Abstract
Setting: Patients with multidrug-resistant tuberculosis (MDR-TB) registered for treatment (2011–2012 cohort) using the standard 24-month regimen, under the Revised National TB Control Programme's programmatic management of drug-resistant TB (PMDT), Maharashtra, India.
Objectives: To assess the treatment outcomes and the timing and risk factors for unfavourable treatment outcomes, with a focus on death and loss to follow-up (LTFU).
Method: This was a retrospective cohort study involving a review of PMDT records. Treatment outcomes were reported on 31 December 2014.
Results: Of 4024 patients, treatment success was recorded in 1168 (29%). Unfavourable outcomes occurred in 2242 (56%), of whom 857 (21%) died and 768 (19%) were lost to follow-up. Treatment outcomes were missing on record review for 375 (9%) patients, and 239 (6%) were still undergoing treatment. Half of LTFU occurred within 3 months, and more than four fifths of deaths occurred after 6 months of treatment. Human immunodeficiency virus infection, being underweight, age ⩾ 15 years, male sex and pulmonary TB were the main risk factors for death, LTFU or other unfavourable treatment outcomes.
Conclusion: The study found poor treatment outcomes in patients with MDR-TB registered for treatment in Maharashtra, India. Interventions are required to address the high rates of LTFU and death.
Keywords: multidrug-resistant tuberculosis, loss to follow-up, death, operational research, India
Abstract
Contexte: Les patients atteints d'une tuberculose multi-résistante (TB-MDR) enregistrés en vue d'un traitement (cohorte de décembre 2011) recourant au protocole standard de 24 mois de la prise en charge programmatique de la TB pharmacorésistante (PMDT) sous l'égide du Programme national révisé de lutte contre la TB, à Maharashtra, Inde.
Objectifs: Evaluer les résultats du traitement, et le timing et les facteurs de risque de résultats défavorables du traitement en particulier, le décès et les pertes de vue.
Méthode: Etude rétrospective de cohorte impliquant la revue des dossiers du PMDT. Les résultats du traitement ont été rapportés au 31 décembre 2014.
Résultats: Sur 4024 patients, 1168 (29%) patients ont connu un succès, tandis que 2242 (56%) ont eu un résultat défavorable : 857 (21%) sont décédés et 768 (19%) ont été perdus de vue. Les résultats du traitement ont été manquants lors de la revue des dossiers pour 375 (9%) patients et 239 (6%) patients étaient toujours sous traitement. La moitié des pertes de vue est survenue dans les 3 mois et plus de quatre décès sur cinq sont survenus après 6 mois de traitement. Le virus de l'immunodéficience humaine, la maigreur, l'âge ⩾ 15 ans, le sexe masculin et la TB pulmonaire ont été des facteurs de risque de décès ou de perte de vue ou de résultat défavorable du traitement.
Conclusion: Cette étude a découvert des résultats médiocres du traitement chez des patients atteints de TB-MDR enregistrés pour traitement à Maharashtra, Inde. Des interventions sont requises pour combattre ce taux élevé de pertes de vue et de décès.
Abstract
Marco de referencia: Los pacientes con diagnóstico de tuberculosis multidrogorresistente (TB-MDR) registrados para tratamiento (cohorte de 2011 y 2012) con el régimen normalizado de 24 meses del tratamiento programático de la TB farmacorresistente (PMDT, por su equivalente en inglés) en el marco del Programa Nacional Revisado contra la TB en Maharashtra, en la India.
Objetivos: Evaluar los desenlaces terapéuticos, la evolución cronológica y los factores de riesgo de alcanzar un desenlace desfavorable, en especial la muerte y la pérdida durante el seguimiento.
Método: Un estudio retrospectivo de cohortes con análisis de los registros del PMDT. Los desenlaces terapéuticos se notificaron el 31 de diciembre del 2014.
Resultados: De los 4024 pacientes tratados, 1168 alcanzaron el éxito terapéutico (29%). Se observaron desenlaces desfavorables en 2242 pacientes (56%), así: 857 fallecieron (21%) y 768 se perdieron durante el seguimiento (19%). En el examen de los registros, faltaba el desenlace terapéutico de 375 pacientes (9%) y 239 estaban aun recibiendo tratamiento (6%). La mitad de las pérdidas durante el seguimiento ocurrió durante los primeros 3 meses y más de cuatro quintos de las muertes ocurrieron después de 6 meses de tratamiento. Los factores que se asociaron con la muerte, la pérdida durante el seguimiento u otros desenlaces desfavorables fueron la infección por el virus de la inmunodeficiencia humana, el peso insuficiente, la edad ⩾ 15 años, el sexo masculino y la TB de localización pulmonar.
Conclusión: En el presente estudio se observaron desenlaces terapéuticos deficientes en los pacientes registrados en tratamiento por TB-MDR en Maharashtra, en la India. Es necesario introducir intervenciones que aborden la alta tasa de pérdida durante el seguimiento y de muertes.
Globally, multidrug-resistant tuberculosis (MDR-TB) poses a major threat to ongoing efforts for TB control. There were 580 000 estimated new cases of MDR-TB and rifampicin (RMP) resistant TB worldwide in 2015; among these, 125 000 (20%) were enrolled into treatment. The MDR-TB treatment success rate was 52% for the 2013 cohort worldwide.1 India, China and the Russian Federation accounted for 45% of all estimated MDR-TB and RMP-resistant TB cases (henceforth referred to as MDR-TB). India, a TB high-burden country, has an estimated 79 000 MDR-TB cases among all notified pulmonary TB cases.1
The Revised National TB Control Programme (RNTCP) of India has made substantial progress in diagnosing and treating MDR-TB since 2007 through the Programmatic Management of Drug-resistant TB (PMDT). Accelerated scale-up of PMDT was implemented in all districts in India by March 2013. Laboratory-confirmed patients with MDR-TB are initiated on a standardised 24–27-month regimen for drug-resistant TB (DR-TB).2 The most recent recorded national treatment success rate, for the 2013 cohort, is 46%.1,3
Maharashtra, situated in western India, is the second largest state in India, with a population of 118 million.4 There are 79 district-level reporting units under the RNTCP,5 which has its headquarters in Mumbai, the city with the highest burden of MDR-TB in India.6
In this article we report on the treatment outcomes, and the timing and risk factors for unfavourable treatment outcomes, with a focus on death and loss to follow-up (LTFU) among a large cohort of patients with MDR-TB registered for treatment between 2011 and 2012 using the standard 24-month regimen under the PMDT in Maharashtra state, India.
METHODS
Study design and population
This was a retrospective cohort study involving a review of PMDT records of patients with MDR-TB registered for treatment between January 2011 and December 2012 at all DR-TB centres (n = 11) across Maharashtra state, India.
Study setting
MDR-TB was diagnosed in 10 culture and drug susceptibility testing (C-DST) laboratories certified under the RNTCP for the following World Health Organization (WHO) endorsed diagnostics—Xpert® MTB-RIF (Cepheid, Sunnyvale, CA, USA), line probe assay (LPA) and liquid and solid culture DST—for the majority of patients and in four Xpert standalone laboratories initiated in 2012. Follow-up culture was performed using liquid or solid media. Sputum samples from presumptive MDR-TB cases and laboratory-confirmed patients with MDR-TB were transported from the collection centres of all the districts to the linked laboratories, either by courier agency or by programme personnel, in properly packed Falcon tubes maintained in cold chain under a bio-safety environment. Since 2015, five laboratories have also begun to offer baseline second-line DST in liquid culture.
All laboratory confirmed patients with MDR-TB underwent pre-treatment evaluation and were initiated on a standardised treatment regimen at 11 DR-TB centres suitably upgraded with airborne infection control measures and managed by a team of trained clinical specialists. After an initial short period of hospitalisation, patients were discharged to continue the remainder of their standard DR-TB treatment on an ambulatory basis. The duration of treatment was 24–27 months, which consisted of ⩾6 months and a maximum of 9 months of an intensive phase (IP) of kanamycin, levofloxacin, ethionamide, pyrazinamide, ethambutol and cycloserine, followed by an 18-month continuation phase (CP) of levofloxacin, ethionamide, ethambutol and cycloserine. The patients were monitored by sputum culture examination at 3, 4, 5 and 6 months during the IP and quarterly in the CP at 7, 9, 12, 15, 18, 21 and 24 months.2 Patients were reviewed after 6 months and treatment was shifted to the CP if the most recent culture result was culture-negative. If the most recent culture was positive at the end of 6 months of treatment (4 months with solid culture; 5 months with liquid culture, in some cases), the IP was extended by 1 month; a further extension beyond 1 month was based on the culture results at 5, 6 and 7 months as the results became available. The IP could be extended for a maximum of 3 months based on positive culture results at 4, 5, 6 and 7 months.
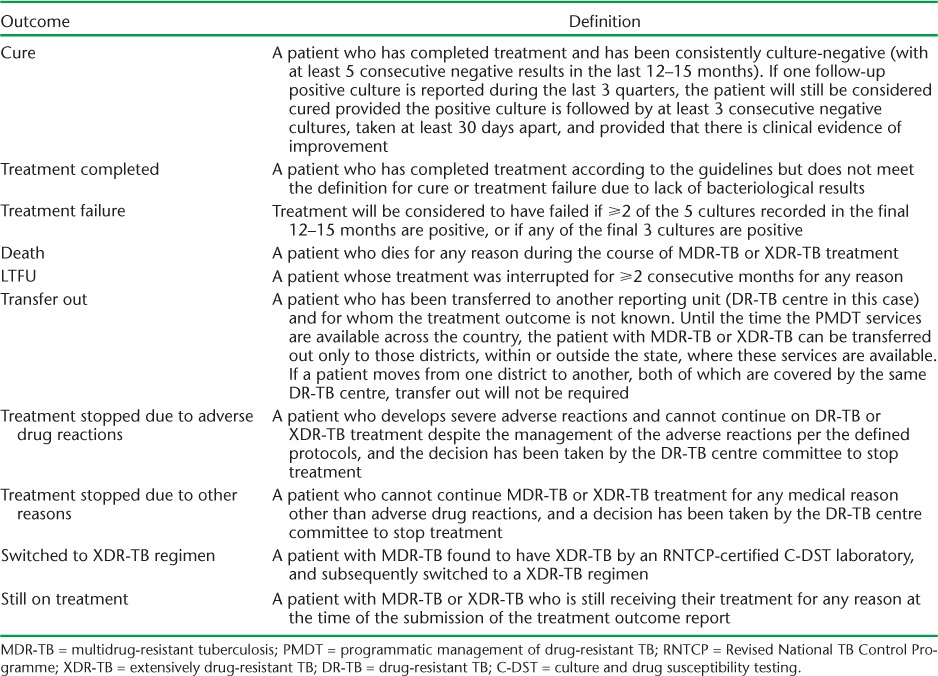
Treatment outcomes were defined as per the 2012 national PMDT guidelines (Table 1).2 ‘Cured’ and ‘treatment completed’ were classified as treatment success. The 2014 revised WHO MDR-TB treatment outcomes were adopted in the national PMDT guidelines in 2016. 7
TABLE 1.
MDR-TB treatment outcomes according to PMDT guidelines, RNTCP, India2
Variables and source of data
Data were collected from the PMDT treatment registers maintained at all the DR-TB centres of Maharashtra between April and June 2015. The variables included registration number, DR-TB centre, date of registration, age, sex, weight, human immunodeficiency virus (HIV) status, site of TB, previous TB category, treatment outcome and date of outcome. The time between diagnosis and treatment registration was derived in days from the respective dates. Treatment outcomes were reported as of 31 December 2014.
Data management and analysis
Data were collected and entered by the statistical assistants of all the DR-TB centres, with support from the district DR-TB supervisors, under the supervision of the district TB officer or the medical officer in charge. All of the staff were trained in data entry. The data from each DR-TB site were double-entered, validated, appended and analysed using EpiData v. 3.1 for entry and validation and v. 2.2.2.183 for appending and analyses (EpiData Association, Odense, Denmark).
The treatment outcomes among all registered patients were summarised using frequencies and proportions. Death and LTFU were stratified by treatment phase (<3 months, 3–6 months, 6–24 months and end of treatment). Risk factors were identified separately for unfavourable treatment outcomes, death and LTFU. Those with treatment outcomes ‘missing (not recorded)’ and ‘still on treatment’ were excluded from the risk factor analysis. Unadjusted relative risks and 95% confidence intervals (CI) were used to summarise and infer the associations after removing those records with variables of interest missing. We decided against performing a regression analysis due to the significant amount of data missing for key exposure variables.
Ethics
Ethics approval was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and the Ethics Committee of the National Tuberculosis Institute (Bengaluru, India). As this study involved retrospective record review of routinely collected programme data, the need for informed consent was waived. Permission from the TB state programme manager of Maharashtra was obtained for the conduct of the study.
RESULTS
Treatment outcomes and timing are shown in the Figure. Of 4024 patients, 828 (21%) were declared cured and 340 (8%) as treatment completed, 857 (21%) died during treatment, 768 (19%) were lost to follow-up, 98 (2%) failed treatment and 190 (5%) were switched to Category V treatment after being diagnosed with extensively drug-resistant TB (XDR-TB). Treatment was stopped due to adverse drug reactions for 3 patients (<1%) and due to other causes in 29 (<1%); 672 (16%) patients were not evaluated, 297 (7%) after transfer out and 375 (9%) who were not recorded (missing). Treatment was still ongoing for 239 patients (6%). Overall, treatment success was observed in 29% (1168/4024) of the patients.
FIGURE.
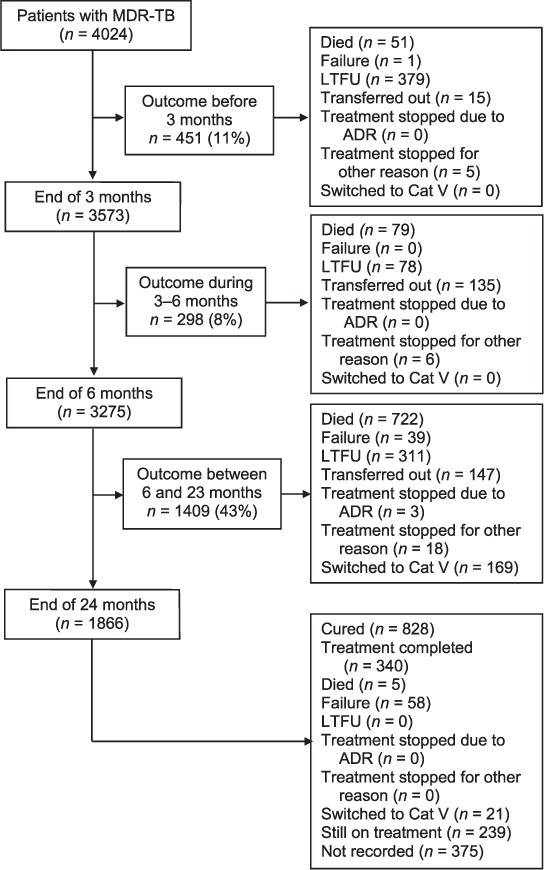
Flowchart describing the timing of treatment outcomes of patients with MDR-TB registered for treatment in Maharashtra, India, 2011–2012. MDR-TB = multidrug-resistant tuberculosis; LTFU = loss to follow-up; ADR = adverse drug reactions: Cat = category.
Among patients with dates available (n = 3614), the median (interquartile range [IQR]) time in days to register after diagnosis was 40 (IQR 24–72), with 6% of patients (232/3614) being registered within 14 days.
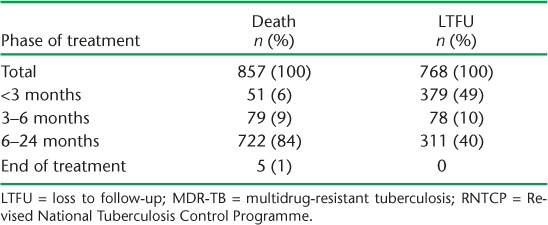
Table 2 summarises the timing of the deaths and LTFU during treatment. Among the 768 LTFU patients, 379 (49%) occurred within the first 3 months. Among the 857 patients who died, 722 (84%) deaths occurred in the continuation phase.
TABLE 2.
Timing of deaths and LTFU among patients with MDR-TB registered for treatment under the RNTCP between 2011 and 2012, Maharashtra, India (N = 4024)
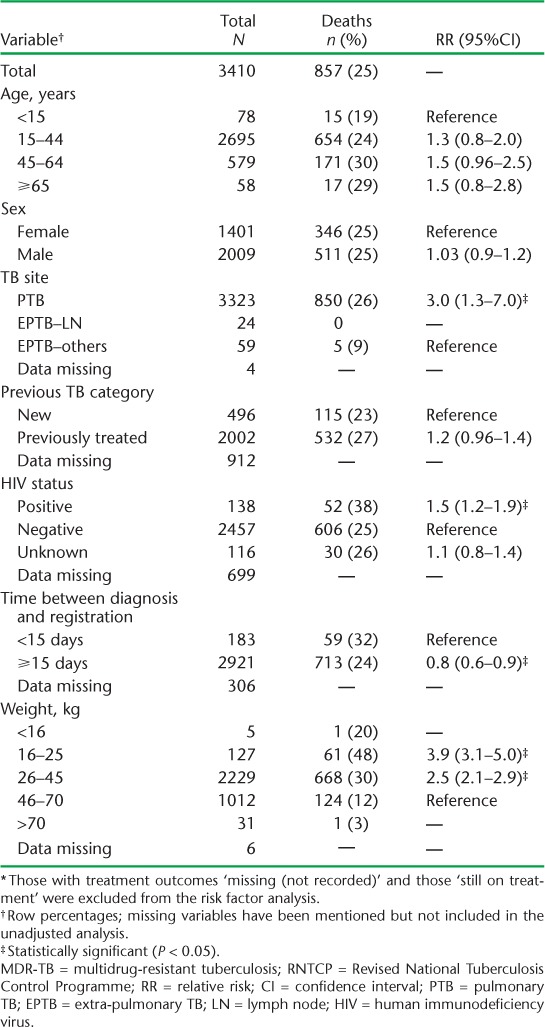
Factors associated with unfavourable outcomes were all age groups ⩾ 15 years (compared to <15 years), male sex (compared to female sex), pulmonary TB (compared to extra-pulmonary TB, no lymph node involvement), and weight bands 16–25 kg and 26–45 kg (compared to >70 kg). Factors associated with death were weight bands 16–25 kg and 26–45 kg (compared to 46–70 kg), pulmonary TB (compared to extra-pulmonary TB, no lymph node involvement) and HIV co-infection. Treatment initiation within 14 days was associated with death. Male sex was associated with LTFU and 16–25 kg weight was associated with a reduced risk for LTFU compared to >70 kg (Tables 3 and 4).
TABLE 3.
Risk factors associated with unfavourable outcomes * among patients with MDR-TB registered for treatment under the RNTCP between 2011 and 2012, Maharashtra, India (n = 3410)
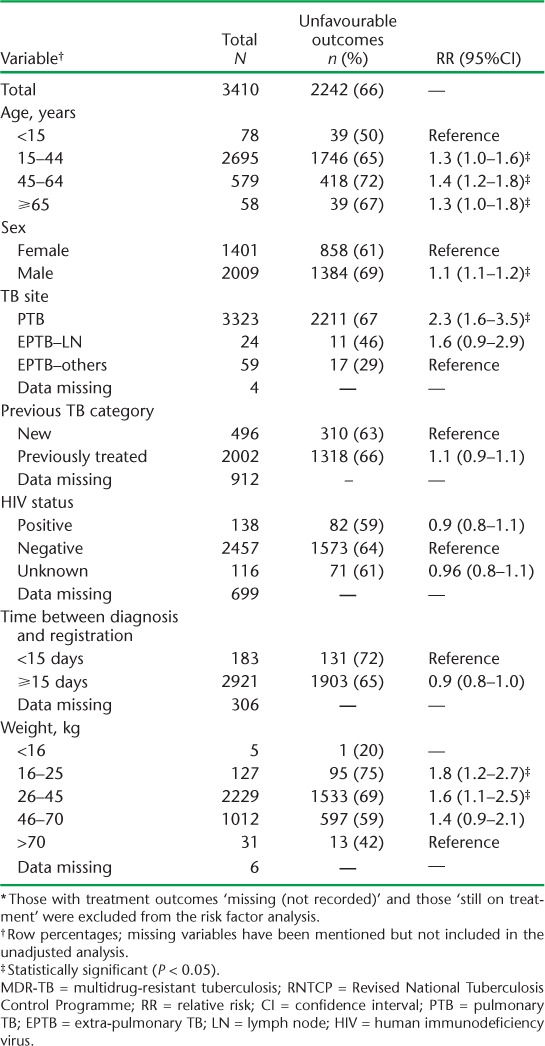
TABLE 4.
Risk factors associated with death among patients with MDR-TB registered for treatment under the RNTCP between 2011 and 2012, Maharashtra, India (n = 3410) *
DISCUSSION
In this large cohort of patients with MDR-TB in Maharashtra, India, the turnaround times to registering after diagnosis were long and there was a high proportion of unfavourable treatment outcomes. More than two thirds of unfavourable outcomes were due to death and LTFU. Significant numbers of patients were lost to follow-up in the first 3 months of treatment. Risk factors associated with unfavourable treatment outcomes were identified.
Strengths and limitations
This was a very large cohort that included all patients registered for DR-TB treatment over a period of 2 years in one of the largest states of India. As we studied the entire population of patients without any sampling, the results are likely to be representative and reflect the reality on the ground. Double data entry and validation ensured quality-assured, robust data. This operational research was conducted without additional funding within existing programme settings. We followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines to report the findings of the study.8
Record review studies have inherent limitations, but the records in the RNTCP are monitored and supervised, which includes periodic data validation. Despite this, the key limitation was the fact that a significant number of records were missing for exposure variables (Tables 2–4) and treatment outcomes (n = 375). This prevented us from utilising the entire cohort in our analysis and from performing regression analyses for adjusted analysis. The fact that second-line DST was performed among MDR-TB patients only if they satisfied the criteria for presumptive XDR-TB is a major limitation. Those still on treatment were excluded from risk factor analyses (n = 239). The RNTCP reports 31–33 month end-of-treatment outcomes for patients with MDR-TB. Of the 239 patients, most (n = 233) had not completed a period of 31–33 months after registration as of 31 December 2014. We used date of registration, as the date of treatment initiation was not uniformly available.
Key findings
Overall, the treatment success rate of 29% for Maharashtra state was very low compared to the WHO-reported global estimate of 52% and the national figure of 46% for India.1,3 The WHO 2015 target is for at least 75% treatment success for MDR-TB.9 Similar low treatment success rates were reported from Tamil Nadu state, in southern India, in the range of 30–41%.10
TABLE 5.
Risk factors associated with LTFU among patients with MDR-TB registered for treatment under the RNTCP between 2011 and 2012, Maharashtra, India (n = 3410) *
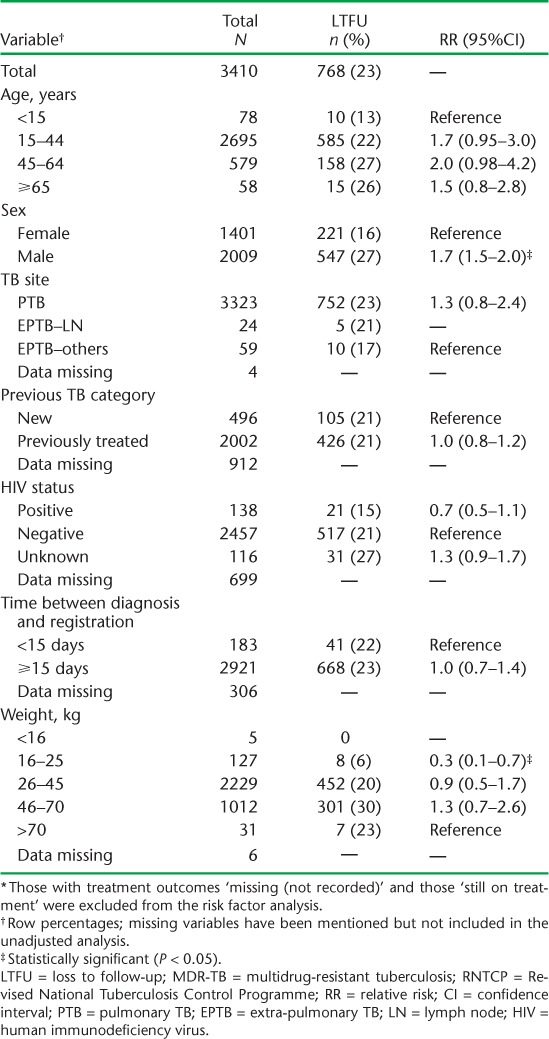
Six of 10 LTFU cases occurred in the intensive phase of treatment, the majority within the first 3 months. Considering the high proportions of patients lost to follow-up early during treatment, there is a need for counselling for patients undergoing DR-TB treatment, at least in the intensive phase of treatment. Eight of 10 deaths occurred in the continuation phase of treatment.
The risk factors identified for unfavourable treatment outcomes in the study require special attention within the programme. Poor treatment outcomes among a cohort of HIV co-infected patients have been described in studies from Mumbai, India, and Johannesburg, South Africa.11,12 Malnutrition has also been associated with unfavourable treatment outcomes.13,14 Timely initiation of antiretroviral therapy (ART) or of continuation of ART for patients already on treatment, through effective TB-HIV linkage, nutritional supplementation for patients in low weight groups and treatment adherence support has helped in reducing deaths and LTFU, ensuring a treatment success of 79%.15
Only one in 16 patients were initiated on treatment within 14 days, and this was a risk factor for death. We assume that many of these patients would have been very ill and contributed to a higher risk of death. As previously mentioned, due to missing data, an adjusted analysis was not possible to determine the independent effect. The 16–25 kg weight group was associated with a reduced risk of LTFU. This can be explained by the fact that patients with low baseline weight would have been more closely followed. Deaths were more common than LTFU among this weight group.
In our study, second-line DST was performed among MDR-TB patients only if they satisfied the criteria for presumptive XDR-TB.2 A total of 190 (5.2%) patients were identified as XDR and switched to a Category V XDR-TB regimen. We assume that many patients with undetected XDR-TB either died or were lost to follow-up because second-line DST was not performed at baseline. Given the high number of unfavourable outcomes, from 2015 onwards, all patients with MDR-TB in Maharashtra underwent second-line DST at baseline. As a result, 899 patients with XDR-TB were registered on treatment in Maharashtra alone—42% (899/2130) of total XDR-TB cases detected in the country. According to the RNTCP's 2016 annual report, the baseline for second-line DST has been introduced as a policy for all patients with MDR-TB.3 Future research is required to document its implementation and impact on MDR-TB treatment outcomes.
In May 2016, the WHO recommended a standardised 9–12 month regimen for all patients (excluding pregnant women) with pulmonary MDR-TB that is not resistant to second-line drugs. Clofazimine and linezolid are now recommended as core second-line drugs in the MDR-TB regimen, and bedaquiline and delamanid have been included in a specific subgroup of add-on agents.1,16 At least 23 countries in Asia and Africa have introduced shorter regimens for MDR-TB treatment and have documented high treatment success rates (87–90%) under operational research conditions.1 In India, bedaquiline has been given approval for use for the first 6 months of treatment along with the background 24-month regimen under conditional access through the RNTCP PMDT programme in six referral sites, to assess the safety profile of the drug among the Indian population.3
Policy implications
There are many policy implications from this study. First, there is a need to improve routine data recording at the DR-TB centres in Maharashtra. Second, the programme needs to address the long delays in registering patients. Third, one priority for the PMDT in Maharashtra is to reduce deaths during treatment by addressing high-risk groups, such as those with HIV co-infection, through better implementation of TB-HIV collaborative services, or those with malnutrition through nutritional supplementation; reduce LTFU by providing counselling services for patients with MDR-TB, at least during the intensive phase; and ensure high coverage of baseline DST among patients with MDR-TB to detect underlying fluoroquinolone resistance or XDR-TB. The Union has supported the provision of MDR-TB counsellors at DR-TB centres in Maharashtra state; the effect of this on treatment outcomes needs to be evaluated in the future. The RNTCP has also placed counsellors. Considering the poor outcomes of the current 24-month treatment regimen for MDR-TB in the state, and in the country as a whole, the programme may consider implementing the WHO-recommended shorter MDR-TB regimen, which has found high success rates in other operational settings around the world.16–19
CONCLUSION
This study found poor treatment outcomes in patients with MDR-TB registered for treatment in a large state in India. Interventions are required to address the high rates of LTFU and death for the programme to achieve the target of ending the epidemic of TB by 2030 and 2035, in line respectively with the recently released Sustainable Development Goals and the End TB strategy.20,21
Acknowledgments
The authors would like to acknowledge the support from the Revised National Tuberculosis Control Programme (New Delhi, India) staff who helped us with performing the record review. The study was undertaken as part of the TB Operations Research Training Project conducted by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France), with funding support from The Global Fund (Geneva, Switzerland) through the Axshya Project. This training project was conceived and implemented by The Union South-East Asia Office (New Delhi, India) in collaboration with the Central TB Division of the Ministry of Health and Family Welfare (New Delhi, India), the National TB Institute (Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India, Bangalore, India), the World Health Organization (WHO) India Country Office (New Delhi, India) and the Centers for Disease Control and Prevention Division of TB Elimination (CDC, Atlanta, GA, USA). The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO, The Union or the CDC. We also thank the Department for International Development (London, UK) for funding the Global Operational Research Fellowship Programme at The Union where HDS works as an operational research fellow.
The database, along with the codebook and the programme used for analysis, can be obtained upon request to the corresponding author.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Revised National Tuberculosis Control Programme. . Guidelines on programmatic management of drug-resistant TB (PMDT) in India. New Delhi, India: Ministry of Health and Family Welfare, 2012. [Google Scholar]
- 3. Revised National Tuberculosis Control Programme. . TB India 2016. Annual status report. New Delhi, India: Ministry of Health and Family Welfare, 2016. [Google Scholar]
- 4. Office of Registrar General and Census Commissioner. . Census of India 2011. New Delhi, India: Ministry of Home Affairs, 2011. http://www.censusindia.gov.in/2011-common/census_2011.html Accessed May 2017. [Google Scholar]
- 5. Revised National Tuberculosis Control Porgramme. . TB India 2013. Annual status report. New Delhi, India: Ministry of Health and Family Welfare, 2013. [Google Scholar]
- 6. Mistry N, Tolani M, Osrin D.. Drug-resistant tuberculosis in Mumbai, India: an agenda for operations research. Operations Research for Health Care 2012; 1: 45– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. . Definitions and reporting framework for tuberculosis, 2013 revision. WHO/HTM/TB/2013.2 Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 8. von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P.. The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. . The Global Plan to Stop TB: 2011–2015. WHO/HTM/TB/2010.7 Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 10. Nair D, Navneethapandian P D, Tripathy J P, . et al. Impact of rapid molecular diagnostic tests on time to treatment initiation and outcomes in patients with multidrug-resistant tuberculosis, Tamil Nadu, India. Trans R Soc Trop Med Hyg 2016; 110: 534– 541. [DOI] [PubMed] [Google Scholar]
- 11. Isaakidis P, Paryani R, Khan S, . et al. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLOS ONE 2013; 8: e68869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Umanah T, Ncayiyana J, Padanilam X, Nyasulu P S.. Treatment outcomes in multidrug resistant tuberculosis-human immunodeficiency virus co-infected patients on anti-retroviral therapy at Sizwe Tropical Disease Hospital Johannesburg, South Africa. BMC Infect Dis 2015; 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicks R M, Padayatchi N, Shah N S, . et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis 2014; 18: 1074– 1083. [DOI] [PubMed] [Google Scholar]
- 14. Podewils L J, Holtz T, Riekstina V, . et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect 2011; 139: 113– 120. [DOI] [PubMed] [Google Scholar]
- 15. Meressa D, Hurtado R M, Andrews J R, . et al. Achieving high treatment success for multidrug-resistant TB in Africa: initiation and scale-up of MDR-TB care in Ethiopia—an observational cohort study. Thorax 2015; 70: 1181– 1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. . WHO treatment guidelines for drug-resistant tuberculosis. 2016 update. WHO/HTM/TB/2016.04 Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 17. Kuaban C, Noeske J, Rieder H L, Aït-Khaled N, Abena Foe J L, Trébucq A.. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 2015; 19: 517– 524. [DOI] [PubMed] [Google Scholar]
- 18. Piubello A, Harouna S H, Souleymane M B, . et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis 2015 2014; 18: 1188– 1194. [DOI] [PubMed] [Google Scholar]
- 19. Van Deun A, Maug A K J, Salim M A H, . et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2010; 182: 684– 692. [DOI] [PubMed] [Google Scholar]
- 20. Word Health Organization. . Health in 2015: from Millennium Development Goals (MDGs) to Sustainable Development Goals (SDGs). Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 21. World Health Organization. . The End TB strategy. WHO/HTM/TB/2015.19 Geneva, Switzerland: WHO, 2015. [Google Scholar]


