Abstract
Cerebrospinal fluid gamma interferon (IFN-γ) and interleukin-10 levels in 39 patients with tuberculous meningitis were serially measured. Cytokine levels did not predict intracranial granuloma (IG) development, but IFN-γ levels in the top quartile after 1 month of therapy were highly associated (odds ratio = 18) with detection of an IG by computed tomography scanning.
Cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha play a pivotal role in the development of granulomas in pulmonary tuberculosis (1, 11, 13). Similar mechanisms presumably mediate intracranial granuloma (IG) formation in patients with tuberculous meningitis (TBM), but as of yet, the relationship has not been well characterized. We therefore sought to determine whether IG formation could be predicted on the basis of cerebrospinal fluid levels of these two cytokines.
Study patients were a subset of a TBM treatment trial conducted at the Abbassia Fever Hospital in Cairo, Egypt. For the treatment trial, patients received daily isoniazid (INH) (10 mg/kg of body weight/day; maximum, 300 mg/day), rifampin (20 mg/kg/day; maximum, 600 mg/day), pyrazinamide (40 mg/kg/day; maximum, 1,000 mg), and streptomycin (15 mg/kg/day; maximum, 1,000 mg) for 2 months followed by daily INH and rifampin. Oral dexamethasone (0.4 mg/kg/day) was administered for the first month of therapy, and then the dosage was tapered over the next month.
Inclusion in the present study required a pretreatment cerebrospinal fluid (CSF) culture positive for Mycobacteria tuberculosis plus an aliquot of CSF collected prior to initiation of antituberculous therapy and 1 month later. When available, CSF collected 6 months after initiation of therapy was also tested for cytokines. CSF IFN-γ and interleukin-10 (IL-10) concentrations were determined at the University of Arkansas by use of enzyme-linked immunoassay kits (R and D Systems, Minneapolis, Minn.). The detection limits of the assays were 8 pg/ml for IFN-γ and 4 pg/ml for IL-10. All samples were analyzed undiluted in duplicate according to the manufacturer's instructions and reanalyzed when variability between duplicates was greater than 20%, which occurred in <3% of the samples tested.
Computed tomography (CT) brain scans, obtained prior to and after administration of urographin contrast material, were performed on all patients before initiation of antituberculous therapy and repeated 1 month and 6 months later with a Toshiba TCT 600 HQ CT scanner (Toshiba Company, Tokyo, Japan). A single experienced radiologist, blinded to the diagnosis of the patient, evaluated all CT scan results.
Clinical characteristics and cytokine levels were compared between patients who did and did not develop IG after completing 1 month and 6 months of therapy. Differences in proportions of categorical variables were evaluated using chi-square or Fisher's exact test when expected cell values were lower than 5. Continuous variables were analyzed using either two-sample Wilcoxon rank-sum (Mann-Whitney) testing for unmatched data or Wilcoxon signed-rank testing for matched data (e.g., baseline versus follow-up). An odds ratio with a P value from χ2 distribution was used to assess association between IFN-γ and granuloma at one month.
A total of 39 patients (56% male), all human immunodeficiency virus negative, with a mean age of 24 ± 12 years (mean ± standard deviation) were studied (Table 1). Of the patients, 90% exhibited British Research Council stage II or stage III disease at hospital admission and were ill for a mean of 53 days (range, 17 to 90) prior to hospitalization. Intracranial granulomas were detected in 5 of 39 patients prior to the initiation of therapy, but this group was clinically indistinguishable from the 34 patients without an IG on the pretreatment scan.
TABLE 1.
Pretreatment characteristics of study population (n = 41)
| Characteristic | Result |
|---|---|
| Mean age (yrs) ± SD | 24 ± 12 |
| Male gender | 23 (56)a |
| Mean days ill before admission ± SD | 53 ± 52 |
| Tuberculosis stage on admission | |
| 1 | 4 (10)a |
| 2 | 25 (61)a |
| 3 | 12 (29)a |
| History of seizures | 11 (27)a |
| Neurologic deficit on admission (physical examination) | 26 (63)a |
| Cerebral granuloma on admission (CT scan) | 5 (12)a |
Total number (percent).
Prior to initiation of therapy, mean levels of IFN-γ were 794 ± 530 pg/ml (range, 0 to 1,500) whereas mean levels of IL-10 were 165 + 112 pg/ml (range, 4.7 to 638 pg/ml). After 1 month of therapy, IFN-γ levels dropped to 179.5 ± 216 pg/ml (mean plus standard deviation) (range, 0 to 901) but IL-10 levels remained relatively unchanged. At completion of therapy, CSF levels of both IFN-γ and IL-10 were low or undetectable for all patients tested.
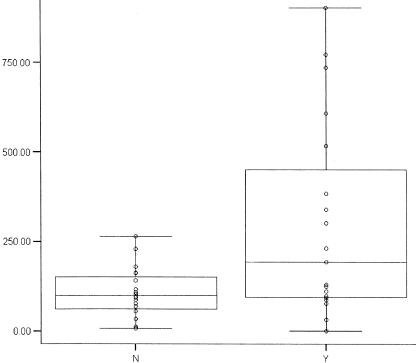
A total of 14 patients (39%) developed an IG within 1 month of starting treatment. While no pretreatment factor was predictive for development of an IG, mean levels of IFN-γ after 1 month of therapy were significantly (P = 0.008) increased among patients who had developed an IG during the first month of therapy (Table 2). Post hoc analysis found that patients with CSF IFN-γ levels in the upper quartile (>230 pg/ml) after 1 month of therapy had a relative risk of 18 (P = 0.01) to have an IG detected by CT (Fig. 1). Three additional patients developed an IG between 1 and 6 months of therapy, but again, no factors were found to be predictive of or associated with the granuloma development. Of the 19 patients with an IG detected by the 1-month CT scan, 13 still had the IG after 6 months of therapy. However, the levels of both IFN-γ and IL-10 in the CSF at this time point were extremely low or undetectable, indicating nearly complete or complete resolution of meningeal inflammation.
TABLE 2.
Mean CSF IFN-γ and IL-10 levels pretherapy and after 1 month of therapy stratified by cerebral granuloma detected by 1-month CT scan
| No granuloma at 1 mo (n = 22)a
|
Granuloma present at 1 mo (n = 14)a
|
|||
|---|---|---|---|---|
| IFN-γ | IL-10 | IFN-γ | IL-10 | |
| Pretherapy | 877 ± 537 | 178 ± 134 | 718 ± 545 | 137 ± 75 |
| 1 mo | 101 ± 70b | 152 ± 161 | 289 ± 297b | 219 ± 169 |
Picograms per milliliter (mean ± standard deviation).
P = 0.008 (calculated using the two-sample Wilcoxon rank-sum [Mann-Whitney] test).
FIG. 1.
Gamma interferon levels (in picograms per milliliter) in CSF stratified by the absence (N) or presence (Y) of cerebral granuloma after 1 month of therapy.
In agreement with the literature, we found that pretreatment CSF concentrations of IFN-γ were markedly elevated, as would be expected in the presence of an inflammatory process, suggesting that the cytokine is an important component of the immune response to TBM (4, 7, 12). The CSF levels of IFN-γ detected with our patients were similar to those described for patients with aseptic meningitis but far higher than those seen with children with bacterial meningitis (3, 5, 8, 10). However, while IFN-γ and IL-10 remained detectable in the CSF of patients with TBM for weeks, the cytokines were present for only days in patients with viral or bacterial meningitis (3, 5, 8, 10). After 6 months of therapy, both IL-10 and IFN-γ were undetectable in the CSF of 90% of our patients, in contrast to the results reported in the literature, according to which IFN-γ was commonly detectable in the CSF at this time point (6, 12). While the fact that our patients received corticosteroids during the first 2 months of therapy could explain the more rapid drop in cytokine levels, corticosteroids did not affect cytokine levels in children with TBM (4). In contrast to IFN-γ, IL-10 is an anti-inflammatory mediator that down-regulates the host immune response (9). Whether down-regulation of the immune response mediated by IL-10 has an overriding beneficial or deleterious effect for the host during the course of TBM remains to be investigated.
Intracranial granulomas were found in 12% of our patients at the time of admission, and as with previous studies, we found that both the clinical stage of the patient's meningitis and the presence of abnormal neurological findings on admission were unrelated to the presence of cerebral granulomas detected by the pretreatment CT scanning (2).
A main aim of our study was to determine whether CSF cytokine levels could be used to predict the development of IG. Although no factors were found to be predictive of IG development, high levels of IFN-γ measured at 1 month of therapy predicted that patients would show evidence of IG at that time. Patients with CSF IFN-γ concentrations in the top quartile at 1 month were 18 times more likely to have an IG detected, suggesting that ongoing CSF inflammation is important in the formation of IG. The absence of an association of increased IFN-γ levels in pretherapy CSF specimens with detection of IG at later time points suggests that a delayed decrease in inflammation, rather than the height of the early inflammatory response, determines the local histological outcome in the brain. The presence of low levels of CSF IFN-γ after 1 month of treatment in 10 patients with an IG suggests that factors other than IFN-γ contribute to presence of an IG. Further work is warranted to determine whether modulation of the cytokine response may be useful in improving outcome of patients with tuberculous meningitis.
Acknowledgments
This work was supported by the Naval Medical Research Command (NMRC), Silver Spring, Md., Work Unit no. 60000.000.000.E0016, and the Horace C. Cabe Foundation and the Bates-Wheeler Foundation, Arkansas Children's Hospital Research Institute.
The protocol was approved by the NAMRU-3 IRB in compliance with all federal regulations governing the protection of human subjects. Informed consent was obtained from each subject, and the human use guidelines of U.S. Departments of Defense and Health and Human Services were strictly followed in the conduct of this trial. No author has a commercial or other association that might pose a conflict of interest in publication of the manuscript. Medhat El-Shafeey, Mohamed F. Abd-El Wahab, and Hassan Soliman (University of Ain Shams, School of Medicine, Cairo, Egypt) are gratefully acknowledged for their scientific supervision as well as Manal Mustafa (U.S. Naval Medical Research Unit no. 3, Cairo, Egypt) for her statistical assistance.
The opinions and assertions contained herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the Navy Department, Department of Defense, the U.S. government, or the Egyptian Ministry of Health.
REFERENCES
- 1.Algood, H. M., J. Chan, and J. L. Flynn. 2003. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 14:467-477. [DOI] [PubMed] [Google Scholar]
- 2.Altunbasak, S., E. Alhan, V. Baytok, N. Aksaray, and N. Onenli. 1996. Intracerebral tuberculoma in children with tuberculous meningitis. Turk. J. Pediatr. 38:323-327. [PubMed] [Google Scholar]
- 3.Chonmaitree, T., and S. Baron. 1991. Bacteria and viruses induce production of interferon in the cerebrospinal fluid of children with acute meningitis: a study of 57 cases and review. Rev Infect. Dis. 13:1061-1065. [DOI] [PubMed] [Google Scholar]
- 4.Donald, P. R., J. F. Schoeman, N. Beyers, E. D. Nel, S. M. Carlini, K. D. Olsen, and G. H. McCracken. 1995. Concentrations of interferon gamma, tumor necrosis factor alpha, and interleukin-1 beta in the cerebrospinal fluid of children treated for tuberculous meningitis. Clin. Infect. Dis. 21:924-929. [DOI] [PubMed] [Google Scholar]
- 5.Kornelisse, R. F., C. E. Hack, H. F. Savelkoul, T. C. van der Pouw Kraan, W. C. Hop, G. van Mierlo, M. H. Suur, H. J. Neijens, and R. de Groot. 1997. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect. Immun. 65:877-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastroianni, C. M., L. Lancella, F. Mengoni, M. Lichtner, P. Santopadre, C. D'Agostino, F. Ticca, and V. Vullo. 1998. Chemokine profiles in the cerebrospinal fluid (CSF) during the course of pyogenic and tuberculous meningitis. Clin. Exp. Immunol. 114:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastroianni, C. M., F. Paoletti, M. Lichtner, C. D'Agostino, V. Vullo, and S. Delia. 1997. Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin. Immunol. Immunopathol. 84:171-176. [DOI] [PubMed] [Google Scholar]
- 8.Minamishima, I., S. Ohga, E. Ishii, C. Miyazaki, K. Hamada, K. Akazawa, and K. Ueda. 1991. Aseptic meningitis in children: correlation between fever and interferon-gamma level. Eur. J. Pediatr. 150:722-725. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann, T. R., and K. W. Moore. 1991. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today 12:A49-A53. [DOI] [PubMed] [Google Scholar]
- 10.Ohga, S., T. Aoki, K. Okada, H. Akeda, K. Fujioka, A. Ohshima, T. Mori, I. Minamishima, and K. Ueda. 1994. Cerebrospinal fluid concentrations of interleukin-1 beta, tumour necrosis factor-alpha, and interferon gamma in bacterial meningitis. Arch. Dis. Child. 70:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutlas, P. N., A. Unal, H. Forta, S. Senol, and D. Kirbas. 2003. Tuberculous meningitis in adults: review of 61 cases. Infection 31:387-391. [DOI] [PubMed] [Google Scholar]
- 12.Thwaites, G. E., C. P. Simmons, N. Than Ha Quyen, T. Thi Hong Chau, P. Phuong Mai, N. Thi Dung, N. Hoan Phu, N. P. White, T. Tinh Hien, and J. J. Farrar. 2003. Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J. Infect. Dis. 188:1105-1115. [DOI] [PubMed] [Google Scholar]
- 13.Verdon, R., S. Chevret, J. P. Laissy, and M. Wolff. 1996. Tuberculous meningitis in adults: review of 48 cases. Clin. Infect. Dis. 22:982-988. [DOI] [PubMed] [Google Scholar]