Abstract
Patient: Male, new born
Final Diagnosis: Congenital chloride diarrhea
Symptoms: Diarrhea
Medication: —
Clinical Procedure: —
Specialty: Obstetrics and Gyneolcogy
Objective:
Congenital defects/diseases
Background:
Congenital chloride diarrhea (CCD) is a rare autosomal recessive disorder that is difficult to distinguish from fetal lower intestinal obstruction. A prenatal diagnosis will make a contribution to the prognosis of the newborn.
Case Report:
We report a rare case of congenital chloride diarrhea (CCD) prenatally suspected by ultrasound and MRI. The prenatal ultrasound revealed signs of intestinal dilatation suggesting lower intestinal obstruction. MRI findings also revealed intestinal dilatation that continued from the rectum. On T1-weighted images, fluid accumulation within the bowel was hypointense. CCD was strongly suspected rather than obstruction of the lower intestinal tract or Hirschsprung’s disease. At 37 weeks and 3 days’ gestation, cesarean section was performed because of fetal distress. The newborn was a 2396 g male with the Apgar scores 8 (1 min) and 9 (5 min). Watery diarrhea subsequently persisted, and on the 3rd day, hyponatremia and hypochloremia were present. The infant stool was hyperchloremic, so we diagnosed CCD.
Conclusions:
When ultrasonography reveals signs of fetal gastrointestinal dilatation, lower intestinal obstruction is considered first. CCD is infrequent and has signs of intestinal dilatation similar to those of other intestinal ailments, so distinguishing these conditions is difficult. However, if CCD is suspected early, then use of MRI will allow its diagnosis. If the condition is diagnosed before birth, the prognosis of the newborn will improve.
MeSH Keywords: Diarrhea, Infantile; Intestinal Obstruction; Polyhydramnios
Background
Congenital chloride diarrhea (CCD) is a condition that manifests primarily as watery diarrhea and is known to be caused by an autosomal recessive disorder. The condition is due to impaired active transport of Cl-/HCO3- in the ileum and colon [1–5]. The problem with this disease is that the large amount of watery diarrhea includes high chloride and sodium levels. If dehydration remains untreated, electrolyte imbalances and metabolic alkalosis may occur and can be life-threatening. However, the long-term outcome is good if the CCD is diagnosed early and managed appropriately [6–8].
Characteristic ultrasound findings of CCD include prominent signs of intestinal dilatation and polyhydramnios, but the condition is often hard to distinguish from others (e.g., meconium peritonitis, lower intestinal obstruction, and Hirschsprung’s disease) [9–12]. With CCD, the Cl level in amniotic fluid rises, but this does not provide a definitive diagnosis [13]. In recent years, Colombani et al. reported that MRI in addition to ultrasound findings helped to diagnose the condition [14].
In this case, signs of fetal intestinal dilatation were noted before birth. Based on ultrasonography and an MRI scan, CCD was strongly suspected. The course of the newborn, and the usefulness of diagnostic imaging, are reported here, along with a discussion of the literature.
Case Report
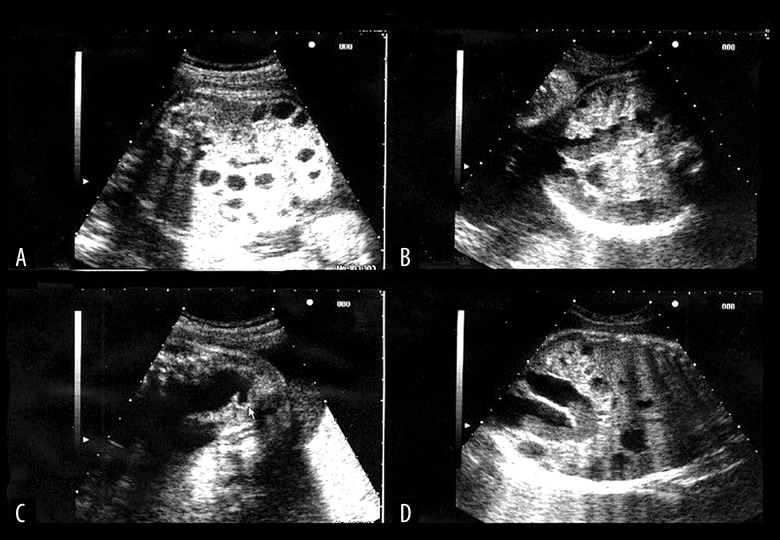
The patient was 36-year-old, gravida 0, para 0 woman. There was no consanguinity in the family. There was no family history of intestinal problems or related to CCD symptoms. No obvious abnormalities were noted in the first trimester, but ultrasonography revealed signs of fetal intestinal dilatation at 26 weeks’ gestation, so the patient was referred to our department at 27 weeks’ gestation. Ultrasonography examination revealed fetal bowel dilatation with the honeycomb sign (Figure 1). Morphological abnormalities in the fetus and placenta were not otherwise noted. There was a large volume of amniotic fluid but not polyhydramnios. Subsequent ultrasonography revealed signs of an increasing distended bowel from the rectum to the small intestine. The MRI scan revealed intestinal dilatation that continued from the rectum (Figure 2). On T1-weighted images, fluid accumulation within the bowel was hypointense (Figure 3). Based on the nature of fluid accumulation within the bowel, CCD was strongly suspected rather than obstruction of the lower intestinal tract or Hirschsprung’s disease.
Figure 1.
Ultrasonograph views at 27 weeks of gestation, showing multiple dilatation of the bowel loops filled with fluid: (A) Axial view of the fetal abdomen. The multiple dilated bowel loops can be seen in the fetal abdomen. (B) Axial view. The continuity of the dilated bowel loops increased gradually. (C, D) Sagittal and coronal view. The lower bowel loops dilated to the level of the rectum.
Figure 2.
Magnetic resonance image views. Showing hypersignal of multiple bowel dilatation which indicate the content to be fluid: (A) Coronal view of the fetal abdomen. The multiple dilated bowel loops with hypersignal can be seen in the fetal abdomen. (B) Axial view. Almost all of the lower bowel loop is dilated and hypersignal, indicating the contents to be fluid. (C, D) Coronal and sagittal view of the fetal abdomen. The dilated and hypersignal images of the lower bowel loop which continues from the rectum.
Figure 3.
Magnetic resonance image views of dilated bowel loops. Usually, meconium is described by hypersignal in the T1-weighted images: (A, B) Coronal and sagittal view of the normal fetus. Meconium is described by hypersignal. The multiple dilated bowel loops with hypersignal can be seen in the fetal abdomen. (C, D) Coronal and sagittal view of the CCD fetus. The dilated and lower signal images of the lower bowel loop which continues from the rectum.
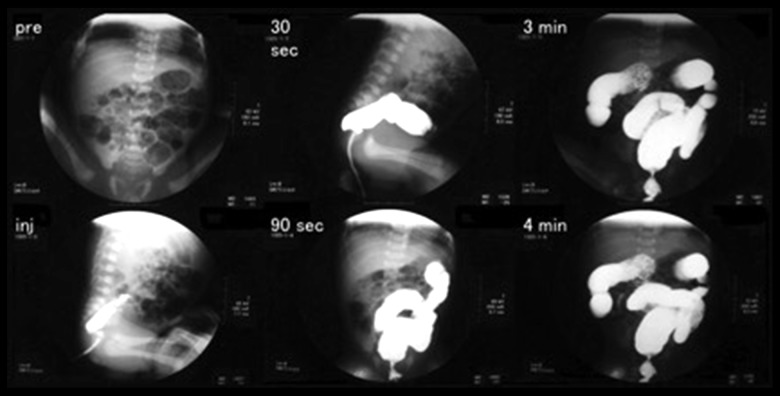
At 37 weeks and 3 days’ gestation, the mother’s labor pains began, followed by rupture of the membrane. In the amniotic fluid, meconium staining was present. A fetal heart monitor revealed frequent severe variable deceleration, so an emergency cesarean section was performed. The newborn was a 2396 g male with Apgar score 8 (1 min) and 9 (5 min). Plain abdominal x-rays of the newborn revealed normal bowel gas (Figure 4). A radiographic contrast enema revealed no intestinal obstruction and Hirschsprung’s disease was also ruled out (Figure 5). Watery diarrhea subsequently persisted, and on 3rd day, hyponatremia and hypochloremia were present (Table 1). The patient was started on an electrolyte solution. The infant stool collected by Nélaton catheter was hyperchloremic, so we diagnosed CCD (Table 2). Since the potassium was reduced from the 5th day, we also started potassium gluconate. The patient’s electrolytes were managed by administering NaCl and potassium gluconate orally and the patient’s subsequent course was satisfactory.
Figure 4.

Abdominal radiography of the neonate (day 1) showing dilated bowel loops.
Figure 5.
Radiographic contrast enema (day 1). There is no caliber change, indicating no Hirschsprung’s disease.
Table 1.
Infant blood electrolytes.
| Day | |||||||
|---|---|---|---|---|---|---|---|
| Electrolytes | 0 | 1 | 2 | 3 | 5 | 6 | 8 |
| Na (mEq/L) | 137 | 134 | 135 | 131 | 137 | 128 | 144 |
| K (mEq/L) | 4.1 | 4.3 | 4.9 | 5.1 | 2.9 | 4.7 | 4.7 |
| Cl (mEq/L) | 101 | 98 | 101 | 96 | 93 | 94 | 107 |
Table 2.
Infant stool electrolytes.
| Day | |||||
|---|---|---|---|---|---|
| Electrolytes | 1 | 4 | 5 | 6 | 11 |
| Na (mMol/L) | 120 | 94 | 98 | 104 | 92 |
| K (mMol/L) | 23.6 | 25.0 | 26.1 | 37.2 | 34.2 |
| Cl (mMol/L) | 144 | 120 | 125 | 132 | 110 |
| Consistency | Watery | Watery | Watery | Watery | Watery |
| Osmotic pressure (mOsm/kgH2O) | 288 | 294 | 278 | ||
Discussion
CCD was first reported by Gamble et al. and Darrow in 1945, known as “Darrow-Gamble disease” [15,16]. The condition manifests primarily as watery diarrhea that starts soon after birth and is accompanied by hypochloremic metabolic alkalosis. The stool contains more Cl than Na and K. CCD is an extremely rare genetic condition caused by impaired active transport of chloride in the ileum and colon. The condition is transmitted by autosomal recessive inheritance disorder and is due to mutation of the SLC26A3 gene on chromosome 7. SLC26A3 gene codes a glycoprotein that passes through the cell membrane into the lumen of epithelial cells of the bowel. This glycoprotein takes up chloride ions (Cl-) and exports bicarbonate ions (HCO3–). This genetic mutation results in active transport failure of Cl- in the intestine. When the absorption inhibition of Cl– occurs, H+/Na+ exchange transport is also inhibited. These defects cause absorption failure of NaCl and fluid from the intestines [1–5]. Therefore, antenatal ultrasonography reveals polyhydramnios and intestinal dilatation, and postnatally, presentation of watery diarrhea, hypochloremic metabolic alkalosis, and elevated stool chloride. The diagnosis of CCD is usually confirmed by clinical symptoms and the stool Cl concentration (>90 mmol/L) after correction of the fluid and electrolytes. Genetic diagnosis for CCD by mutation analysis is also performed [17,18]. In this case, genetic diagnosis was also considered, but the parents did not approve and it was not performed. Therefore, we diagnosed CCD by clinical symptom and the stool Cl concentration.
CCD occurs in 1 in 10 000–40 000 births. There are regional differences in its prevalence, with many reports coming from northern European countries like Finland, Sweden, and Poland, and the Middle East [20,21]. The condition is often detected because of symptoms such as refractory watery diarrhea after birth. Large amounts of chloride from the ileum and colon are lost in the feces soon after birth. CCD is treated by replenishing and restoring lost electrolytes and water. If the condition is diagnosed in the early stages after birth and promptly treated, the infant will grow and develop normally. Diagnosing the condition in utero and treating it soon after birth help to improve prognosis [7].
Ultrasonography findings noted in CCD are polyhydramnios and signs of intestinal dilatation. Watery diarrhea from the fetus increase amniotic fluid and cause polyhydramnios. An excessive amount of watery diarrhea causes fetal intestinal dilatation. Polyhydramnios typically suggests obstruction of the upper gastrointestinal tract, and signs of bowel dilatation suggest lower intestinal obstruction. Thus, polyhydramnios coexisting with intestinal dilatation are findings that are observed in obstruction of the upper or lower small intestine, meconium peritonitis, Bartter syndrome, and Hirschsprung’s disease, so differentiating these conditions from CCD is difficult [9–13,19]. Lundkvist et al. reported that prenatal ultrasound findings distinguishing CCD from lower intestinal atresia were: 1) severe polyhydramnios and the need for repeated amniocentesis; 2) a distended bowel noted throughout the fetus’ abdominal cavity; and 3) normal peristalsis in the distended bowel [9].
Colombani et al. reported that MRI helped to diagnose the condition. Normally, meconium within the bowel is hyperintense on T1-weighted images. With CCD, hyperintensity within the bowel is absent due to watery diarrhea [14]. In this case, we carried out MRI with 1.5T system MAGNETOM Symphony (Siemens Healthcare, Germany) as an adjunct to ultrasonography. To evaluate the fetus, we used the images of T2-weighted half-Fourier acquisition single-shot turbo spin echo (HASTE), T2-weighted True fast imaging with steady-state precession (TrueFISP), and T1-weighted two-dimensional fast low angle shot (FL2D). No fetal sedation was performed.
The imaging findings from this case revealed signs of fetal intestinal dilatation, suggesting lower intestinal obstruction. However, signs of gastrointestinal dilatation continuing from the rectum conflict somewhat with the usual images of lower intestinal obstruction. Although some investigators have indicated that a high Cl concentration in amniotic fluid suggests congenital chloride diarrhea, polyhydramnios was not noted in this case, so the Cl concentration in amniotic fluid was not determined [13]. In addition, the degree of CCD in the fetus is affected by reabsorption of watery stool via the mouth, and electrolytes in the amniotic fluid are affected by electrolyte exchange via the placenta and amniotic fluid volume. Given these facts, the Cl level in amniotic fluid has little diagnostic value.
Treatment of CCD is electrolyte replenishment of fluid and NaCl and KCl intravenously or orally. Because of diarrhea throughout life, long-term appropriate replacement therapy is needed. If untreated, CCD will result in chronic kidney disease. An electrolyte abnormality due to diarrhea can cause renal dysfunction associated with interstitial failure [6,22]. Early CCD detection, if the electrolyte and the fluid is sufficiently replenished, can prevent the onset of chronic kidney disease. Therefore, antenatal diagnosis of CCD is very important for treatment. However, the ultrasonographic findings in CCD are infrequent and have signs of intestinal dilatation similar to those of other intestinal obstructions, so distinguishing these conditions is difficult. If polyhydramnios and intestinal obstruction are recognized by ultrasonography, it is necessary to consider CCD as a differential diagnosis, and the use of MRI will help. If CCD is diagnosed before birth, the prognosis for the newborn will improve.
Conclusions
We report a case of CCD suspected by the prenatal ultrasonography and MRI examination. CCD is characterized by a large amount of watery diarrhea causing hypochloremia, hyponatremia, hypokalemia, metabolic alkalosis, and dehydration. Without appropriate treatment, fluid loss and electrolyte disorders can be life-threatening. Therefore, prenatal diagnosis is important and can lead to better outcomes for infants with CCD. Ultrasonography findings in CCD are polyhydramnios and bowel dilatation, but these findings are also observed in bowel obstruction, meconium peritonitis, Bartter syndrome, and Hirschsprung’s disease, so differentiating these conditions from CCD is difficult. In this case, MRI was also performed as well as ultrasonography and was useful in the diagnosis. Our experience suggests that MRI is useful for accurately diagnosing fetal CCD.
Footnotes
Conflicts of interest
No potential conflicts of interest were disclosed.
References:
- 1.Hoglund P, Haila S, Socha J, et al. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–19. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 2.Berni Canani R, Terrin G, Cardillo G, et al. Congenital diarrheal disorders: improved understanding of gene defects is leading to advances in intestinal physiology and clinical management. J Pediatr Gastroenterol Nutr. 2010;50:360–66. doi: 10.1097/MPG.0b013e3181d135ef. [DOI] [PubMed] [Google Scholar]
- 3.Wedenoja S, Hoglund P, Holmberg C. Review article: the clinical management of congenital chloride diarrhoea. Aliment Pharmacol Ther. 2010;31:477–85. doi: 10.1111/j.1365-2036.2009.04197.x. [DOI] [PubMed] [Google Scholar]
- 4.Wedenoja S, Pekansaari E, Hoglund P, et al. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2011;32:715–22. doi: 10.1002/humu.21498. [DOI] [PubMed] [Google Scholar]
- 5.Terrin G, Tomaiuolo R, Passariello A, et al. Congenital diarrheal disorders: An updated diagnostic approach. Int J Mol Sci. 2012;13:4168–85. doi: 10.3390/ijms13044168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hihnala S, Hoglund P, Lammi L, et al. Long-term clinical outcome in patients with congenital chloride diarrhea. J Pediatr Gastroenterol Nut. 2006;42:369–75. doi: 10.1097/01.mpg.0000214161.37574.9a. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Herrera A, Navas-López VM, Redondo-Nevado J, Gutiérrez G. Compound heterozygous mutations inthe SLC26A3 gene in 2 Spanish siblings with congenital chloride diarrhea. J Pediatr Gastroenterol Nutr. 2011;52(1):106–10. doi: 10.1097/MPG.0b013e3181f28d1a. [DOI] [PubMed] [Google Scholar]
- 8.Lechner S, Ruemmele FM, Zankl A, et al. Significance of molecular testing for congenital chloride diarrhea. J Pediatr Gastroenterol Nutr. 2011;53(1):48–54. doi: 10.1097/MPG.0b013e31820bc856. [DOI] [PubMed] [Google Scholar]
- 9.Lundkvist K, Ewald U, Lindgren PG. Congenital chloride diarrhea: A prenatal differential diagnosis of small bowel atresia. Acta Paediatr. 1996;85:295–98. doi: 10.1111/j.1651-2227.1996.tb14019.x. [DOI] [PubMed] [Google Scholar]
- 10.Imada S, Kikuchi A, Horikoshi T, et al. Prenatal diagnosis and management of congenital chloride diarrhea: a case report of two siblings. J Clin Ultrasound. 2012;40:239–42. doi: 10.1002/jcu.21895. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Park YK. Antenatal differential diagnosis of congenital chloride diarrhea: A case report. J Obstet Gynaecol Res. 2012;38:957–61. doi: 10.1111/j.1447-0756.2012.01876.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa M, Hidaka N, Kido S, et al. Congenital chloride diarrhea: Accurate prenatal diagnosis using color doppler sonography to show the passage of diarrhea. J Ultrasound Med. 2015;34(11):2113–15. doi: 10.7863/ultra.15.01011. [DOI] [PubMed] [Google Scholar]
- 13.Tsukimori K, Nakanami N, Wake N, et al. Prenatal sonographic findings and biochemical assessment of amniotic fluid in a fetus with congenital chloride diarrhea. J Ultrasound Med. 2007;26:1805–7. doi: 10.7863/jum.2007.26.12.1805. [DOI] [PubMed] [Google Scholar]
- 14.Colombani M, Ferry M, Toga C, et al. Magnetic resonance imaging in the prenatal diagnosis of congenital chloride diarrhea. Ultrasound Obstet Gynecol. 2010;35(5):560–65. doi: 10.1002/uog.7509. [DOI] [PubMed] [Google Scholar]
- 15.Gamble JL, Fahey KR, Appleton J, et al. Congenital alkalosis with diarrhea. J Pediatr. 1945;26:509–18. [Google Scholar]
- 16.Darrow DC. Congenital alkalosis with diarrhea. J Pediatr. 1945;26:519–32. [Google Scholar]
- 17.Heinz-Erian P, Oberauer M, Neu N, et al. A novel homozygous SLC26A3 nonsense mutation in a Tyrolean girl with congenital chloride diarrhea. J Pediatr Gastroenterol Nutr. 2008;47:363–66. doi: 10.1097/MPG.0b013e318174e818. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg C, Perheentupa J, Launiala K. Colonic electrolyte transport in health and congenital diarrhea. J Clin Invest. 1975;56:302–10. doi: 10.1172/JCI108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer JC, Winthrop AL, Burrows RF, et al. False diagnosis of intestinal obstruction in fetus with congenital chloride diarrhea. J Pediatr Surg. 1991;26:1282–84. doi: 10.1016/0022-3468(91)90599-o. [DOI] [PubMed] [Google Scholar]
- 20.Holmberg C. Congenital chloride diarrhea. Clin Gastroenterol. 1986;15:583–602. [PubMed] [Google Scholar]
- 21.Hoglund P, Auranen M, Socha J, et al. Genetic background of congenital chloride diarrhea in high-incidence populations: Finland, Poland, and Saudi Arabia and Kuwait. Am J Hum Genet. 1998;63(3):760–68. doi: 10.1086/301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmberg C, Perheentupa J, Pasternack A. The renal lesion in congenital chloride diarrhea. J Pediatr. 1977;91:738–43. doi: 10.1016/s0022-3476(77)81026-3. [DOI] [PubMed] [Google Scholar]