Abstract
Setting: Government health centres and hospitals (six urban and 20 rural) providing tuberculosis (TB) treatment for people living with the human immunodeficiency virus (PLHIV) in central and western Uganda.
Objective: To identify and quantify modifiable factors that limit TB treatment success among PLHIV in rural Uganda.
Design: A retrospective cross-sectional review of routine Uganda National Tuberculosis and Leprosy Programme clinic registers and patient files of HIV-positive patients who received anti-tuberculosis treatment in 2014.
Results: Of 191 rural patients, 66.7% achieved treatment success compared to 81.1% of 213 urban patients. Adjusted analysis revealed higher average treatment success in urban patients than in rural patients (OR 3.95, 95%CI 2.70–5.78, P < 0.01, generalised estimating equation model). Loss to follow-up was higher and follow-up sputum smear results were less frequently recorded in TB clinic registers among rural patients. Patients receiving treatment at higher-level facilities in rural settings had greater odds of treatment success, while patients receiving treatment at facilities where drug stock-outs had occurred had lower odds of treatment success.
Conclusion: Lower reported treatment success in rural settings is mainly attributed to clinic-centred factors such as treatment monitoring procedures. We recommend strengthening treatment monitoring and delivery.
Keywords: hospital records, rural, urban, Uganda, PLHIV, TB
Abstract
Contexte: L'étude a été réalisée dans des centres de santé et des hôpitaux publics, six urbains et 20 ruraux, fournissant un traitement de la tuberculose (TB) aux personnes vivant avec le VIH (PVVIH) dans le centre et l'ouest de l'Ouganda.
Objectif: Identifier et quantifier les facteurs modifiables qui limitent le succès du traitement de la TB parmi les PVVIH dans l'Ouganda rural.
Schéma: Une revue rétrospective transversale des registres cliniques et des dossiers de patients du Programme national tuberculose et lèpre d'Ouganda pour les patients VIH positifs qui ont reçu un traitement de TB en 2014.
Résultats: Parmi 191 patients ruraux, 66,7% ont eu un bon résultat de leur traitement, tandis que parmi 213 patients urbains, 81,1% ont eu un bon résultat. Une analyse ajustée a révélé un succès thérapeutique moyen plus élevé chez les patients urbains comparés aux patients ruraux (OR 3,95 ; IC95% 2,70–5,78 ; P < 0,01 ; modèle d'équation d'estimation généralisée). Les pertes de vue ont été plus élevées et les résultats de frottis de crachats de suivi ont été moins souvent enregistrés dans les registres des centres TB pour les patients ruraux. Les patients recevant un traitement dans des structures de plus haut niveau, toujours en zone rurale, avaient plus de chances d'avoir un succès thérapeutique. Les patients recevant leur traitement dans des structures où étaient survenues des ruptures de stock de médicaments avaient moins de chances de succès thérapeutique.
Conclusion: Les taux plus faibles de succès du traitement rapportés en zone rurale sont en majorité attribués à des facteurs liés aux centres de santé, comme les procédures de suivi du traitement. Nous recommandons le renforcement de la fourniture et du suivi du traitement.
Abstract
Marco de referencia: El estudio se llevó a cabo en centros de salud y hospitales del sector público, seis en entornos urbanos y 20 en medio rural y consistió en suministrar el tratamiento antituberculoso a las personas positivas frente al virus de la inmunodeficiencia humana (VIH) en la región central y occidental de Uganda.
Objetivo: Determinar y cuantificar los factores modificables que limitan la eficacia del tratamiento antituberculoso en las personas positivas frente al VIH en las zonas rurales de Uganda.
Método: Fue este un estudio transversal retrospectivo de análisis de los registros corrientes y las historias clínicas de los pacientes positivos frente al VIH, en los consultorios del Programa Nacional contra la Tuberculosis y la Lepra de Uganda en el 2014.
Resultados: De los 191 pacientes de entornos rurales, el 66,7% logró un tratamiento eficaz y en los 213 pacientes en medio urbano esta proporción fue 81,1%. Un análisis ajustado reveló un promedio de éxito terapéutico más alto en los pacientes urbanos en comparación con los pacientes rurales (OR 3,95; IC95% de 2,70 a 5,78; P < 0,01, según un modelo de ecuaciones de estimación generalizadas). En medio rural, se observó una mayor pérdida durante el seguimiento y se consignaban con menor frecuencia los resultados de las baciloscopias de seguimiento en los registros de tuberculosis de los consultorios. Los pacientes que recibían tratamiento en los establecimientos de nivel de atención más alto en medio rural tenían mayores posibilidades de éxito terapéutico. Los pacientes que recibían tratamiento en centros que presentaban desabastecimientos de medicamentos tuvieron menos probabilidades de lograr un tratamiento eficaz.
Conclusión: La menor proporción de éxito terapéutico notificada en los entornos rurales se debe en su mayor parte a factores que dependen del consultorio, como los procedimientos de supervisión del tratamiento. Se recomienda reforzar la supervisión y el suministro del tratamiento antituberculoso.
A sub-Saharan African country with both high tuberculosis (TB) incidence and human immuno-deficiency virus (HIV) prevalence, Uganda is among the 20 countries with the highest TB-HIV burden worldwide.1 TB incidence in Uganda in 2014–2015 was estimated to be 174 smear-positive cases per 100 000 population per year;1 HIV prevalence in 15–49 year olds was 7.4% in 2012–2013.2 TB treatment success rates have greatly improved in Uganda, from 44% in 1995 to 75% in 2014, approaching the 2015 World Health Organization (WHO) target of 85%.1
However, treatment success among TB patients living with HIV (PLHIV) are marginally lower than in their HIV-negative counterparts, both in Uganda (73% vs. 77%)1 and globally (73% vs. 88% in 2014).1 Low TB treatment success among PLHIV has been documented in various studies conducted in Africa.3,4 Rural health care settings report lower treatment success than national rates,3–6 although the introduction of the DOTS strategy may have improved rates.7
A qualitative evaluation identified health system barriers to TB treatment outcomes such as stock-outs of drugs and laboratory supplies, low motivation and poor co-ordination of services, as well as contextual barriers such as the cost of seeking treatment.8 Previous studies in Uganda showed that late presentation, patients lost to follow-up from treatment and inadequate treatment monitoring were associated with negative outcomes.9,10
In the present study, we aimed to use data from routinely collected government facilities to identify factors that limit treatment success in Ugandan urban and rural settings among the vulnerable population of PLHIV.
METHODS
Study design, setting and population
A retrospective cross-sectional study of Uganda National Tuberculosis and Leprosy Programme (NTLP) routine data was conducted in 26 government health facilities in Kampala and western Uganda. The Ugandan health system is structured into five health facility levels, ranging from village health team (HC-I) to specialised services at the national referral hospital. TB treatment and diagnosis is available from HC-III facilities upwards, and most TB-HIV integrated care is available from HC-IV facilities upwards. Facilities (HC-III and upwards) were randomly sampled from rural and urban sites supported by the Infectious Diseases Institute (IDI), Kampala, Uganda. Six of eight urban Kampala City Council Authority (KCCA) clinics were selected. Twenty rural facilities from Western Uganda were selected from 44 available (Appendix Tables A.1 and A.2). Eligible participants were new pulmonary TB cases, PLHIV, aged ⩾14 years, who initiated anti-tuberculosis treatment in 2014. Participants were ineligible if they had multidrug-resistant TB (MDR-TB) or extensively drug-resistant TB (XDR-TB), or if their records were missing date of birth, age, TB diagnosis, TB regimen or date of treatment initiation. During the target period, the Uganda guidelines criteria for antiretroviral therapy (ART) were PLHIV with a CD4 count of <350 cells/μl regardless of WHO stage, or all PLHIV diagnosed with TB.11,12 A rural setting was defined as an area outside a city or big commercial town, while an urban setting was defined as a city or big commercial town.
Anti-tuberculosis treatment
All clinics enrolled in the study followed the Uganda NTLP guidelines13 for TB diagnosis and treatment. New pulmonary TB patients were given first-line anti-tuberculosis treatment comprising a 2-month intensive phase of rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E) (2RHZE) and either a 6-month continuation phase comprising ethambutol (E) and isoniazid (H) (as combination: 6EH), or 4 months of rifampicin (R) and isoniazid (H) (4RH). The 4RH regimen was standard care for people aged ⩽15 years, and was used during drug stock-outs in some clinics.
Participants and data collection
Records were extracted between December 2015 and January 2016. To attain a targeted sample size of 396 cases (198 cases each from rural and urban areas), we required at least 33 cases per clinic. To allow for missing data, we targeted 40 cases and therefore included all eligible cases in facilities with ⩽40 cases, while in those with >40 cases, systematic sampling with probability proportional to size was used.
Participants' TB and HIV data were obtained from registers, HIV care cards in patient files and ART registers. Patients' HIV care identification clinic numbers (IDCNO) were used to match TB and HIV data, but when these were missing from TB registers, demographic characteristics were used for matching. If a patient's file could not be traced, the next eligible patient in the register was considered. Clinic-level data on drug stock-outs, staffing, geographic location, possession of microscopy and other clinic activities such as patient tracing were obtained from review of annual reports and from staff at the TB clinic.
Data were double-entered into Epi Info™ 7 statistical software (Centers for Disease Control and Prevention, Atlanta, GA, USA), verified for consistency, and transferred to Stata 13.1 software (Stata LP, College Station, TX, USA) for analysis.
Statistical analysis and outcomes
With 80% power, a significance of 5% and a sampling ratio of 1:1 in urban:rural clinics, 322 patients were required to detect a difference in treatment success, from 71%14 in urban clinics to 56% in rural clinics, based on an estimated difference of 15% between success rates. To allow for clustering effects, the sample size was inflated by 20%, making a target sample size of 396.
The primary outcome was the proportion of participants with treatment success (defined as cure or treatment completed) comparing rural and urban participants. Secondary outcomes were standard end of treatment measures, defined according to the Uganda NTLP and the WHO1,13 (Appendix Table A.3). Logistic generalised estimating equations (GEE) models with clinics as clusters, odds ratios (ORs) and 95% confidence intervals (CIs) were used to examine measured potential predictors of TB treatment success. Factors examined in the analysis included patient characteristics at TB treatment start (age, sex, CD4 cell count, body mass index [BMI], ART treatment history, living in a different subcounty from the clinic, and nature of TB diagnosis: bacteriologically confirmed or clinically diagnosed), status during TB treatment (timing of ART initiation among the ART-naïve, comorbidities that interfere with patient adherence, such as stomach ulcer and malaria, missed TB doses, missed clinic appointments, received 4 weeks' supply of anti-tuberculosis drugs for the intensive phase) and clinic factors (health facility level, TB drug stock-outs, number of staff at TB clinic by qualification, patient load at TB clinic). All analyses used inverse probability weighting to account for unequal sampling probability of participants at each clinic. Sex, age and being on ART at the start of anti-tuberculosis treatment were a priori factors included in the adjusted model. All variables with P values <0.3 were then added to the adjusted model and removed if P > 0.3 in the adjusted model. All remaining variables were tested again one by one, and included if P < 0.3 in the adjusted model.
Ethical considerations
The ethics committees of the London School of Hygiene & Tropical Medicine, London, UK (LSHTM reference 9761), The AIDS Support Organization (TASO), Kampala, Uganda (number: TASO-REC/062/15-UG-REC-009) and the Uganda National Council for Science and Technology, Kampala, Uganda (UNCST number: HS 1965) granted ethical approval for the study. Due to the retrospective study design and strict anonymity of participants, the need for patient consent was waived. However, written consent was obtained from TB clinic staff who provided clinic-level data.
RESULTS
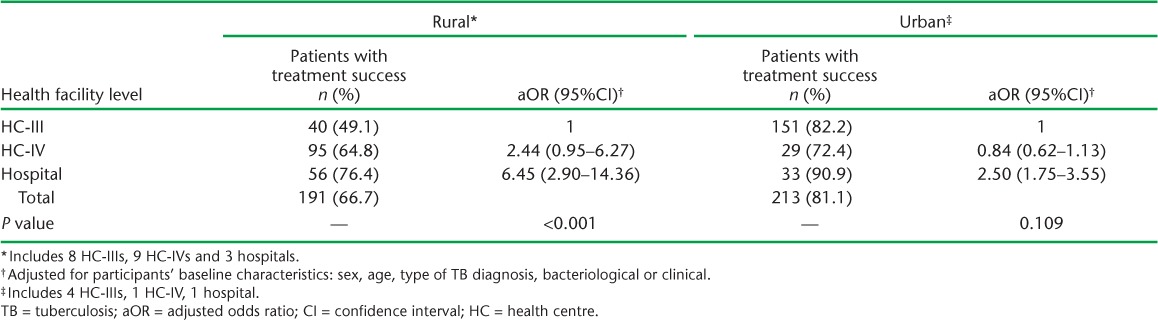
Of 2810 individuals initiated on anti-tuberculosis treatment during the study period in 26 clinics studied, 871 met the inclusion criteria: TB and HIV data were obtained for 191 rural and 213 urban participants (Figure 1). The majority were male (61.1%), and had been prescribed 2RHZE/6EH (92.0%); the mean age was 35 years (standard deviation 10) and 40.7% were on ART at the start of anti-tuberculosis treatment (Table 1). At 2 months, 235 (51.2%) participants had follow-up sputum smear results, 183 (42.4%) at 5 months and 174 (40.9%) at 8 months (Figure 2). Treatment was successful for 81.1% of 213 participants in urban facilities and 66.7% of 191 participants in rural facilities (Table 2). Urban patients were more likely to have achieved treatment success than rural patients (adjusted OR [aOR] 3.95, 95%CI 2.70–5.78, P < 0.01; Table 3). There was no evidence of an independent association of sex, age and ART status with treatment success (Table 3). Analysis of secondary outcomes revealed that 41.4% were cured (17.0% in rural, 65.7% in urban areas), 32.5% completed treatment (49.7% in rural, 15.4% in urban areas), 1% had failed and 8.1% had died, whereas 6.9% of patient outcomes could not be evaluated and 10.1% of the participants had become lost to follow-up (LTFU), with rural clinics having a higher proportion of cases LTFU than urban clinics (16.6% vs. 3.5%; Table 2).
FIGURE 1.
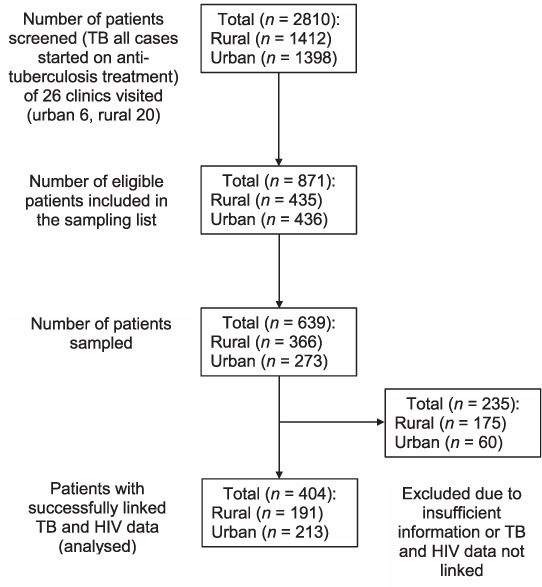
Numbers screened and analysed. TB = tuberculosis; HIV = human immunodeficiency virus.
TABLE 1.
Sociodemographic, clinical and facility characteristics by urban or rural location *
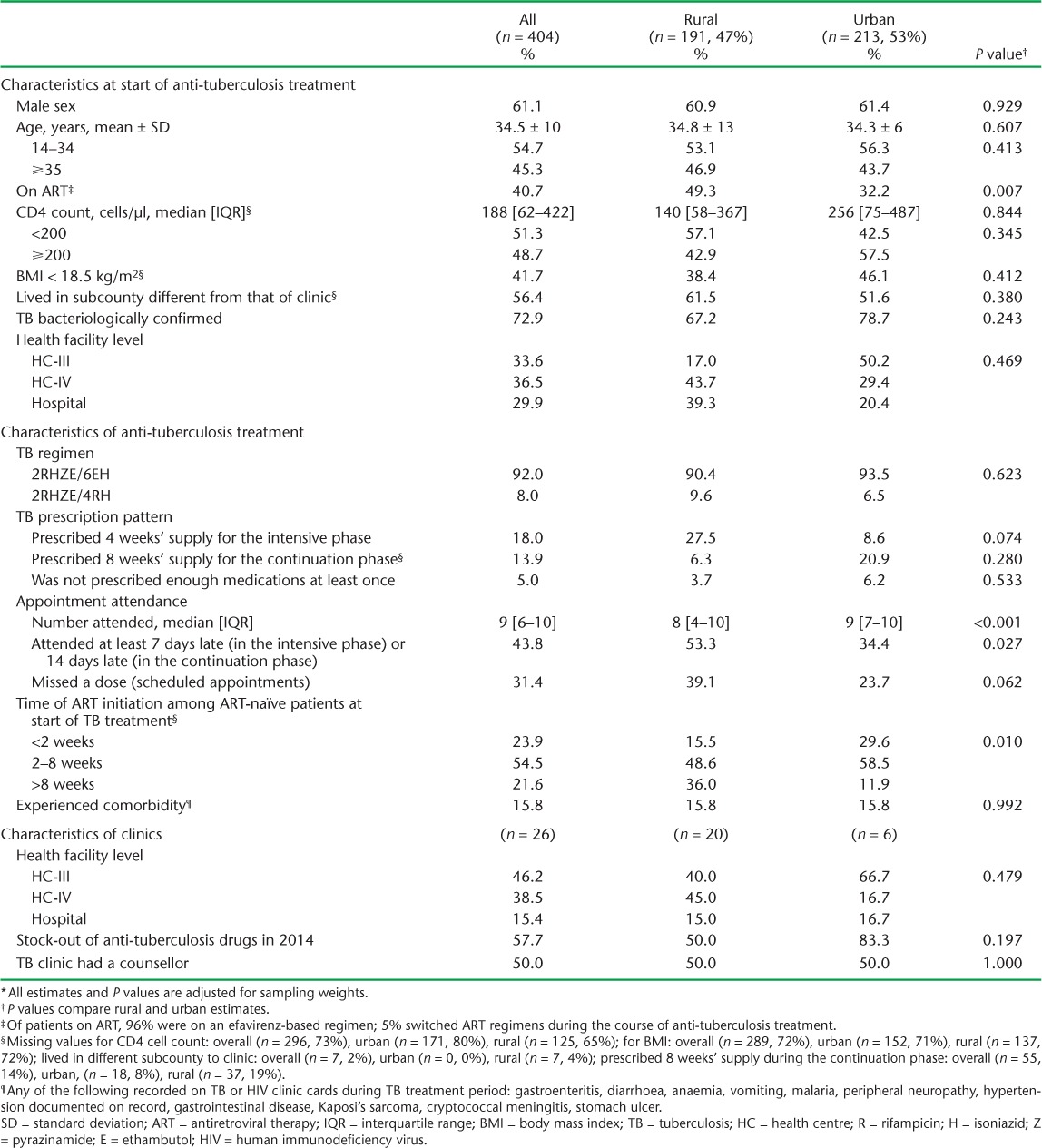
FIGURE 2.
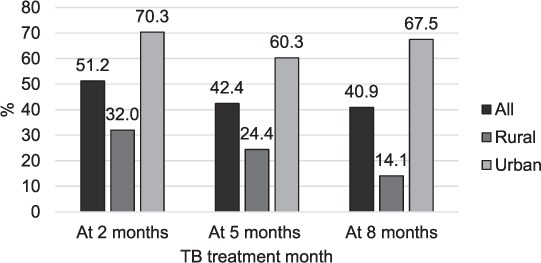
Percentage of patients with sputum smear results recorded in TB registers: all patients (n = 404), rural patients, (n = 191), urban patients (n = 213). Comparison of urban and rural percentage at 2 months P value = 0.003, at 5 months P < 0.001 and at 8 months P < 0.001. TB = tuberculosis.
TABLE 2.
Percentage of patients with anti-tuberculosis treatment outcomes by urban or rural location
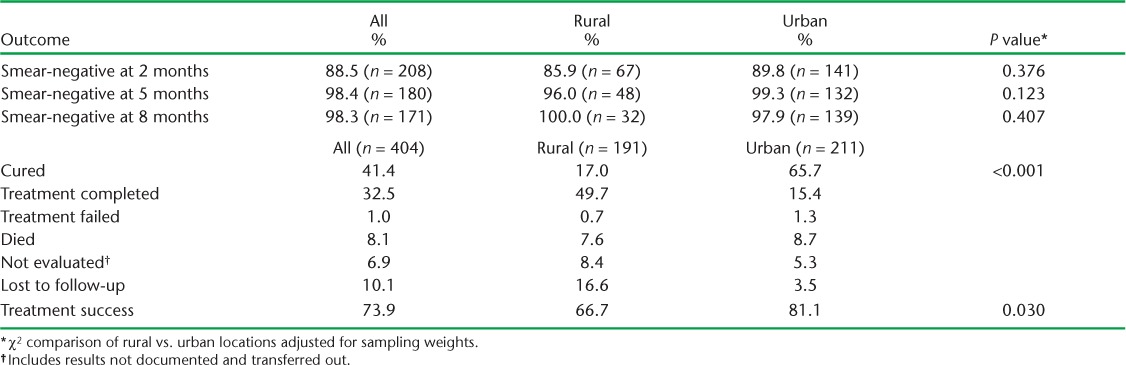
TABLE 3.
Sociodemographic, clinical and facility characteristics associated with TB treatment success by urban or rural location
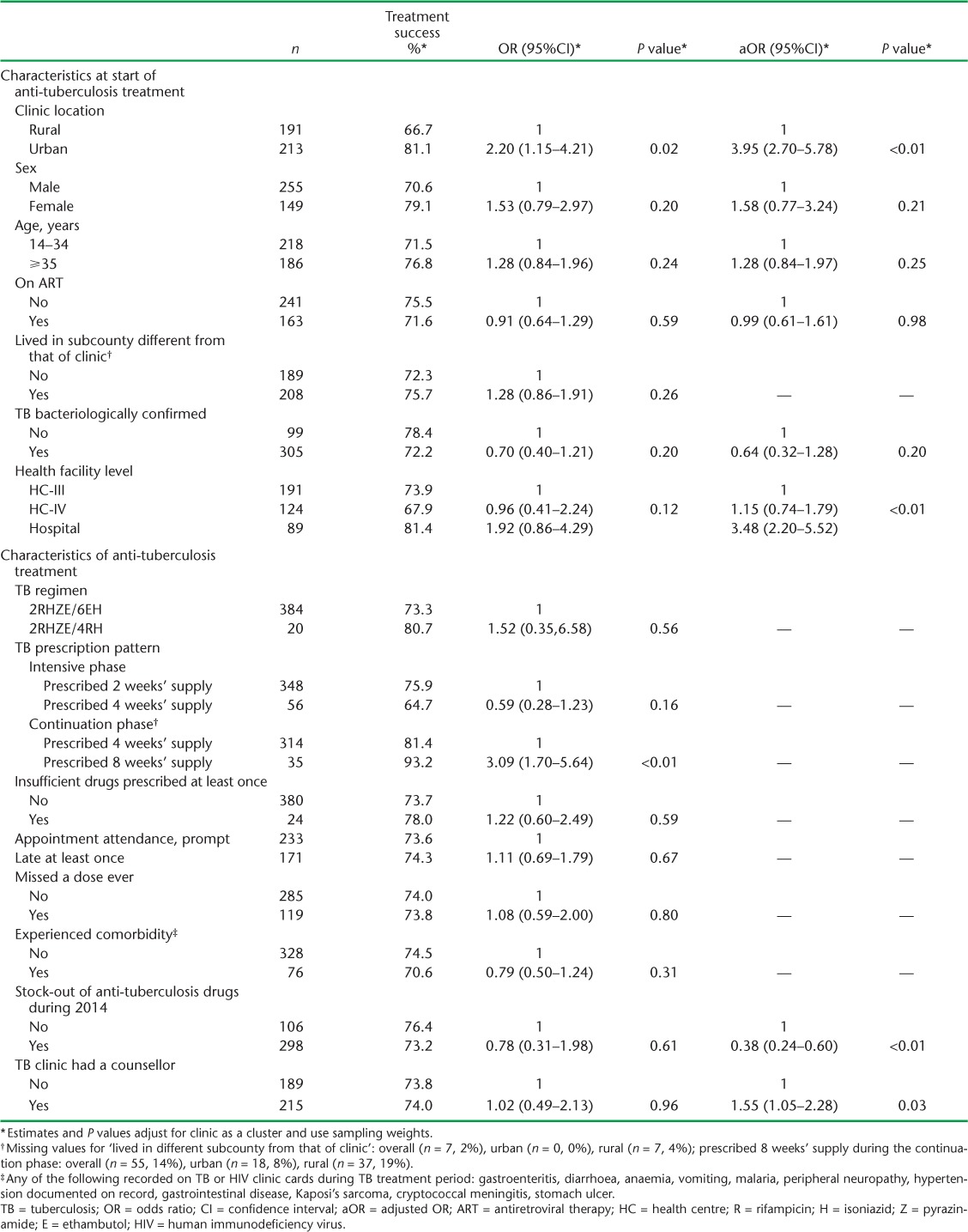
The median number of participants per clinic was nine (inter-quartile range [IQR] 4–13) in rural and 34 (IQR 32–39) in urban facilities. Sputum smear results in TB registers were significantly more likely to be recorded in urban than in rural facilities at all times (P < 0.001), and did not depend on the level of health facility (P = 0.480). Clinic-level treatment success ranged from 72% to 91% in urban and 0% to 100% in rural facilities. The two clinics with 0% success had less than five patients; one had no counsellor and one reported TB drug stock-outs during the period studied. Having a TB drug stock-out was associated with lower treatment success (aOR 0.38, 95%CI 0.24–0.60, P < 0.01), while having a counsellor in the clinic was associated with on average 55% higher odds of treatment success (aOR 1.55, 95%CI 1.05–2.28, P = 0.03; Table 3). Treatment success increased with health facility level in rural areas: 49.1% at HC-III, 64.8% at HC-IV and 76.4% at hospital level (P value for interaction <0.01: Appendix Table A.4). For urban clinics, treatment success was highest in hospitals (90.9%) and lowest in HC-IV facilities (72.4%). TB-HIV integrated services were being implemented in five of the six urban health facilities and nine of the 20 rural health facilities during the study period.
DISCUSSION
TB treatment success rates among PLHIV vary widely among sub-Saharan African countries,15–21 and our study findings fit within this range. Studies that reported better treatment success rates than our study had more well-organised community-based DOTS programmes and better patient tracing7,17,22–24 than most rural clinics in our study. The higher proportion of patients reported as LTFU by rural clinics was likely related to patient characteristics: such patients lived further from health facilities, presented later for treatment, were more likely to be malnourished, and had lower CD4 cell counts at diagnosis than their urban counterparts and received less treatment monitoring than urban patients, consistent with other studies in Uganda and elsewhere in sub-Saharan Africa.3,5,8,21,25
Treatment monitoring was key to the improved treatment success in urban clinics. The availability of follow-up sputum smear results was less common for rural patients, despite the presence of microscopes in all facilities. Facilities reported lack of laboratory supplies such as reagents, which limited the performance of sputum testing. In addition, data management was poor in most rural clinics, resulting in missing sputum results and treatment outcomes in TB registers even when the tests were performed. Efforts were made to obtain missing outcome data, but we were limited by what was recorded in the TB registers. A comparable rural Ugandan study found that of 264 511 patient encounters, 1.8% had sputum smear microscopy prescribed, of which 60% underwent a complete evaluation.26 Fewer rural health facilities had TB-HIV integrated care than urban facilities. Rural clinics may have tailored TB appointments to coincide with HIV clinic appointments for HIV patients on anti-tuberculosis treatment, specifically due to the longer distances to the health facility. This could have contributed to poor adherence to anti-tuberculosis treatment among rural patients due to a lack of adequate monitoring.
Stock-outs in the facilities studied were most common for the drug combination administered during the intensive phase (RHZE), and lasted on average for 2 months (maximum 8 months). As in other studies,27 causes of reported stock-outs were delayed supply of drugs from national medical stores and poor forecast of requirements of drugs by the clinics. Solutions reported were borrowing from nearby clinics or issuing alternative drugs. Drug stock-outs of antiretroviral or anti-tuberculosis drugs were also reported in 25% of 2454 South African health facilities studied in 2014.27 Adequate funding and commitment among the stakeholders responsible for procurement, custody and issuing of drugs may reduce stock-outs of essential drugs in public health facilities in sub-Saharan Africa.27
Furthermore, the presence of counsellors in the team of TB clinic staff is undoubtedly related to better-resourced—and probably higher level—clinics. Counselling services have been previously shown to improve treatment success.9 The higher treatment success rates in urban sites may be attributable to programmes being implemented in KCCA clinics (TB Care 128 and TRACK-TB [project ongoing until 2017]), but not in rural clinics. These programmes aimed to improve the quality of TB treatment care, adherence, treatment outcomes, data quality/documentation and drug management. The reports from such programmes have shown significant improvements in documentation in patients' medical records as a result of TB staff training and support supervision.28 Such programmes should be extended to rural settings to achieve nationwide improvements in treatment success. The interrelationship between patient-level and clinic-level factors highlights the obstacles to improving treatment success.29,30
Both a strength and a weakness of this study was that it used data routinely collected from public health facilities at primary health care level. The inclusion of public health facilities from outlying rural areas provided good representation of routine TB care in Uganda, although routine hospital data may suffer from incomplete recording, resulting in missing data, including outcome data. We correctly analysed the data using sampling weights to account for multiple site sampling. Because we restricted data collection to clinics supported by IDI and to patients with linked TB-HIV data, this may have limited the generalisability of our findings to clinics that were not providing these services. This may have led to an estimation of greater treatment success than would be expected in non-IDI clinics. However, this should not affect the urban-rural comparison, as this restriction was imposed in both areas. Furthermore, many proxy variables had to be used, for example distance to clinic (not collected in data sources used), which was approximated by residence in another subcounty. Linkage of TB and HIV health records was less likely in rural settings, among males and among LTFU patients, which may have overestimated treatment success due to the exclusion of high numbers of LTFU patients that would otherwise be categorised as unfavourable outcomes.
CONCLUSION
The lower reported treatment success rates in rural clinics are likely a combination of patient-centred factors, such as late presentation and distance to clinic, and clinic-centred factors, such as staff unavailability for treatment monitoring and follow-up. We recommend the reinforcement of community-based TB treatment, especially in rural settings, active tracing of patients who miss appointments and increased staff training on treatment monitoring and delivery, and good data management.
Acknowledgments
This research was funded by a European & Developing Countries Clinical Trial Partnership Master's Training Fellowship for JM (grant number MF.2013.40205.025). AMR and JB are additionally supported by the UK Medical Research Council through a grant to the LSHTM Tropical Epidemiology Group, London, UK (grant code MR/K012126/1).
The authors would like to thank the health centres and staff for their support with data collection, research assistants O Nampewo, I Nabwire, B Oribakiriho and G Anguzu, and the Infectious Disease Institute, Kampala, Uganda, for supporting JM in applying for this fellowship and providing their facilities.
APPENDIX
TABLE A.1.
List of sampled health facilities
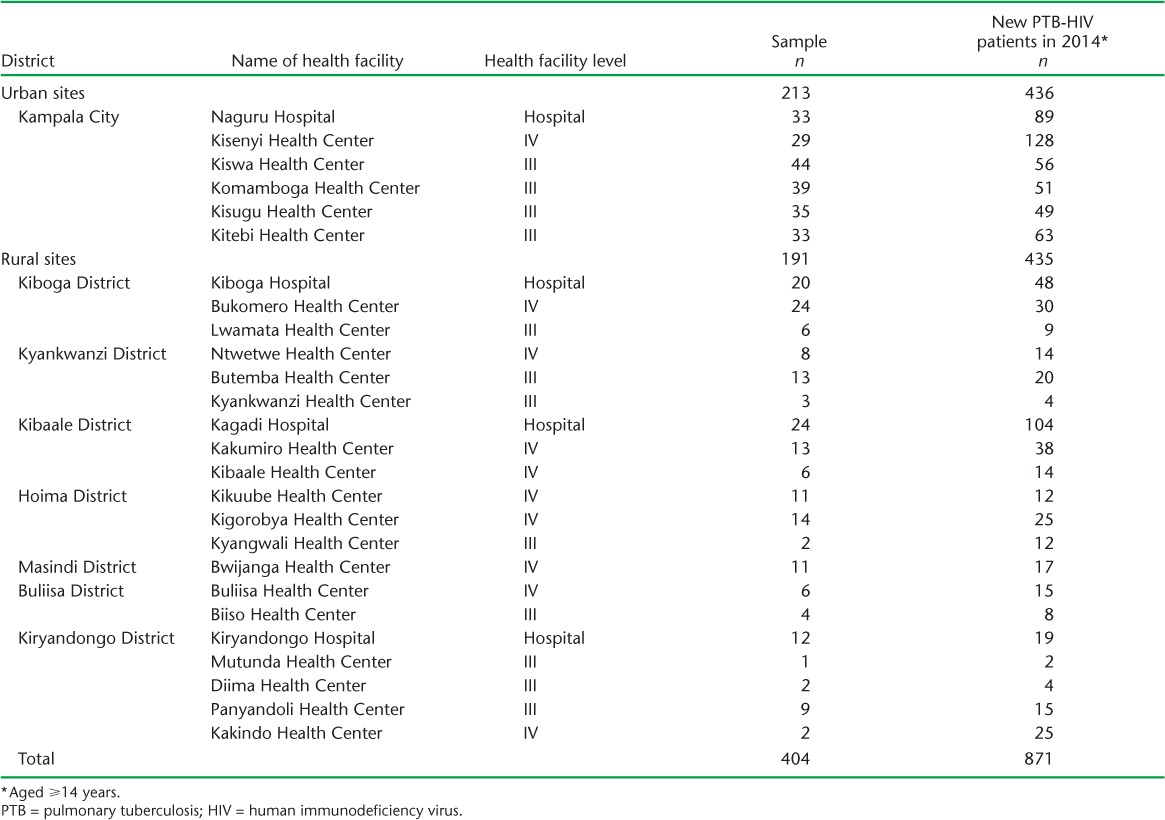
TABLE A.2.
Districts in Uganda and accredited government health facilities for HIV care where the Infectious Diseases Institute (Kampala, Uganda) offered support up to May 2015

TABLE A.3.
Definition of key terms taken from WHO guidelines1
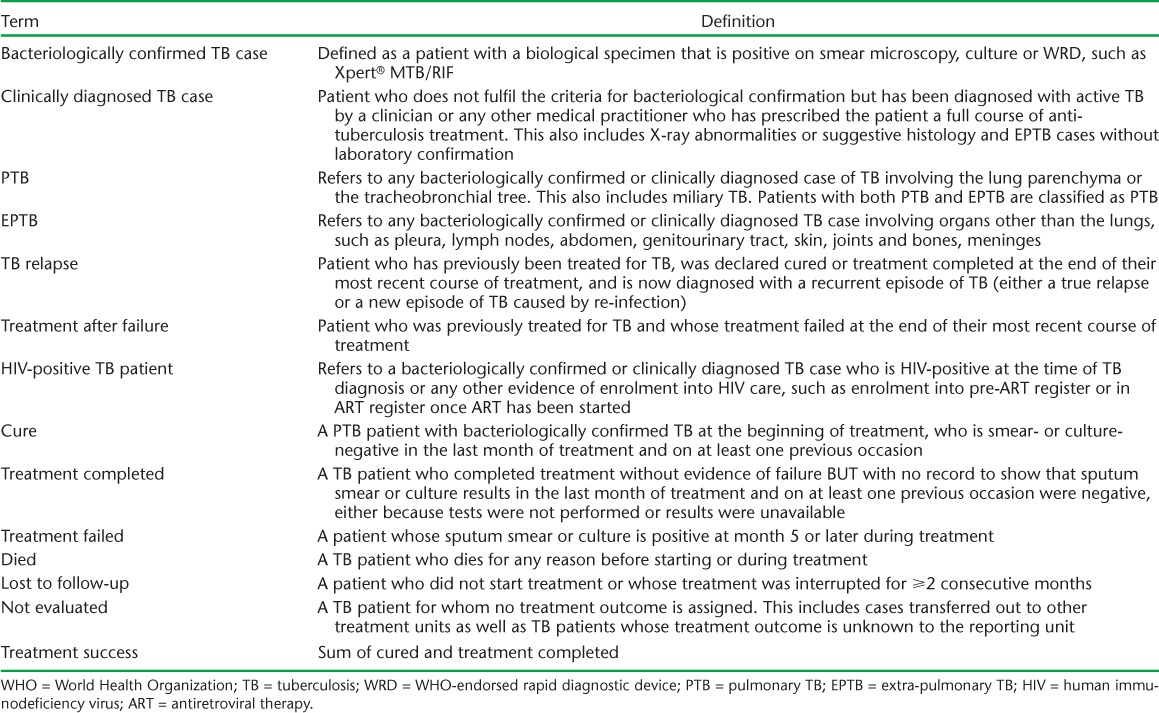
TABLE A.4.
TB treatment success across health facility level stratified by rural/urban
References
- 1. World Health Organization. . Definitions and reporting framework for tuberculosis: 2013 revision. WHO/HTM/TB/2013.2 Geneva, Switzerland: WHO, 2014. [Google Scholar]
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Republic of Uganda Ministry of Health. . The HIV and AIDS Uganda Country Progress Report 2014. Kampala, Uganda: Ministry of Health, 2015. [Google Scholar]
- 3. Ukwaja K N, Oshi S N, Alobu I, Oshi D C.. Profile and determinants of unsuccessful tuberculosis outcome in rural Nigeria: implications for tuberculosis control. World J Methodol 2016; 6: 118– 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zenebe Y, Adem Y, Mekonnen D, . et al. Profile of tuberculosis and its response to anti-TB drugs among tuberculosis patients treated under the TB control programme at Felege-Hiwot Referral Hospital, Ethiopia. BMC Public Health 2016; 16: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Achen E. Improving tuberculosis treatment completion rates: experience from Gulu Regional Referral Hospital, Uganda. Chevy Chase, MD, USA: University Research Co, 2012. [Google Scholar]
- 6. Nuwaha F. Factors influencing completion of treatment among tuberculosis patients in Mbarara District, Uganda. East Afr Med J 1997; 74: 690– 693. [PubMed] [Google Scholar]
- 7. Adatu F, Odeke R, Mugenyi M, . et al. Implementation of the DOTS strategy for tuberculosis control in rural Kiboga District, Uganda, offering patients the option of treatment supervision in the community, 1998–1999. Int J Tuberc Lung Dis 2003; 7 Suppl 1: S63– S71. [PubMed] [Google Scholar]
- 8. Cattamanchi A, Miller C R, Tapley A, . et al. Health worker perspectives on barriers to delivery of routine tuberculosis diagnostic evaluation services in Uganda: a qualitative study to guide clinic-based interventions. BMC Health Serv Res 2015; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bulage L, Sekandi J, Kigenyi O, Mupere E.. The quality of tuberculosis services in health care centres in a rural district in Uganda: the providers' and clients' perspective. Tuberc Res Treat 2014; 2014: 685982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olle-Goig J E. Tuberculosis in rural Uganda. Afr Health Sci 2010; 10: 226– 229. [PMC free article] [PubMed] [Google Scholar]
- 11. Republic of Uganda Ministry of Health. . National antiretroviral treatment and care guidelines for adults and children. Kampala, Uganda: MoH, 2008. [Google Scholar]
- 12. Republic of Uganda Ministry of Health. . Addendum to the national antiretroviral treatment guidelines. Kampala, Uganda: MoH, 2013. [Google Scholar]
- 13. Republic of Uganda Ministry of Health. . Manual of the National Tuberculosis and Leprosy Programme. Kampala, Uganda: MoH, 2010. [Google Scholar]
- 14. World Health Organization. . Global tuberculosis report, 2013. WHO/HTM/TB/2013.11 Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 15. Endris M, Moges F, Belyhun Y, Woldehana E, Esmael A, Unakal C.. Treatment outcome of tuberculosis patients at Enfraz health center, northwest Ethiopia: a five-year retrospective study. Tuberc Res Treat 2014; 2014: 726193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gebremariam G, Asmamaw G, Hussen M, . et al. Impact of HIV status on treatment outcome of tuberculosis patients registered at Arsi Negele Health Center, Southern Ethiopia: a six year retrospective study. PLOS ONE 2016; 11: e0153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobson K B, Moll A P, Friedland G H, Shenoi S V.. Successful tuberculosis treatment outcomes among HIV/TB coinfected patients down-referred from a district hospital to primary health clinics in rural South Africa. PLOS ONE 2015; 10: e0127024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minaleshewa B, Belay Y, Hailay A, Senafekesh B, Fisseha Z A.. Treatment outcomes of tuberculosis and associated factors in an Ethiopian university hospital. Adv Public Health 2016; 2016 http://www.academia.edu/26690106/Treatment_Outcomes_of_Tuberculosis_and_Associated_Factors_in_an_Ethiopian_University_Hospital. Accessed April 2017. [Google Scholar]
- 19. Onyebuchi S O, Bethrand B O.. Treatment outcome of tuberculosis patients at National Hospital Abuja, Nigeria: a five year retrospective study. S Afr Fam Pract 2015; 57: 50– 56. [Google Scholar]
- 20. Shaffer D N, Obiero E T, Bett J B, . et al. Successes and challenges in an integrated tuberculosis/HIV clinic in a rural, resource-limited setting: experiences from Kericho, Kenya. AIDS Res Treat 2012; 2012: 238012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yimer S A, Bjune G A, Holm-Hansen C.. Time to first consultation, diagnosis and treatment of TB among patients attending a referral hospital in Northwest, Ethiopia. BMC Infect Dis 2014; 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gebrezgabiher G, Romha G, Ejeta E, Asebe G, Zemene E, Ameni G.. Treatment outcome of tuberculosis patients under directly observed treatment short course and factors affecting outcome in Southern Ethiopia: a five-year retrospective study. PLOS ONE 2016; 11: e0150560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melese A, Zeleke B, Ewnete B.. Treatment outcome and associated factors among tuberculosis patients in Debre Tabor, Northwestern Ethiopia: a retrospective study. Tuberc Res Treat 2016; 2016: 1354356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurt A O, Sasmaz T, Bugdayci R, Oner S, Yapici G, Ozdemir O.. A five year retrospective surveillance; monitoring and evaluation for the regional tuberculosis control programme in Mersin, Turkey, 2004–2008. Cent Eur J Public Health 2012; 20: 144– 149. [DOI] [PubMed] [Google Scholar]
- 25. Gebreegziabher S B, Bjune G A, Yimer S A.. Total delay is associated with unfavorable treatment outcome among pulmonary tuberculosis patients in West Gojjam Zone, Northwest Ethiopia: a prospective cohort study. PLOS ONE 2016; 11: e0159579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross J M, Cattamanchi A, Miller C R, . et al. Investigating barriers to tuberculosis evaluation in Uganda using geographic information systems. Am J Trop Med Hyg 2015; 93: 733– 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Treatment Action Campaign, Doctors without Borders/Médecins San Frontières, Rural Health Advocacy Project, Rural Doctors Association of Southern Africa, SECTION27, Southern Africa HIV Clinician's Society. . Stock outs in South Africa. Second Annual Report: 2014 stock outs survey. Cape Town, South Africa: Treatment Action Campaign, 2015. [Google Scholar]
- 28. TB CARE 1 Project. . Documentation of lessons learnt TB CARE 1 Uganda Project. Washington, DC, USA: USAID, 2013. http://www.tbcare1.org/publications/toolbox/tools/country/Lessons_Learnt_TB%20CARE_I_Uganda_Project.pdf Accessed April 2017. [Google Scholar]
- 29. Herrero M B, Ramos S, Arrossi S.. Determinants of non adherence to tuberculosis treatment in Argentina: barriers related to access to treatment. Rev Bras Epidemiol 2015; 18: 287– 298. [DOI] [PubMed] [Google Scholar]
- 30. Wasti S P, Simkhada P, Randall J, Freeman J V, van Teijlingen E.. Factors influencing adherence to antiretroviral treatment in Nepal: a mixed-methods study. PLOS ONE 2012; 7: e35547. [DOI] [PMC free article] [PubMed] [Google Scholar]


