-four patients were treated with avelumab and followed for a median of 16.5 months (interquartile range, 15.8 to 16.7 months). The data cutoff was March 19, 2016. The most frequent treatment-related adverse events of any grade were fatigue/asthenia (31.8%), infusion-related reaction (20.5%), and nausea (11.4%). Grades 3 to 4 treatment-related adverse events occurred in three patients (6.8%) and included asthenia, AST elevation, creatine phosphokinase elevation, and decreased appetite. The confirmed objective response rate by independent central review was 18.2% (95% CI, 8.2% to 32.7%; five complete responses and three partial responses). The median duration of response was not reached (95% CI, 12.1 weeks to not estimable), and responses were ongoing in six patients (75.0%), including four of five complete responses. Seven of eight responding patients had PD-L1–positive tumors. The median progression-free survival was 11.6 weeks (95% CI, 6.1 to 17.4 weeks); the median OS was 13.7 months (95% CI, 8.5 months to not estimable), with a 12-month OS rate of 54.3% (95% CI, 37.9% to 68.1%).
Conclusion
Avelumab was well tolerated and associated with durable responses and prolonged survival in patients with refractory metastatic UC.
INTRODUCTION
Urothelial carcinoma of the bladder is a leading cause of cancer deaths worldwide, with an estimated 429,800 new cases reported in 2012.1 Patients with metastatic urothelial carcinoma are typically treated with cisplatin-based combination chemotherapy in the first-line setting, with a median overall survival (OS) of 14 to 15 months.2,3 Prognostic factors associated with shorter OS include poor performance status, visceral metastasis, and low albumin or hemoglobin level.4 Second-line chemotherapies, such as paclitaxel, pemetrexed, docetaxel, and vinflunine, have limited efficacy, with median survival of approximately 7 months.5-8
Immunotherapy with immune checkpoint inhibitors, particularly agents targeting the programmed death-ligand 1/programmed death-1 (PD-L1/PD-1) axis, has improved treatment outcomes in several tumor types.9 Immunotherapy for urothelial carcinoma began in 1990 with approval of the intravesical bacillus Calmette-Guérin vaccine for non–muscle-invasive disease.10,11 The rationale for assessing immune checkpoint inhibitors in advanced urothelial cancer is supported by a high prevalence of tumor somatic mutations,12 which may generate neoantigens that are recognized by activated antitumor T cells.13,14 Such mutational signatures have been shown to correlate with response to PD-L1/PD-1 antibodies in a range of advanced solid tumors, including metastatic urothelial carcinoma.13,15 Atezolizumab, an anti–PD-L1 agent, showed clinical activity in patients with locally advanced or metastatic urothelial carcinoma after platinum-based chemotherapy in a single-arm, phase II trial,16 which led to its approval in the United States.17 Although levels of PD-L1 expression on tumors and infiltrating lymphocytes in the tumor microenvironment have been associated with response to atezolizumab in urothelial carcinoma in the second-line setting, the role of PD-L1 as a predictive biomarker remains unclear.18,19
Avelumab (MSB0010718C) is an investigational fully human anti–PD-L1 IgG1 antibody that inhibits PD-1/PD-L1 interactions while leaving the PD-1/PD-L2 pathway intact20 and enhances immune activation against tumor cells, as shown in preclinical studies.21,22 Unlike other anti–PD-L1/PD-1 antibodies that are approved or in advanced clinical development, avelumab induces lysis of tumor cells via antibody-dependent cell-mediated cytotoxicity in vitro, suggesting an additional mechanism of action.23 Importantly, avelumab has not shown antibody-dependent cell-mediated cytotoxicity against immune cell subsets in humans.21,24 A large, international, multicohort, phase I study was conducted to assess the safety and clinical activity of avelumab in patients with refractory advanced solid tumors. In the dose-escalation part of the study, intravenous infusion of avelumab every 2 weeks was safe and had a predictable pharmacokinetic profile at doses ≤ 20 mg/kg.20 The 10 mg/kg dose was selected for study in phase Ib expansion cohorts in a range of tumor types. Herein, we describe results in a dose-expansion cohort of patients with metastatic urothelial carcinoma.
METHODS
Study Design and Participants
JAVELIN Solid Tumor is an ongoing, phase I, open-label, multiple ascending–dose trial designed to investigate the safety, tolerability, pharmacokinetics, and biologic and clinical activity of avelumab in patients with metastatic or locally advanced solid tumors, with expansion in selected tumor types. In this phase Ib dose-expansion cohort, eligible patients had metastatic urothelial carcinoma of the renal pelvis, ureter, urinary bladder, or urethra, as confirmed by histology or cytology. Eligible patients were required to have relapsed, refractory, or progressive disease, measurable by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,25 following at least one previous line of treatment. The enrollment of cisplatin-ineligible patients (as a result of impaired renal function, hearing loss of 25 dB over two contiguous frequencies, or grade ≥ 2 peripheral neuropathy) was permitted after protocol amendment on November 19, 2014.
Other eligibility criteria included age ≥ 18 years; life expectancy ≥ 3 months; Eastern Cooperative Oncology Group performance status of 0-1; and adequate hepatic, renal (creatinine clearance > 30 mL/min per the Cockcroft-Gault formula or measured 24-hour creatinine clearance), and hematologic functions. Patients were not preselected on the basis of biomarkers, including PD-L1 expression on tumor cells or tumor-associated immune cells. Concurrent anticancer treatment, immunosuppressive or hormonal agents, or prior therapy with any antibody/drug targeting T-cell coregulatory proteins were not permitted. Patients with adrenal insufficiency could continue corticosteroids or topical steroids; patients were tapered off all other steroids and immunosuppressive agents before receiving study treatment (Data Supplement provides full inclusion and exclusion criteria).
The study protocol (Clinical Trials.gov identifier: NCT01772004) was approved by the institutional review board or independent ethics committee at each center, and patients were enrolled per international standards of good clinical practice and institutional safety monitoring. Patients or their representatives provided written informed consent before study entry, and all investigators signed Good Clinical Practice compliance forms.
Procedures
Patients received avelumab (EMD Serono, Rockland, MA) 10 mg/kg by 1-hour intravenous infusion once every 2 weeks until the criterion for withdrawal occurred. Adverse events (AEs) were coded per Medical Dictionary for Regulatory Activities terminology. The severity of an AE was classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Immune-related AEs and infusion-related reactions were of special interest. Potential immune-related AEs were identified using a prespecified list of Medical Dictionary for Regulatory Activities search terms followed by medical review. An infusion-related reaction was classified by investigators as an AE in patients who had signs and symptoms of a potential infusion-related reaction (eg, fever, chills, or rigors) on the day of treatment or the next day. All patients were premedicated with an antihistamine and acetaminophen (Data Supplement). Dose reductions were not permitted; however, interruptions that resulted in < 90% of the planned dose were considered dose reductions. Dose delays were specified following the first occurrence of a grade 2 treatment-related adverse event (TRAE) that did not resolve to grade ≤ 1 by the last day in a treatment cycle.
Avelumab treatment was permanently discontinued following any grade 3 or 4 TRAE except for single laboratory values out of normal range that were considered unlikely to be as a result of trial treatment, did not have any clinical correlate, and resolved to grade ≤ 1 within 7 days with medical management. Other exceptions were transient (≤ 6 hours) grade 3 flu-like symptoms or fever controlled with medical management; grade 3 fatigue, local reactions, headache, nausea, or emesis lasting ≤ 24 hours; grade ≥ 3 amylase or lipase abnormality not associated with symptoms or clinical manifestations of pancreatitis; tumor flare phenomena (local pain, irritation, or rash at site of tumor); and increase in Eastern Cooperative Oncology Group performance status to ≥ 3.
Clinical activity was assessed by cross-sectional imaging (computed tomography or magnetic resonance imaging) performed at baseline and every 6 weeks and evaluated using RECIST version 1.1 to determine best overall response and duration of response from the start of treatment until documented disease progression. Images were reviewed locally by the investigator and centrally by a blinded independent review committee. Stable disease at the first postbaseline tumor assessment after 6 weeks was required to qualify for a best response of stable disease.
PD-L1 expression in archived or fresh tumor biopsies was assessed independently and blinded to any clinical data by immunohistochemistry using a proprietary assay (Dako, Carpinteria, CA) on the basis of an anti–PD-L1 rabbit monoclonal antibody clone (73-10), under license to Merck KGaA. Tumors were categorized on the basis of quantity and intensity of PD-L1 staining using percentage thresholds of ≥ 1% (any intensity), ≥ 5% (any intensity), or ≥ 25% (≥ 2+ staining intensity) in tumor cell membranes, and ≥ 10% in hotspots of tumor-associated immune cells (any intensity). Tumor-associated immune cells were identified as nonmalignant cells on the basis of conventional morphologic features detected in the PD-L1–stained section.
Outcomes
The primary objective was to assess the safety and tolerability of avelumab, and the primary end point was occurrence of dose-limiting toxicities during the first 3 weeks of treatment in the initial dose-escalation part of the trial; these data are reported elsewhere.20 Key secondary end points included best overall response according to RECIST by investigator assessment and independent review committee, duration of response, progression-free survival (PFS), OS, and evaluation of PD-L1 expression. Other secondary end points (pharmacokinetic and pharmacodynamic profile and immunogenicity of avelumab) will be analyzed across multiple cohorts of this phase Ib study and reported elsewhere. Subgroup analyses on the basis of patient and disease characteristics at baseline were conducted post hoc.
Statistical Analyses
The enrollment of approximately 50 patients was planned in this cohort. Safety and clinical activity were analyzed in all patients who received at least one dose of avelumab. The study was designed to estimate the objective response rate (ORR; proportion of patients with a confirmed best response of complete or partial response) using 95% Clopper-Pearson CIs. Time-to-event outcomes, such as duration of response, PFS, and OS, were estimated using Kaplan-Meier methodology, and corresponding CIs were calculated using Brookmeyer-Crowley methodology. Because of favorable preliminary clinical activity, enrollment was stopped after 44 patients to open a larger urothelial cohort with an additional primary end point of best overall response according to independent central review per RECIST within the same multicohort study.
Role of the Funding Source
The sponsor, Merck KGaA (Darmstadt, Germany), provided the study drug and helped investigators design the trial, collect and analyze data, and interpret results. All authors participated fully in the development of the manuscript for publication. Funding for a professional medical writer with access to the data was provided by the sponsor and Pfizer (New York, NY), for initial drafts of the manuscript.
RESULTS
Between September 3, 2014, and December 9, 2014, 66 patients were screened. Of these, 44 patients with metastatic urothelial carcinoma that had progressed following platinum-based chemotherapy were enrolled and treated at 24 sites in the United States and Europe (Data Supplement). No cisplatin-ineligible patients were enrolled. At the time of data cutoff on March 19, 2016, patients had been followed for a median of 16.5 months (interquartile range [IQR], 15.8-16.7 months) and a minimum of 15.0 months; six of 44 patients (13.6%) remained on treatment. Disease progression was the most common reason for discontinuation (31 patients [70.5%]; Data Supplement).
The median age of patients was 68.0 years (IQR, 63.0-73.0 years); 30 of 44 patients (68.2%) were male (Table 1). The subsite of the primary tumor was in the lower tract (bladder or urethra) in 37 patients (84.1%) and the upper urinary tract (renal pelvis or ureter) in seven patients (15.9%). Thirty-three patients (75.0%) had visceral metastases (defined as lung, liver, bone, or other nonlymph node sites).
Table 1.
Select Baseline Characteristics
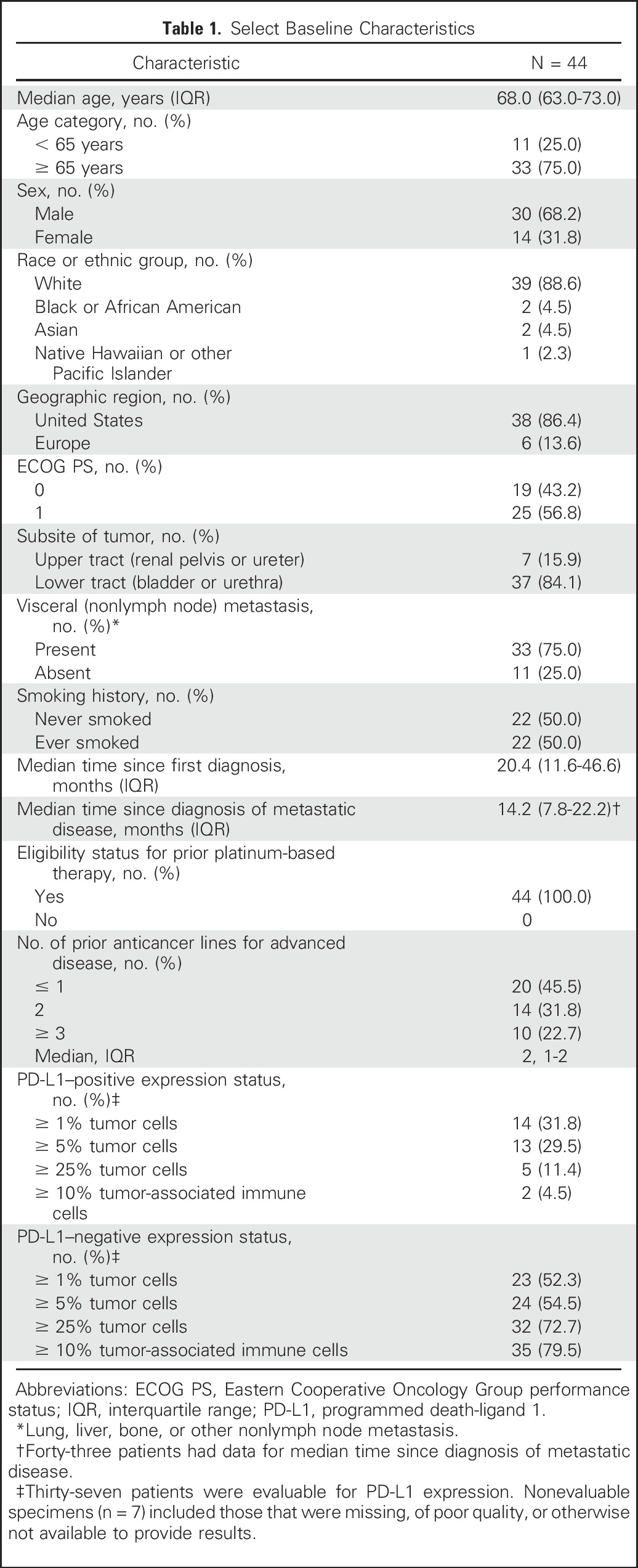
All patients were eligible for platinum-based chemotherapy and had been treated with at least one prior line of systemic therapy (Data Supplement). Twenty-four patients (54.5%) had received at least two prior anticancer regimens for advanced disease (Table 1).
Specimens from 37 patients (84.1%) were evaluable for PD-L1 expression (Table 1 and Data Supplement). Across all expression thresholds, most tumors were PD-L1– on the basis of assessment of tumor cells or tumor-associated immune cells. Using a ≥ 5% staining threshold in tumor cells, 13 patients (29.5%) had PD-L1–positive tumors.
Patients received a median of seven doses of avelumab (IQR, 3-17 doses) for a median duration of 14.1 weeks (IQR, 6.0-35.1 weeks). Dosing was delayed in 17 of 44 patients (38.6%), for 3-6 days in four patients (9.1%), and for ≥ 7 days in 13 patients (29.5%). The planned dose was reduced once in one patient (2.3%).
All patients had an AE (Data Supplement); 29 of 44 (65.9%) had a TRAE (Table 2 and Data Supplement). The most common TRAEs of any grade were fatigue (n = 9 [20.5%]), infusion-related reaction (n = 9 [20.5%]), asthenia (n = 5 [11.4%]), and nausea (n = 5 [11.4%]). Symptoms of infusion-related reactions included chills/rigors, fever, hypertension, headache, flushing, dizziness, sweating, and leg cramps within 24 hours of infusion. Treatment modifications following infusion-related reactions were as follows: grade 1, infusion rate decreased by 50% with close monitoring for worsening; and grade 2, infusion stopped and resumed at 50% of the previous rate after resolution or decrease to grade 1, with close monitoring for worsening.
Table 2.
Treatment-Related Adverse Events Occurring At Any Grade in > 5% of Patients, or Grade ≥ 3 in Any Patient, or Classified As Immune Related in Any Patient
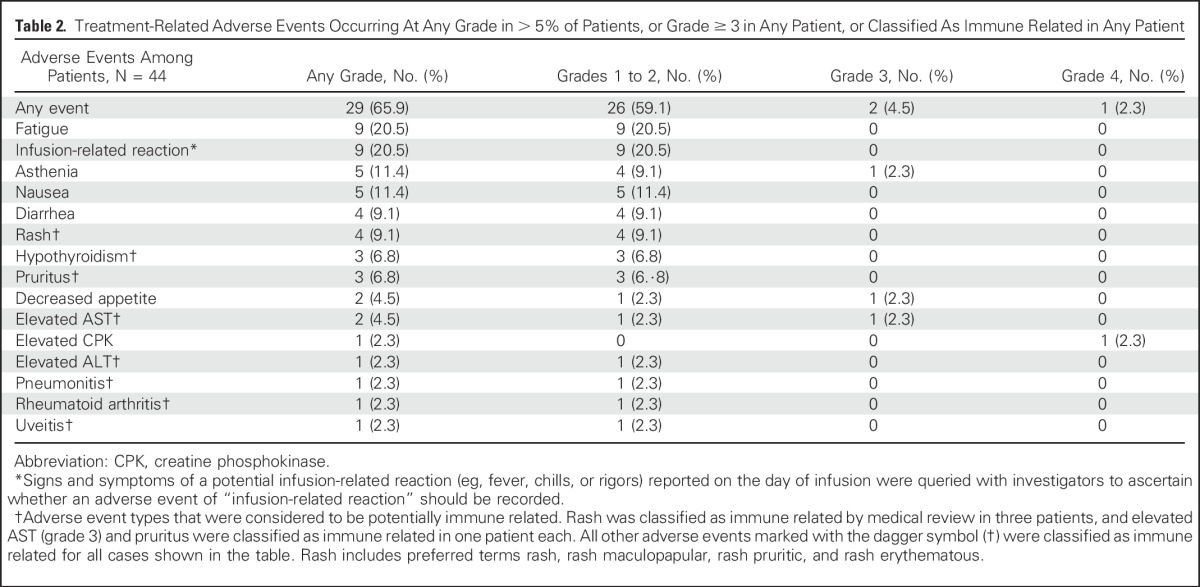
Four grade 3 to 4 TRAEs occurred in three patients (6.8%): grade 4 elevated blood creatine phosphokinase and grade 3 AST elevation (both events occurred in the same patient, who also experienced grade 2 hypothyroidism), grade 3 asthenia, and grade 3 decreased appetite. Nineteen of 44 patients (43.2%) had a serious AE (Data Supplement), considered a TRAE in two patients: elevated creatine phosphokinase and AST (same patient) and asthenia. Avelumab was permanently discontinued in four patients (9.1%) following a TRAE: grade 3 elevated AST, grade 2 infusion-related reaction, grade 2 uveitis, and grade 2 arthralgia, respectively. No treatment-related renal toxicities were reported. Nine patients (20.5%) had a TRAE of any grade that was potentially immune related, most commonly hypothyroidism (n = 3 [6.8%]; Table 2). There were no treatment-related deaths. Seven deaths (15.9%) occurred that were not treatment-related, with six (13.6%) as a result of disease progression and one (2.3%) attributed to respiratory failure as a result of disease progression.
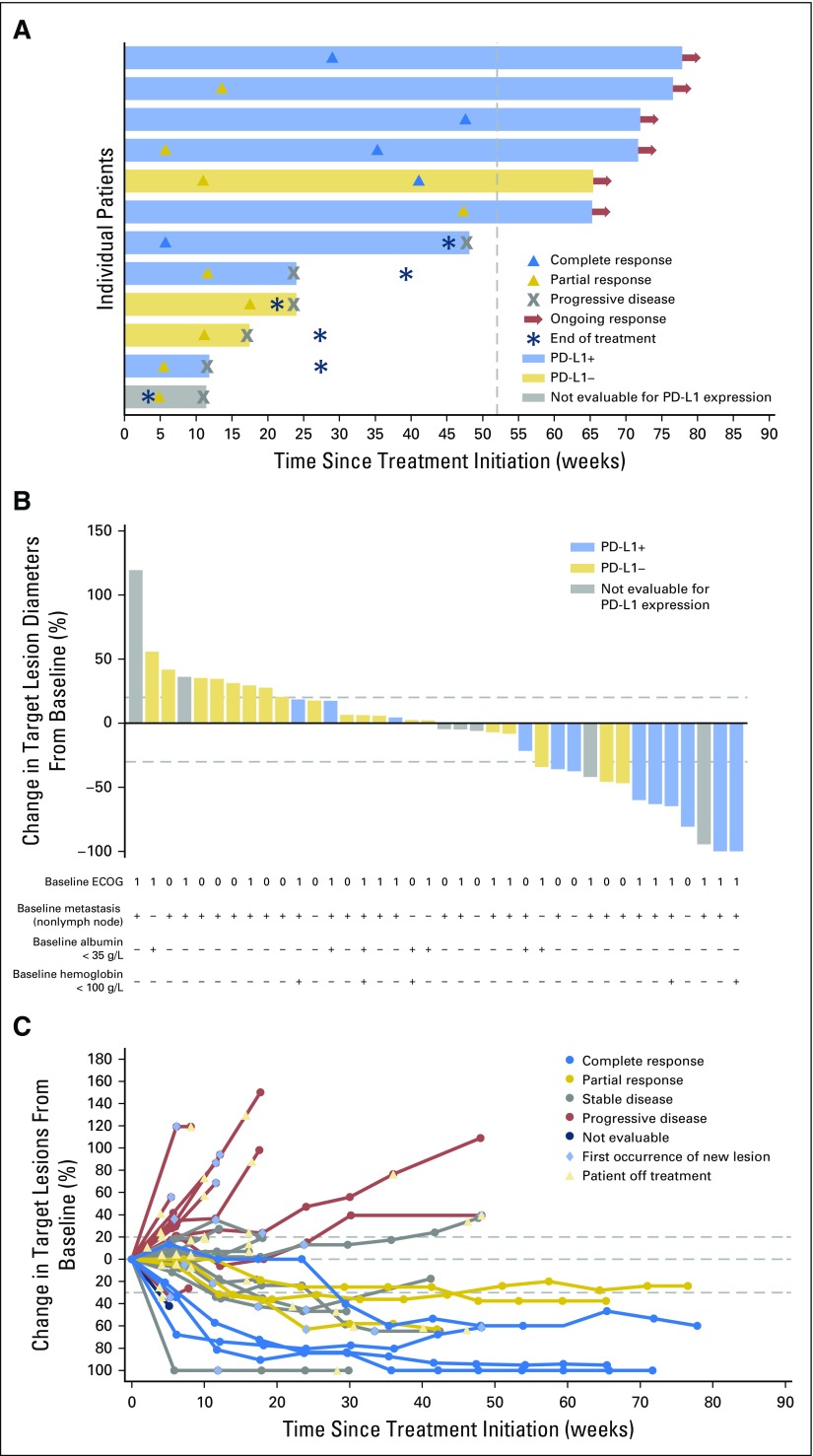
On the basis of independent central review assessment, treatment with avelumab resulted in a confirmed ORR of 18.2% (95% CI, 8.2 to 32.7) and a disease control rate of 52.3%, including five patients (11.4%) with a complete response, three patients (6.8%) with a partial response, and 15 patients (34.1%) with stable disease as best response (Table 3 and Fig 1A). Five of the eight responding patients (62.5%) had visceral (nonlymph node) metastases (Fig 1B). The unconfirmed ORR was 27.3% (95% CI, 15.0 to 42.8), including five complete responses and seven partial responses. Reasons for lack of response confirmation were development of new lesion (n = 3) and no subsequent on-study tumor assessment (n = 1).
Table 3.
Clinical Activity of Avelumab
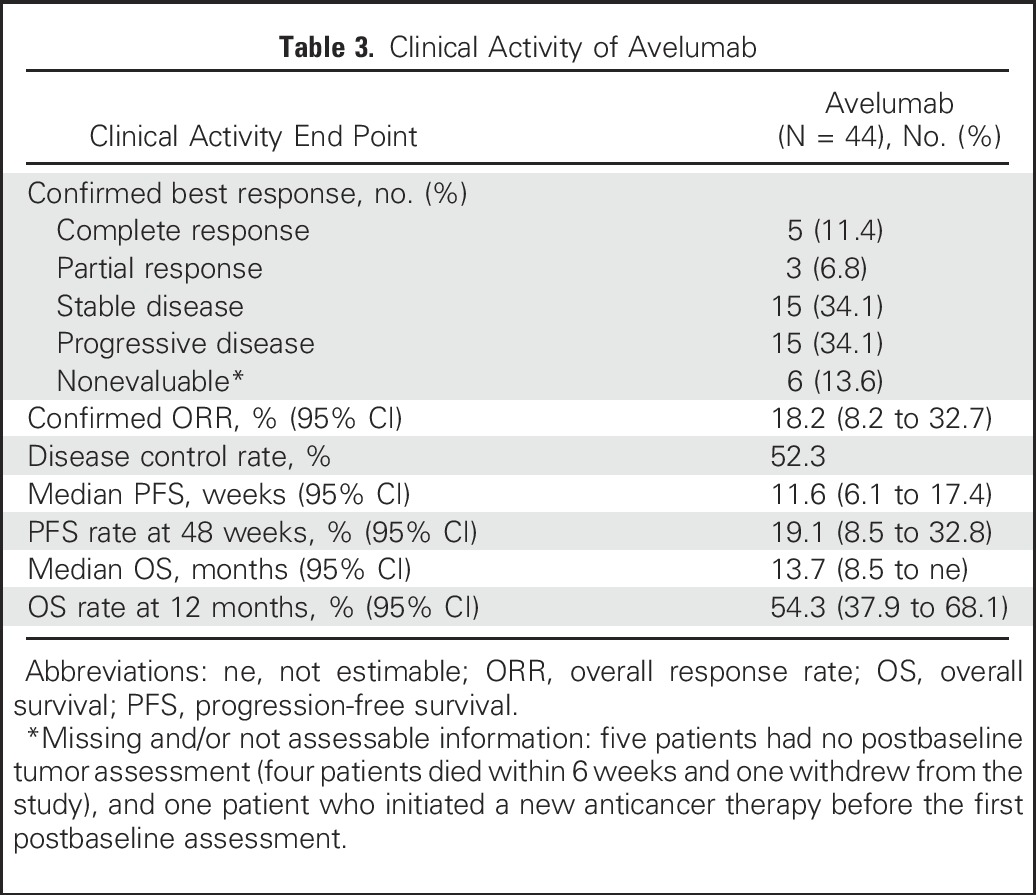
Fig 1.
Clinical activity of avelumab. (A) Time to response, duration of treatment, and duration of response to avelumab (eight confirmed responses and four unconfirmed responses as of data cutoff), with programmed death-ligand 1 (PD-L1) expression status indicated (on the basis of a ≥ 5% staining threshold on tumor cells; nonevaluable specimens [n = 7] included those that were missing, of poor quality, or otherwise not available to provide results). The vertical dotted line represents 1 year from the initiation of treatment. (B) Plot of tumor regression from baseline as measured by Response Evaluation Criteria in Solid Tumors (RECIST) in all assessable patients (n = 38), with PD-L1 expression status indicated (on the basis of a ≥ 5% staining threshold on tumor cells). Eastern Cooperative Oncology Group (ECOG) performance status, presence of nonlymph node metastasis, and albumin and hemoglobin levels at baseline are shown for each patient. The upper dotted line represents progression at 20% and the lower dotted line represents the RECIST boundary for complete response or partial response at 30%. (C) Percentage change in sum of target lesion diameters from baseline over time for all assessable patients (n = 38), defined as those patients with baseline tumor assessments and at least one postbaseline assessment. The upper dotted line represents progression at 20% and the lower dotted line represents the RECIST boundary for complete response or partial response at 30%.
In patients with a confirmed response, the median time to response on the basis of independent review was 13.0 weeks (IQR, 8.8-38.6 weeks), and the median duration of response was not reached (95% CI, 12.1 weeks to not estimable). Responses were ongoing at the time of analysis in six of eight patients (75.0%; durations of ≥ 17.7 to ≥ 65.7 weeks), including four of five patients with a complete response, and were maintained for ≥ 48 weeks in 70.0% of patients (95% CI, 22.5% to 91.8%) by Kaplan-Meier estimates. Thirteen of 44 patients (29.5%) had a reduction in target lesions by ≥ 30% from baseline; six patients were nonevaluable (Figs 1B and 1C).
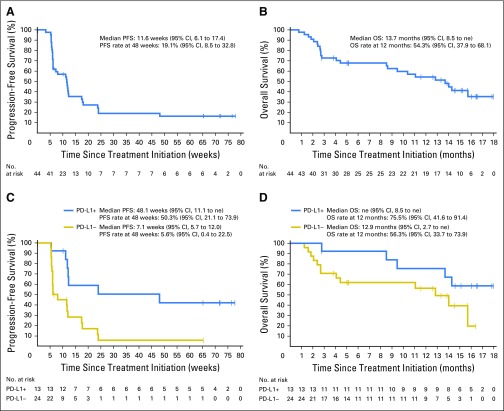
The median PFS was 11.6 weeks (95% CI, 6.1 to 17.4 weeks); the proportion of patients who were free of disease progression at 48 weeks was 19.1% (95% CI, 8.5% to 32.8%; Table 3 and Fig 2A). The median OS was 13.7 months (95% CI, 8.5 months to not estimable), and the 12-month OS rate was 54.3% (95% CI, 37.9% to 68.1%; Table 3 and Fig 2B). Sixteen patients (36.4%) received anticancer therapy after discontinuing avelumab; of these, 13 (29.5%) had drug therapy, including cytotoxic chemotherapy (n = 9; 20.5%), kinase inhibitor (n = 5; 11.4%), and investigational antibody (n = 2; 4.5%).
Fig 2.
Progression-free survival (PFS) and overall survival (OS). Kaplan-Meier estimate of PFS (A) and OS (B) for the overall study population (N = 44). Kaplan-Meier estimate of PFS (C) and OS (D) on the basis of programmed death-ligand 1 (PD-L1) expression on tumor cells (n = 37). Vertical lines show censored events. Positive PD-L1 expression was defined as ≥ 5% of tumor cells expressing PD-L1 at any intensity. ne, not estimable.
Among 37 patients evaluable for PD-L1 expression, responses occurred in patients with PD-L1–positive and PD-L1–negative tumors at all prespecified PD-L1 expression–level thresholds, although trends toward higher response rates and longer PFS and OS were seen in patients with PD-L1–positive tumors (Figs 1A, 2C and 2D, and Data Supplement). On the basis of a ≥ 5% threshold for staining on tumor cells, seven of eight responding patients (87.5%) had PD-L1–positive tumors. The confirmed ORR by independent review was 53.8% in PD-L1–positive tumors (seven of 13) and 4.2% in PD-L1–negative tumors (one of 24).
Responses occurred in patients who had factors associated with poor prognosis. Confirmed ORRs by independent central review were 15.2% (five of 33) and 27.3% (three of 11) in patients with or without visceral (nonlymph node) metastases at baseline, respectively, and 30.0% (three of 10) and 10.0% (two of 20) in patients with ≥ 3 or ≤ 1 prior anticancer lines for advanced disease, respectively. There were no confirmed responses in patients with low albumin or hemoglobin levels (0.0% [zero of eight] and 22.2% [eight of 36] in patients with baseline albumin levels < 35 g/L or ≥ 35 g/L, respectively, and 0.0% [zero of seven] and 21.6% [eight of 37] in patients with baseline hemoglobin levels < 100 g/L or ≥ 100 g/L, respectively). The median PFS in patients with or without visceral (nonlymph node) metastases (11.4 v 11.6 months) was similar to that in the overall cohort.
DISCUSSION
In this study of 44 patients with refractory metastatic urothelial carcinoma who were followed for a median of 16.5 months, avelumab was well tolerated and showed promising clinical activity, which was characterized by durable responses, disease stabilization, and prolonged OS. The confirmed ORR of 18.2% is notably higher than a historical control rate of 10% for chemotherapy8 and included a complete response in 11.4% of patients. Importantly, responses were ongoing at the time of analysis (minimum 15 months of follow-up) in 75.0% of responding patients, including four of the five patients with a complete response. Responses were also notable in patients with visceral (nonlymph node) metastases (15.2%) and heavily pretreated patients (30.0% in those patients who had received at least three prior anticancer lines in the advanced setting), indicating that avelumab may have clinical benefit in subpopulations with a poor prognosis. However, no confirmed response to avelumab occurred in patients with low albumin or hemoglobin levels, each of which is a known poor prognostic feature in metastatic urothelial carcinoma. The median OS was 13.7 months. The 12-month OS rate was 54.3%, which is highly encouraging compared with vinflunine8 and other chemotherapy regimens26 in the second-line setting, and is consistent with other anti–PD-L1/PD-1 antibodies.16,27-29 A potential trend toward greater clinical activity of avelumab was seen in patients with PD-L1–positive tumors, although patients with PD-L1–negative tumors also had responses. These analyses are exploratory, and the potential use of PD-L1 as a predictive biomarker for avelumab is being further analyzed.
Key safety findings included a low rate of grade ≥ 3 TRAEs (6.8%), a low incidence of immune-related AEs (20.5%, most of which were grade 1 or 2), and no treatment-related renal toxicities or treatment-related deaths. In studies of other anti–PD-L1/PD-1 antibodies in advanced urothelial carcinoma, rates of grade 3 to 4 TRAEs ranged from 4.9% to 21.8%.16,27-29 The most common TRAEs with avelumab were fatigue, infusion-related reaction, asthenia, and nausea, which were mostly grades 1 or 2. One patient discontinued treatment as the result of a grade 2 infusion-related reaction. The mechanism leading to infusion-related reaction during avelumab treatment is currently unknown; detailed analysis across various tumor types is ongoing.
In conclusion, findings from this study suggest that avelumab could become a potential treatment option for patients with advanced urothelial carcinoma. On the basis of the promising clinical activity seen in this cohort of the JAVELIN Solid Tumor trial, an additional expansion cohort of approximately 200 patients with urothelial carcinoma has been enrolled to further characterize the efficacy and safety of avelumab in this patient population. In addition, a randomized phase III trial of avelumab plus best supportive care versus best supportive care alone as maintenance therapy in patients with metastatic urothelial carcinoma not progressing after first-line platinum-based therapy is underway (NCT02603432).
ACKNOWLEDGMENT
We thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers and at Merck KGaA (Darmstadt, Germany) and EMD Serono (Billerica, MA; a US subsidiary of Merck KGaA). We also thank Dr Debra Hanks (Agilent Technologies, Carpinteria, CA) for expert pathology support. This trial was sponsored by Merck KGaA and is part of an alliance between Merck KGaA and Pfizer (New York, NY). Medical writing support was provided by ClinicalThinking (Hamilton, NJ) and funded by Merck KGaA and Pfizer.
Footnotes
Supported by Merck (Darmstadt, Germany) and Pfizer (New York, NY).
Interim analyses were presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium, San Francisco, CA, January 7-9, 2016 (abstract no. 367) and at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016 (abstract no. 4514).
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT01772004.
See accompanying Oncology Grand Rounds on page 2109
AUTHOR CONTRIBUTIONS
Conception and design: Andrea B. Apolo, Ding Wang, Howard Safran, Michael Schlichting, Kevin Chin, James L. Gulley
Administrative support: Andrea B. Apolo, Kevin Chin, James L. Gulley
Provision of study materials or patients: Andrea B. Apolo, Jeffrey R. Infante, Ani Balmanoukian, Manish R. Patel, Ding Wang, Karen Kelly, Anthony E. Mega, Carolyn D. Britten, Alain Ravaud, Alain C. Mita, Howard Safran, Thomas E. Stinchcombe, James L. Gulley
Collection and assembly of data: Andrea B. Apolo, Jeffrey R. Infante, Ani Balmanoukian, Manish R. Patel, Ding Wang, Karen Kelly, Anthony E. Mega, Carolyn D. Britten, Alain Ravaud, Alain C. Mita, Howard Safran, Thomas E. Stinchcombe, Marko Srdanov, Arnold B. Gelb, Michael Schlichting, Kevin Chin, James L. Gulley
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Avelumab, an Anti–Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Andrea B. Apolo
No relationship to disclose
Jeffrey R. Infante
Consulting or Advisory Role: EMD Serono
Research Funding: EMD Serono, Celldex (Inst), ARMO BioSciences (Inst), BioMed Valley Discoveries (Inst), Novartis (Inst), Janssen Oncology (Inst), GlaxoSmithKline (Inst), Immunocore (Inst), Calithera Biosciences (Inst), Phosplatin Therapeutics (Inst), Genentech (Inst), Roche (Inst), Aileron Therapeutics (Inst), AstraZeneca (Inst), eFFECTOR Therapeutics (Inst), MedImmune (Inst), Pfizer (Inst), Bristol-Myers Squibb (Inst), TESARO (Inst), Merck (Inst)
Ani Balmanoukian
Speakers’ Bureau: Merck, Bristol-Myers Squibb, Genentech
Manish R. Patel
Honoraria: Medivation/Astellas, Genentech, Bristol-Myers Squibb, Exelixis, Gilead Sciences, Guardant Health
Speakers’ Bureau: Medivation/Astellas, Bristol-Myers Squibb, Genentech, Gilead Sciences, Exelixis
Ding Wang
No relationship to disclose
Karen Kelly
Honoraria: Roche, Bristol-Myers Squibb
Consulting or Advisory Role: Clovis Oncology, Transgene, Celgene, Synta, AstraZeneca, Eli Lilly, Clovis Oncology, AstraZeneca, ARIAD, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Eli Lilly
Research Funding: Millennium (Inst), Novartis (Inst), Synta (Inst), EMD Serono (Inst), Eli Lilly (Inst), Genentech (Inst), AbbVie (Inst), Gilead Sciences (Inst), Celgene (Inst), Five Prime Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Author royalties for UpToDate, an evidence-based, peer-reviewed information resource, available via the Web, desktop, and PDA.
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol-Myers Squibb, Celgene, ARIAD, Genentech
Anthony E. Mega
Honoraria: Bayer, Sanofi-Aventis, Medivation/Astellas, Bristol-Myers Squibb
Consulting or Advisory Role: Bayer
Speakers’ Bureau: Bayer, Sanofi-Aventis, Medivation/Astellas, Bristol-Myers Squibb
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Sanofi-Aventis, Bayer, Medivation/Astellas, Bristol-Myers Squibb
Carolyn D. Britten
Consulting or Advisory Role: Cleave Biosciences
Research Funding: AstraZeneca (Inst), Bayer (Inst), Boston Biomedical (Inst), Eli Lilly (Inst), EMD Serono (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Plexxikon (Inst), Roche (Inst), Array BioPharma (Inst), Five Prime Therapeutics (Inst)
Travel, Accommodations, Expenses: Five Prime Therapeutics
Alain Ravaud
Consulting or Advisory Role: Pfizer, Roche, Novartis, Bristol-Myers Squibb
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Bristol-Myers Squibb
Alain C. Mita
Speakers’ Bureau: Genentech
Howard Safran
No relationship to disclose
Thomas E. Stinchcombe
Consulting or Advisory Role: AbbVie, ARIAD, Boehringer Ingelheim
Research Funding: EMD Serono (Inst), Genentech (Inst), Bristol Meyers (Inst)
Marko Srdanov
No relationship to disclose
Arnold B. Gelb
Employment: EMD Serono
Michael Schlichting
Employment: Merck KGaA
Travel, Accommodations, Expenses: Merck KGaA
Kevin Chin
Employment: Merck KGaA
James L. Gulley
Speakers’ Bureau: CancerNet
Research Funding: EMD Serono (Inst), Bavarian Nordic (Inst), Celgene (Inst), Medivation/Astellas (Inst)
REFERENCES
- 1.Ervik M, Lam F, Ferlay J, et al: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2016. http://gco.iarc.fr/today
- 2.Kaufman D, Raghavan D, Carducci M, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000;18:1921–1927. doi: 10.1200/JCO.2000.18.9.1921. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 4.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. 2013;105:499–503. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002;20:937–940. doi: 10.1200/JCO.2002.20.4.937. [DOI] [PubMed] [Google Scholar]
- 6.Galsky MD, Mironov S, Iasonos A, et al. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25:265–270. doi: 10.1007/s10637-006-9020-9. [DOI] [PubMed] [Google Scholar]
- 7.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15:1853–1857. doi: 10.1200/JCO.1997.15.5.1853. [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. TheraCys (BCG live), intravesical [package insert]. Toronto, Ontario, Canada: Sanofi Pasteur Limited; 2013.
- 11.Fuge O, Vasdev N, Allchorne P, et al. Immunotherapy for bladder cancer. Res Rep Urol. 2015;7:65–79. doi: 10.2147/RRU.S63447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. TECENTRIQ: (atezolizumab) injection [package insert]. South San Francisco, CA: Genentech, Inc; 2016.
- 18. McDaniel AS, Alva A, Zhan T, et al: Expression of PDL1 (B7-H1) before and after neoadjuvant chemotherapy in urothelial carcinoma. Eur Urol Focus 1:265-268, 2016. [DOI] [PubMed]
- 19.Jiang L, Zhao Z, Jiang S, et al. Immunological markers predict the prognosis of patients with squamous non-small cell lung cancer. Immunol Res. 2015;62:316–324. doi: 10.1007/s12026-015-8662-0. [DOI] [PubMed] [Google Scholar]
- 20. Heery CR, O’Sullivan Coyne GH, Madan RA, et al: Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. J Clin Oncol 32:5s, 2014 (suppl; abstr 3064) [Google Scholar]
- 21.Grenga I, Donahue RN, Lepone LM, et al. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunology. 2016;5:e83. doi: 10.1038/cti.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandeveer AJ, Fallon JK, Tighe R, et al. Systemic immunotherapy of non-muscle invasive mouse bladder cancer with avelumab, an anti-PD-L1 immune checkpoint inhibitor. Cancer Immunol Res. 2016;4:452–462. doi: 10.1158/2326-6066.CIR-15-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepone LM, Donahue RN, Farsaci B, et al. Evaluation of immune cell subsets of cancer patients treated with a fully human IgG1 anti-PD-L1 MAb (MSB0010718C) capable of mediating ADCC of human tumor cells. Cancer Res. 2015;75(15) Suppl abstr 1316. [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal N, Bellmunt J, Maughan BL, et al. Six-month progression-free survival as the primary endpoint to evaluate the activity of new agents as second-line therapy for advanced urothelial carcinoma. Clin Genitourin Cancer. 2014;12:130–137. doi: 10.1016/j.clgc.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18:212–220. doi: 10.1016/S1470-2045(17)30007-4. [DOI] [PubMed] [Google Scholar]