Abstract
Background
Major depressive disorder (MDD) is a recurrent mental illness worldwide. The glutamatergic neurotransmission system is now a target for antidepressant therapy because it takes part in synaptic plasticity and cognition in physical condition and has a potential excitatory neurotoxicity in pathological conditions. Glial glutamate transporter EAAT2 performs 90% of Glu neurotransmission. Therefore, the aim of the study was to evaluate the effect of acupuncture on depressive behaviors and EAAT2 in CUMS.
Material/Methods
We randomly divided 56 male SD rats into a normal group, a model group, an acupuncture group, and a riluzole group. Rats in the model group, acupuncture group, and riluzole group underwent chronic unpredictable mild stress (CUMS) exposure for 21 days. The acupuncture group received electro-acupuncture stimulation on LI4 and LR3 for 5 continuous days per week for 4 weeks, and rats in the riluzole group received 4 mg/kg of riluzole orally (Sanofi, J20140092) for 4 weeks after undergoing CUMS stimulation.
Results
Rats showed significantly increased sucrose consumption in the sucrose preference test paradigm, and showed elevated food intake and shortened latency in the novelty-suppressed feeding test paradigm after undergoing acupuncture therapy and riluzole treatment. The amelioration of depressive behavioral actions was consistent with increasing number of positive cells, protein, and mRNA expression of glial glutamate transporter EAAT2 in the hippocampus and PFC.
Conclusions
The results suggest that acupuncture and riluzole are both effective in improving sucrose consumption, latency, and food intake in CUMS rats. However, acupuncture appears to achieve an antidepressant effect later than riluzole does because it might need accumulated stimulation by enhancing EAAT2 expression. Enhance glial glutamate transporter EAAT2 in the hippocampus and PFC is a mechanism underlying the antidepressant effect of acupuncture.
MeSH Keywords: Acupuncture, Depressive Disorder, Excitatory Amino Acid Transporter 2
Background
Major depressive disorder (MDD) is a recurrent mental illness worldwide, which imposes a heavy medical and economic burden on society [1]. According to the WHO, MDD will become the second leading cause of disability by 2020 due to suicidal ideation [2]. Despite years of efforts to develop more effective treatment, there are few available pharmaceutical options and therapies for depressed patients. The great need for effective and rapid-acting antidepressant therapies is underscored by the moderate effect, significant time-lag, and partial responsiveness of currently used drug [3].
Although it is important to develop effectual therapies and to better understand the pathogenesis, the heterogeneity of MDD makes this difficult. The monoamine neurotransmission system was targeted in antidepressant drug development for decades. However, release of core depressive symptoms begins several days or weeks after elevating synaptic monoamine levels by use of monoamine antidepressants [4–6]. This limitation means that our understanding of the role of the monoamine system in the pathogenesis of MDD remains incomplete.
To address this question, increasing research attention has focussed on the glutamatergic neurotransmission system. Glutamate (Glu) is the most common excitatory neurotransmitter in the central nervous system and it takes part in synaptic plasticity and cognition in physical condition as well as having potential excitatory neurotoxicity in pathological conditions [7]. Glu transmission in the mammalian brain is mainly transported by glial cells to maintain homeostasis through tripartite glutamatergic synapses [8,9]. Glu transmission between synapses is handled by excitatory amino acid glutamate transporters (EAATs) located in neurons and glial cells. As glial cells are the most numerous cell types in CNS, glutamate is transported mainly by EAATs in astrocytes in the mammalian brain. The dysfunction of EAATs induced by stress and depression can directly affect Glu transmission, and the excess Glu accumulation in the synaptic cleft results in neuronal atrophy and synapse loss in cerebral regions involved in emotion [10,11]. Hence, enhancing glutamatergic transmission to achieve neuron protection or synapse plasticity gives us a new insight into novel antidepressant development. And EAATs then also become an important target because it binds and transports Glu into astrocytes to convert to glutamine. Riluzole, the only drug used for treatment of ALS, exerts its neuroprotective effect by reducing the excitotoxic effect of excessive accumulation of extracellular Glu. The beneficial effects of riluzole on anhedonia induced by CUMS and resulting from Glu modulation show the antidepressant effect of riluzole in rats.
Acupuncture, a therapy widely used in China for thousands of years, is still used in psychological clinical practice. According to our previous clinical and animal studies, acupuncture had shown alleviation effect on depressive behaviors [12–14]. The underlying mechanism may implicate astrocytes protection effect of acupuncture that astrocytes account for over 90% of the uptake of the glutamate in brain. It also is involved in increasing soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins, which enhance the glutamatergic transmission in astrocytes for remission of depression [15]. Moreover, given the ubiquitous nature of Glu and glial cells in the brain of depressed subjects, the mechanism of the antidepressant effect acupuncture is quite complex. To further identify how acupuncture affects glutamate transmission to alleviate depressive syndromes, we used behavioral parameters, immunohistochemistry, Western blot, and real-time polymerase chain reaction to evaluate the transfer of EAAT2 in Glu on astrocytes in the CNS.
Material and Methods
Animals
We used 56 male SD rats purchased from the Laboratory Animal Center of Guangdong Province, weighting 260–280 g at the beginning of the experiment (permit no. 440072000000348). The rats were housed in a controlled environment (22°C, 12-h light cycle starting at 8: 00 AM), with ad libitum food and water. All experimental protocols were approved by local animal ethics committee and followed the guidelines of animal bioethics. Rats were randomly divided into a normal group, a model group, an acupuncture group, and a riluzole group. Rats in the model group, acupuncture group, and riluzole group underwent chronic unpredictable mild stress (CUMS) exposure for 21 days [16]. A total of 7 different stressors were used, including food and water deprivation, cages tilted and rotated, lights on/off, cold stress, and crowded and isolation housing. Sucrose preference test and novelty-suppressed feeding test were used to assess the model rats after CUMS stimulation.
Acupuncture and administration
Rats in the acupuncture group received electro-acupuncture stimulation on LI4 and LR3 for 5 continuous days per week for 4 weeks, immediately after CUMS by a skilled acupuncture practitioner, as reported in previous studies [15,17]. LI4 is located on the depression between the first and second metacarpal of the forelimbs, and while LR3 is located on the depression of the first and second metacarpal of the hind limbs. We used stainless needles 0.25 mm in diameter and 30 mm in length (Hua Tuo Medical Instruments Co. Ltd., Suzhou, China). The needles were inserted 2 mm into the skin. The electric acupuncture apparatus G-6805II was employed for electro-acupuncture stimulation at a frequency 2 Hz for 30 min 1 time. Rats in the riluzole group received 4 mg/kg of riluzole (Sanofi, J20140092) orally for 4 weeks. The administration of the drug was performed orally by gastric gavage with rat biomedical needles.
Behavior parameters
Sucrose preference test
The sucrose preference test (SPT) was used to evaluate anhedonia behavior caused by CUMS, as previously described [18]. Rats were caged individually and had free access to 1% sucrose solution for 48 h followed by 4 h of water deprivation and 1 h of exposure to 2 bottles (sucrose solution and water). The sucrose preference was assessed by weighting the bottles and calculated by the equation: volume of sucrose/total volume of (sucrose + water) ×100%.
Novelty-suppressed feeding test
The novelty-suppressed feeding test (NSFT) was performed, as previously described [19,20] for rat behavior changes after CUMS. Rats were fasted for 12 h before the test. The test was done in a wooden box (80×80×40 cm) with a small amount of food in the center. Each rat was removed from the home cage and placed in a corner of the box. The latency to feed (the time the animal took to approach and take the first bite of the food) was recorded by an observer who was blind to the grouping.
Immunohistochemistry
Rats were euthanized under anesthesia, followed by transcardial perfusion with saline and 4% paraformaldehyde in 0.1 M phosphate-buffer at 4°C. Then, the left hippocampus and prefrontal cortex were quickly removed and post-fixed in 12% formalin solution for 24 h at room temperature. A 3-mm coronal block was cut and embedded in paraffin, then sectioned at 3 μm each. Sections were dried at 65°C after unfolding in polysine and were kept in 37°C. Then the sections prepared for subsequent examination of immunohistochemistry. Paraffin-embedded sections were dewaxed in dimethylbenzene and hydrated in gradient ethanol and water. After been submerged in a mixed solution of hydrogen peroxide and methanol for endogenous peroxidase inactivation, sections were heated in citrate buffer for 5 min by microvan to antigen retrieval followed by washing in 0.01 M PBS 3 times. The blocked slices were then incubated in EAAT2 primary antibodies (Abcam, USA) dissolved in 0.01 MPBS with the dilution 1: 500 overnight at 4°C. The secondary antibody was then used on the second day at room temperature for 15 min and stained by DAB. Negative controls received the same treatment, omitting the primary antibodies, and showed no specific staining. We selected 5 visual regions in the hippocampus and prefrontal cortex for counting immune-positive cells under 10× and 20× microscopes.
Western blot
Rats were sacrificed and right prefrontal cortex and hippocampus tissue samples quickly removed. Protein concentrations were measured by BCA kits (Thermo Scientific) and 15 μg of protein was loaded onto 7.5% polyacrylamide gels for electrophoresis. Proteins were transferred to a nitrocellulose membrane and blocked for 1 h with 2% BSA. Membranes were incubated overnight with anti-EAAT2 (Sigma, USA, 1: 3000) antibody at 4°C. Then, membranes were washed 3 times with TBST and incubated for 1 h with IgG HRP anti-rabbit secondary antibody (Jinqiao, Beijing, 1: 5000) in 5% milk at room temperature, and proteins were detected with enhanced chemiluminescence. Bands were quantified using Bio-Rad Image Lab software and normalized to GAPDH.
Real-time polymerase chain reaction
Total RNA was extracted from the hippocampus and prefrontal cortex by use of an RNA extraction kit (Invitrogen, USA) according to manufacturer’s instructions. The complementary DNA was synthesized by Shanghai Science & Technical Co. (Shanghai, China). The forward and reverse primers of EAAT2 were 5′-GAACTTCGGTCAATGTAGTGGGCG-3′ and 5′-TGGACTGCGTCTTGGTCATTTCG-3′, and 5′-GGAGATTACTGCCCTGGCTCCTA-3′ and 5′-GACTCATCGTACTCCTGCTTGCTG-3′ for actin. All samples were normalized by actin. Each sample was tested twice. The 2−ΔΔCT method was used for measuring relative gene expression.
Statistical analysis
The results are represented as Mean ±SD. SPSS 18.0 software was used for statistical analysis. Repeated measures ANOVA with subsequent Duncan’s test was used to determine the significant difference in multiple comparisons. A P-value of less than 0.05 was considered significant.
Results
Acupuncture ameliorated depressive behaviors induced by CUMS
The sucrose preference test and novelty-suppressed feeding test reliably demonstrated changes in rat depressive behavior after chronic stress stimulation, acupuncture, and administration.
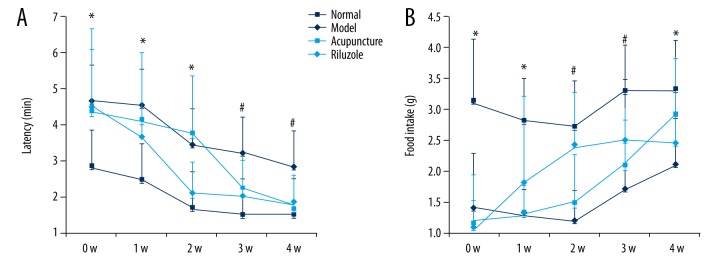
As shown in Figure 1, sucrose consumption of rats in the model group, acupuncture group, and riluzole group was dramatically decreased after undergoing CUMS compared to the normal group (p<0.05) at baseline. There was still no significant amelioration 1 week after acupuncture and drug intervention (p>0.05). Two weeks after intervention, sucrose consumption of rats in the riluzole group was significantly increased compared to the acupuncture group and model group (p<0.05), but was still lower than in the normal group (p<0.05). However, the sucrose consumption of the acupuncture group at the third week was sharply elevated from baseline and the second week (p<0.05), and was at the same level as the riluzole group. At the end of the 4th week, the intervention groups achieved a normal level (p<0.05) and the model group showed a declining trend in SPT (p>0.05).
Figure 1.
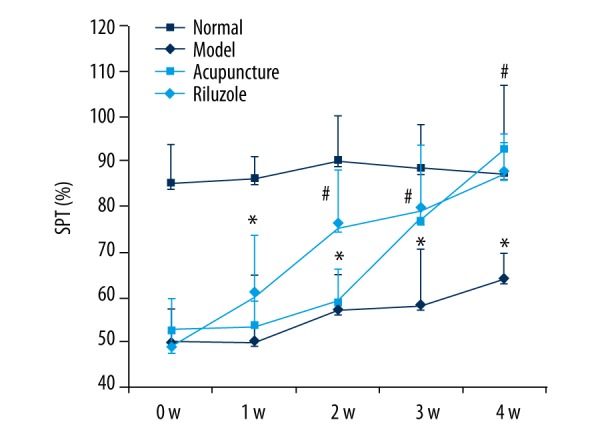
The SPT paradigm: Rats that underwent CUMS showing sharply decreased in sucrose consumption and the change cannot be self-healing. Rats treated by riluzole and acupuncture could rectify the declining of sucrose consumption. Rats in the riluzole group showed significant improvement at the 2nd week, while acupuncture group improved in the 3rd week. Sucrose consumption in the riluzole and acupuncture groups was nearly at the same level at the 3rd week and both reached normal level at the 4th week. * Means significant difference compared to normal group (P<0.05). # Means significant difference compared to model group (P<0.05).
According to the novelty-suppressed feeding test results, we also found remarkable improvement in latency to feeding and food intake, as shown in Figure 2. The CUMS rats had longer latency to feeding and less food intake than the normal control rats. In the riluzole group, the latency to feeding was significantly reduced at the 2nd week (P<0.05) and then maintained a stable level to the 3rd and 4th weeks (Figure 2A), and the same trend was also shown in food intake (Figure 2B). Although latency to feeding gradually improved in the acupuncture group, it sharply decreased in the 3rd week and was maintained to the 4th week. Food intake had an increasing trend from the 2nd week onward (Figure 2A).
Figure 2.
The NSFT paradigm: The longer latency to feeding and lower food intake caused by CUMS was improved by administration and acupuncture. The riluzole group had alleviated SPT deficit after the 2nd week and kept a steady level through the 4th week (P<0.05). In the acupuncture group, a sharp improvement occurred in the 3rd week and then was maintained from the 4th week (P<0.05). * Means significant difference compared to normal group (P<0.05). # Means significant difference compared to model group (P<0.05).
Acupuncture increased EAAT2 expression in the hippocampus and prefrontal cortex
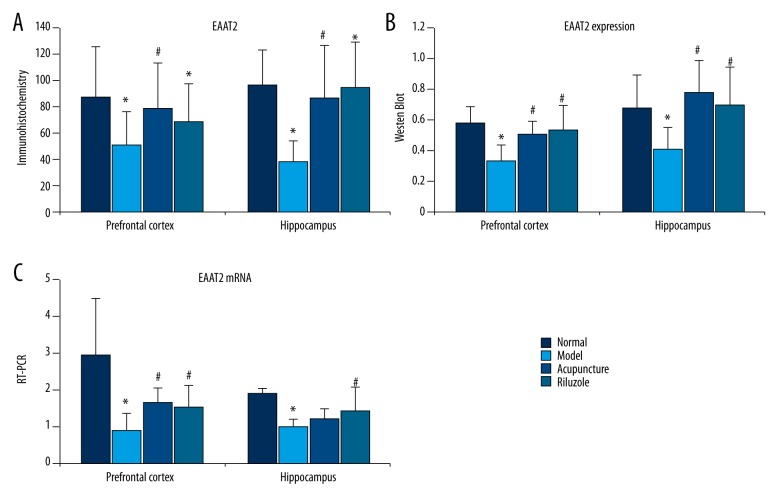
To determine the effects of acupuncture on EAAT2 expression in the hippocampus and prefrontal cortex, immunohistochemistry, Western blot analysis, and RT-PCT were used. As shown in Figure 3, CUMS a had negative influence on EAAT2-positive cell count, protein, and mRNA expression of EAAT2 in the hippocampus and prefrontal cortex. The acupuncture group and the riluzole group had robust increase in EAAT2-positive cell count and protein expression in the HP and PFC groups compared to the model group (P<0.05) in HP and PFC. There was no significant difference in EAAT2-positive cells, protein expression, and mRNA between the acupuncture group and the riluzole group (P<0.05). However, there was no significant change in EAAT2 mRNA in the hippocampus (P>0.05).
Figure 3.
Effects of acupuncture on EAAT2 expression in hippocampus and prefrontal cortex. CUMS had a negative influence on EAAT2-positive cell count, protein, and mRNA expression of EAAT2 in the hippocampus and prefrontal cortex (P<0.05). Acupuncture and riluzole significantly increased EAAT2-positive cells and protein expression in the HP and PFC (P<0.05). EAAT2 mRNA expression in the PFC was also increased, but there was no change in the hippocampus. * Means significant difference compared to normal group (P<0.05). # Means significant difference compared to model group (P<0.05).
Discussion
Our results demonstrated the antidepressant effect of acupuncture on behavioral actions by increased glial glutamate transporter EAAT2 expression in the hippocampus and prefrontal cortex. Rats significantly increased sucrose consumption in the SPT paradigm, and showed elevated food intake and shortened latency in the NSFT paradigm after 3 weeks of acupuncture therapy. The amelioration of depressive behavioral actions was consistent with increased positive cell number, protein, and mRNA expression of glial glutamate transporter EAAT2 in the hippocampus and PFC after interventions using acupuncture and riluzole.
Glutamate (Glu), the major excitatory neurotransmitter in the mammalian brain, is well accepted as a treatment target in psychiatric disorders because it plays a key role in synaptic plasticity and memory. Excessive extracellular Glu is a potent neuronal excitotoxin, triggering neurotoxicity and impairing synaptic and neural plasticity, most commonly induced by abnormal activity of the glutamatergic system [21]. Therefore, correcting abnormal glutamatergic activity by means such as boosting extrasynaptic Glu level and circuitry is crucial to depressive disorder treatment [22]. The neurotransmission of Glu in the CNS is mainly performed by glial transporters. Deficits in glial cells could lead to a mismatch of excitatory and energetic supply, finally developing into neural atrophy [23]. High-affinity excitatory amino acid transporters (EAATs) in glial cells contribute to the most Glu clearance in extracellular clefts [24]. Failure of the EAATs may results in Glu accumulation and cellular damage. EAAT2 is responsible for over 90% of glutamate reuptake among 5 EAATs in the CNS, which predominantly exist in glial membranes [25]. Decreased concentrations of EAAT2 can directly result in extracellular Glu accumulation, according to postmortem studies [26]. Therefore, we chose EAAT2 as an indicator for evaluating the glutamate neurotransmission effect of acupuncture in this study.
Riluzole, the positive control in our study, is a glutamate reuptake enhancer acting by increasing Glu reuptake via EAAT2 and inhibiting Glu release to avoid excitotoxic effects by extracellular Glu concentration [27]. The glutamatergic dysfunction implicated in neural plasticity and cellular resilience may then contribute to the pathophysiology of depressive disorder, and riluzole was evaluated as a treatment for depression in both human and rodents [28]. A recent preclinical, observational, open-label study showed great improvement in the riluzole group compared to the placebo group [29,30]. In the present study, riluzole showed dramatic amelioration of sucrose consumption, latency, and food intake in the behavioral paradigm compared to the CUMS model group. Corresponding with the improvement in behavioral actions, EAAT2-positive cell count and protein expression in the hippocampus and prefrontal cortex were increased (Figures 3–5), and the same tendency was found in the acupuncture group. Thus, we speculated that increased level of glial glutamate transporter EAAT2 may be an underlying mechanism by which acupuncture ameliorates depressive behaviors induced by CUMS.
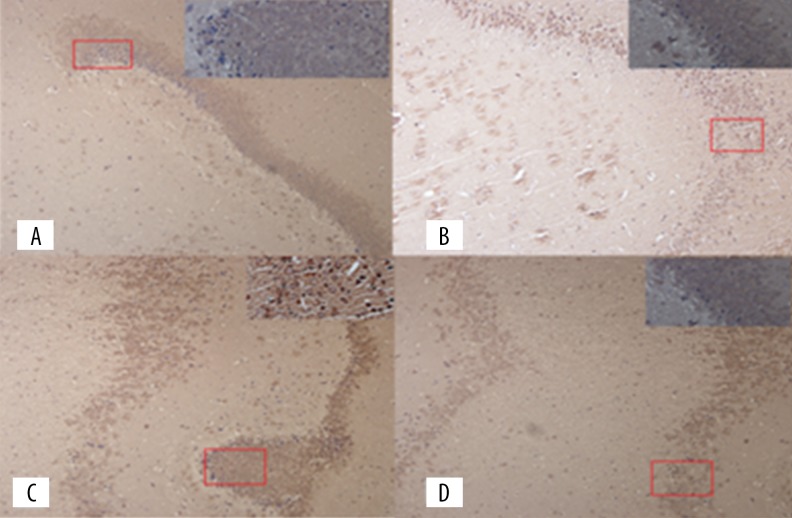
Figure 4.
EAAT2 immunohistochemistry photographs in hippocampus: (A) Normal group; (B) Model group; (C) Acupuncture group; (D) Riluzole group.
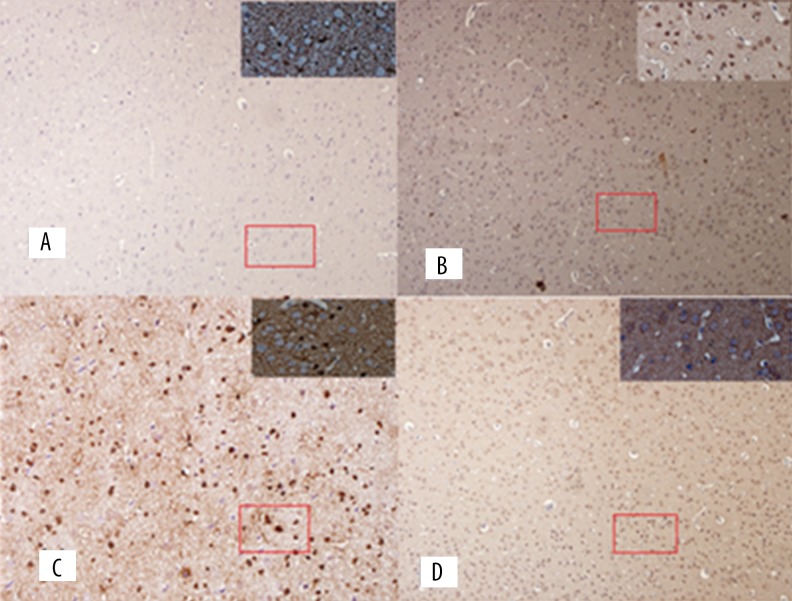
Figure 5.
EAAT2 immunohistochemistry photographs in PFC: (A) Normal group; (B) Model group; (C) Acupuncture group; (D) Riluzole group.
However, it is interesting to note that the time course of depressive behavioral actions remission was different between the riluzole and acupuncture groups. The behavioral actions significantly improved at the 2nd week in the riluzole group, but in the acupuncture group this occurred at the 3rd week. The same trend was also found in EAAT2-positive cells, protein, and mRNA expression. That suggests that a certain minimum level of acupuncture stimulation is needed before the antidepressant effect can be achieved.
These results suggest that an underlying mechanism of the effect of acupuncture treatment on CUMS is by enhancing the glial glutamate transporter EAAT2. The present study has some limitations. First, the dose-effect relationship of acupuncture stimulation and antidepressant effect should be further elaborated. Second, not only glutamate transporter EAAT2, but also the details of glutamate cycling in CNS, should be explored to expand understanding of the underlying mechanism, such as Glu transmission and synaptic plasticity. Therefore, more study is needed.
Conclusions
The results suggest that acupuncture is effective in improving sucrose consumption, latency, and food intake in CUMS rats. Enhanced glial glutamate transporter EAAT2 in the hippocampus and PFC is an underlying mechanism of the antidepressant effect of acupuncture.
Footnotes
Conflict of interests
No conflict of interest.
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81173348) and Guangdong Province Scientific and Technical Programs (No. 2013B032500008 and No. 2014A020221079), and the Guangdong Provincial High-Level University Construction Program
References
- 1.Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76(2):155–62. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 2.Reddy MS. Depression: The disorder and the burden. Indian J Psychol Med. 2010;32(1):1–2. doi: 10.4103/0253-7176.70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–45. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 4.Delgado PL. Depression: The case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- 5.Pomara N, Sidtis JJ. Brain neurotoxic amyloid-beta peptides: Their potential role in the pathophysiology of depression and as molecular therapeutic targets. Br J Pharmacol. 2010;161(4):768–70. doi: 10.1111/j.1476-5381.2010.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 7.Pilc A, Wieronska JM, Skolnick P. Glutamate-based antidepressants: Preclinical psychopharmacology. Biol Psychiatry. 2013;73(12):1125–32. doi: 10.1016/j.biopsych.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist. 2009;15(5):525–39. doi: 10.1177/1073858409336093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 10.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6(3):219–33. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64(10):863–70. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KF, Yeung WF, Yu YM, et al. Acupuncture for residual insomnia associated with major depressive disorder: A placebo- and sham-controlled, subject- and assessor-blind, randomized trial. J Clin Psychiatry. 2015;76(6):e752–60. doi: 10.4088/JCP.14m09124. [DOI] [PubMed] [Google Scholar]
- 13.Fan L, Gong J, Fu W, et al. Gender-related differences in outcomes on acupuncture and moxibustion treatment among depression patients. J Altern Complement Med. 2015;21(11):673–80. doi: 10.1089/acm.2015.0068. [DOI] [PubMed] [Google Scholar]
- 14.Fan L, Fu WB, Xu NG, et al. [Impacts of acupuncture and moxibustion on outcome indeices of depression patients’ subjective reports]. Zhongguo Zhen Jiu. 2012;32(5):385–89. [in Chinese] [PubMed] [Google Scholar]
- 15.Fan L, Chen Z, Fu W, et al. Soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein involved in the remission of depression by acupuncture in rats. J Acupunct Meridian Stud. 2016;9(5):242–49. doi: 10.1016/j.jams.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Banasr M, Valentine GW, Li XY, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62(5):496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y. Effects of electro-acupuncture on chronic stress which induced depression rats’ hippocampal structure and function of AST under TEM. International English Education Research. 2013;49(1):174–79. [Google Scholar]
- 18.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 19.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 20.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, et al. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95(3):298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(5):367–77. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 22.Hasler G, van der Veen JW, Tumonis T, et al. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 23.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmseth S, Scott HA, Real K, et al. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162(4):1055–71. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102(43):15653–58. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SJ, Wang KY, Wang WC. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) Neuroscience. 2004;125(1):191–201. doi: 10.1016/j.neuroscience.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1–21. doi: 10.1037/a0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salardini E, Zeinoddini A, Mohammadinejad P, et al. Riluzole combination therapy for moderate-to-severe major depressive disorder: A randomized, double-blind, placebo-controlled trial. J Psychiatr Res. 2016;75:24–30. doi: 10.1016/j.jpsychires.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Zarate CA, Jr, Payne JL, Quiroz J, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161(10):171–74. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]