Abstract
Setting: Adult pulmonary tuberculosis (TB) patients unable to expectorate quality sputum represent a diagnostic challenge. A private hospital in Pakistan routinely performs gastric aspiration in adults with difficulties expectorating.
Objective: To assess the usefulness of gastric specimens (GS) in diagnosing pulmonary TB (PTB) and drug-resistant TB in adult presumptive TB patients unable to expectorate, and to compare the diagnostic yield and sensitivity of smear, culture and the Xpert® MTB/RIF assay.
Design: This was a comparative cross-sectional study based on retrospective record review.
Results: Of 900, 885 and 877 GS tested by smear, Xpert and culture, respectively, interpretable results were obtained for respectively 900 (100%), 859 (97.1%) and 754 (86.0%), with a diagnostic yield of respectively 23.6%, 30.3% and 24.9%. The yield was significantly higher for Xpert in previously treated patients. There were 313 patients with definite TB, defined as positive on Xpert and/or culture. The 82.8% sensitivity of Xpert was significantly higher than that of smear (61.0%) and culture (67.8%).
Conclusion: GS obtained by aspiration under routine programme conditions is useful for detecting TB and drug-resistant TB in adult patients unable to expectorate. Xpert, with its rapid testing, high proportion of interpretable results and better sensitivity, can substantially improve the diagnosis of bacteriologically confirmed TB and rifampicin resistance.
Keywords: Xpert® MTB/RIF, gastric specimen, adult pulmonary TB
Abstract
Contexte: Les patients adultes atteints de tuberculose pulmonaire (TB) incapables d'expectorer des crachats de qualité posent un problème en matière de diagnostic. Un hôpital privé du Pakistan réalise en routine l'aspiration gastrique chez les adultes qui ont du mal à expectorer.
Objectif: Evaluer l'utilité des échantillons gastriques (GS) chez des patients adultes présumés atteints de TB incapables d'expectorer, pour le diagnostic de la TB pulmonaire et pharmacorésistante et pour comparer le rendement diagnostique et la sensibilité du frottis, de la culture et du test Xpert® MTB/RIF.
Schéma: Etude comparative transversale basée sur une revue rétrospective des dossiers.
Résultats: Un total de 900, 885 et 877 GA ont été testés respectivement par frottis, Xpert et culture et des résultats interprétables ont été obtenus chez 900 (100%), 859 (97,1%) et 754 (86,0%) patients avec un rendement diagnostique respectivement de 23,6%, 30,3% et 24,9%. Le rendement a été significativement plus élevé pour l'Xpert chez les patients déjà traités. Il y avait 313 patients avec une TB, définie comme la positivité de l'Xpert et/ou de la culture. La sensibilité de l'Xpert de 82,8% a été significativement plus élevée que le frottis (61,0%) et la culture (67,8%).
Conclusion: Le GS aspiré sous des conditions de routine de programme est utile pour détecter la TB et la TB pharmacorésistante chez des patients adultes incapables d'expectorer. L'Xpert avec un test rapide, la proportion élevée de résultats interprétables et une meilleure sensibilité, peuvent substantiellement améliorer le diagnostic de la TB confirmée par bactériologie et la résistance à la rifampicine.
Abstract
Marco de referencia: El diagnóstico de la tuberculosis (TB) pulmonar se dificulta en los pacientes adultos que no pueden suministrar muestras de esputo de buena calidad. En un hospital privado del Pakistán se practica de manera sistemática la aspiración gástrica en los adultos con dificultad para expectorar.
Objetivo: Evaluar la utilidad del aspirado gástrico (GS) para el diagnóstico de la TB pulmonar farmacorresistente, en los pacientes con presunción de TB que tienen dificultad para expectorar y comparar el rendimiento diagnóstico y la sensibilidad de la baciloscopia, el cultivo y la prueba Xpert® MTB/RIF.
Método: Un estudio transversal comparativo a partir del examen retrospectivo de las historias clínicas.
Resultados: Se examinaron 900 muestras de GS mediante baciloscopia, 885 con la prueba Xpert y 877 por cultivo; se obtuvieron resultados interpretables en 900 (100%), 859 (97,1%) y 754 muestras (86,0%), con un rendimiento diagnóstico de 23,6%, 30,3% y 24,9%, respectivamente. El rendimiento fue significativamente superior con la prueba Xpert en los pacientes con antecedente de tratamiento. El diagnóstico definitivo de TB, definido como un resultado positivo de la prueba Xpert, el cultivo o ambos, se estableció en 313 pacientes. La prueba Xpert exhibió una sensibilidad de 82,8%, que fue significativamente más alta que la sensibilidad de la baciloscopia (61,0%) y la del cultivo (67,8%).
Conclusión: El examen de las muestras de GS en el marco del programa corriente es útil para detectar la TB y la TB farmacorresistente en los pacientes adultos que no pueden expectorar. La prueba Xpert que ofrece un diagnóstico rápido, alta proporción de resultados interpretables y mejor sensibilidad puede mejorar notablemente el diagnóstico con confirmación bacteriológica de la TB y la resistencia a rifampicina.
While the use of rapid molecular testing is increasing worldwide, only 57% of the pulmonary tuberculosis (PTB) cases reported globally in 2015 were bacteriologically confirmed.1 Diagnostic challenges are encountered when patients are unable to expectorate quality sputum, especially when presumptive TB patients are at risk for drug-resistant TB (DR-TB). Different approaches are being studied to improve the quality of expectorated sputum2 or the sensitivity of microscopy examination.3,4 Sputum induction (SI), bronchoalveolar lavage (BAL) and gastric aspiration (GA) are among the most commonly used procedures to obtain specimens for bacteriological diagnosis. Both SI and BAL have been shown to increase the diagnostic yield, and are considered safe procedures.5–8 The availability of isolation rooms with negative pressure required for SI, and the presence of bronchoscopy facilities only in specialised hospital settings, however, hinders the wider use of these procedures in resource-limited settings. GA is commonly used in children to obtain sputum specimens,6,8–13 but is less common in adults with difficulties in expectoration.
Pakistan is among the top five high-burden countries for TB and DR-TB, with an estimated incidence of 270 per 100 000 population for TB and a rifampicin (RMP) resistance rate of 4.2% in new and 16% in previously treated TB cases.1,14,15 In 2015, 63% of estimated incident TB cases were notified, 81% with PTB and 51% bacteriologically confirmed; only 18% of estimated DR-TB cases were enrolled on treatment.1
In Pakistan, direct smear microscopy is the front-line test for PTB diagnosis, offered through a quality-assured network of more than 1400 microscopy laboratories. Smear-negative patients with an X-ray (CXR) result suggestive of TB are started on empiric anti-tuberculosis treatment. Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) was introduced in Pakistan in 2011, soon after its endorsement by the World Health Organization,16 and had been made available at 60 sites by the end of 2015. Testing is recommended in 1) patients at high risk for DR-TB (previous anti-tuberculosis treatment or contact with a DR-TB patient); 2) presumptive TB patients from vulnerable populations, including children, people living with the human immunodeficiency virus (HIV) and other immune-compromised and/or seriously ill patients; and 3) extra-pulmonary or pulmonary specimens obtained using any invasive or non-invasive procedures. Patients with TB that is RMP-resistant are registered at DR-TB treatment sites and comprehensive phenotypic drug susceptibility testing (DST) is performed on a fresh sample.17
The objectives of this study were to assess the usefulness of gastric specimens (GS) aspirated under routine programme conditions among adult presumptive TB patients unable to expectorate quality sputum for the diagnosis of PTB and DR-TB, and to compare the yield and sensitivity of smear, Xpert and culture.
STUDY POPULATION AND METHODS
Study setting
The Rawalpindi Leprosy Hospital (RLH), a not-for-profit private hospital, receives patients from a large area, many of whom are seriously ill and have previously been treated for TB. The hospital is a long-standing partner of the National Tuberculosis Control Program (NTP) in TB and DR-TB care. The proportion of bacteriological confirmation among notified PTB cases has gradually increased, from 47% in 2012 to 70% in 2015, along with the increase in TB case notification. GA is routinely performed in adult presumptive PTB patients who are unable to expectorate due to poor physical condition (e.g., severe weakness, respiratory distress) when CXR results are moderately or highly suggestive of TB in patients with or without the risk of DR-TB. The procedure is performed routinely in out-patient clinics twice a week on fixed days by a trained nurse. Patients are telephoned early in the morning with instructions to ingest nothing by mouth after midnight. GS is aspirated using a 10-ml syringe. Fluids (such as saline) are not routinely instilled before aspiration in all cases. Only direct sputum microscopy is performed in RLH; for all other tests, the specimens are transported to the national TB reference laboratory (NRL), a 1 h drive from RLH.
Study design and population
This is a comparative cross-sectional study based on retrospective record review. Presumptive PTB patients aged ⩾15 years who had their GA referred to the NRL for TB diagnosis during 2012–2015 were included. Patients with any positive sputum smear, those already undergoing anti-tuberculosis treatment at the time of the GA according to the TB register in the RLH, and specimens labelled as ‘follow-up’ were excluded from the study.
Laboratory methods
The aspirated GS was transferred directly to a Falcon™ tube (Thermo Fisher Scientific, Waltham, MA, USA), without any additive to neutralise the specimen. The GS were transported the same afternoon to the NRL and processed the next morning using Petroff's method; the sediment was used for smear, Xpert and culture on Löwenstein-Jensen medium (LJ) and BAC-TECTM MGIT 960™ (BD, Franklin Lakes, NJ, USA). Auramine-stained smears were examined using light-emitting diode (LED) fluorescence microscopy. Culture isolates were confirmed for Mycobacterium tuberculosis on detection of the MPB64 antigen using the TBC-ID kit (BD). Phenotypic DST was performed on LJ media in 2012–2014 and on MGIT 960 in 2015.
The RLH laboratory participates regularly in quarterly external quality assessment (EQA) of smear microscopy by blinded rechecking. The NRL participates in annual EQA for DST by the Supranational Reference Laboratory (Antwerp, Belgium), and has been quality assured since 2010 for first- and second-line DST.
Data collection and variables
Demographic, clinical and laboratory data were extracted from the main electronic database of the NRL and the notification trend of PTB cases from the RLH quarterly notification reports. The RLH TB register was used to identify patients undergoing treatment and those reported positive on sputum smear.
The data were imported from the electronic register into EpiData software v. 3.1 for entry and v. 2.2.2.182 for analysis (EpiData Association, Odense, Denmark).
Definitions
A ‘definite TB case’ was defined as any patient whose GS was positive for M. tuberculosis on Xpert and/or on culture (a composite reference standard); a ‘probable TB case’ was defined as positive on smear only (including scanty), with Xpert and culture results either negative or not available and no non-tuberculous mycobacteria (NTM) grown on culture.
Data analysis
The diagnostic yield of smear, Xpert and culture was calculated as the percentage of positive results among total valid results for each test, and sensitivity was calculated by cross-tabulation against the composite reference standard using samples with valid results for all three tests. Phenotypic DST was compared with Xpert for the RMP results.
Ethics approval was obtained from the NTP (Islamabad, Pakistan) for the use of the data already available in the NRL database. The Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France) approved the study protocol.
RESULTS
The GS from 1010 adult patients were received in the NRL during 2012–2015. One hundred and ten patients were excluded from the study, including 55 with positive sputum smear, 53 who were on anti-tuberculosis treatment and 2 with no valid result.
Of the 900 patients included in the study, 46% were male, with a mean age of 42 years compared to 36 years in females. There were 617 (69%) patients unable to expectorate spontaneously, 283 (31%) with negative sputum and 705 at increased risk for DR-TB: 622 (69%) with previous anti-tuberculosis treatment and 95 (11%) who were contacts of DR-TB patients (Table 1).
TABLE 1.
Characteristics of presumptive, definite and RMP-resistant TB cases tested using gastric aspirate specimens in Rawalpindi Leprosy Hospital, Rawalpindi, Pakistan, 2012–2015
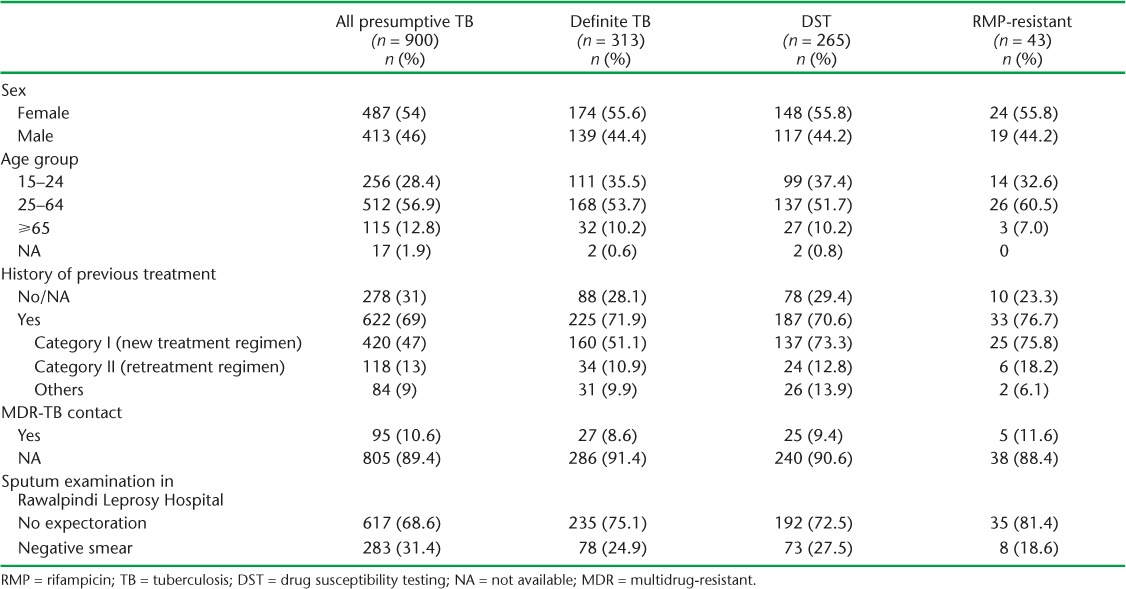
The specimen volume was ⩽5 ml in 595 (66%) cases, including 281 samples (32%) with <1 ml volume. Respectively 900, 885 and 877 samples were tested by smear, Xpert and culture, and interpretable results were obtained in respectively 900 (100%), 859 (97.1%) and 754 (86.0%), with diagnostic yields of 23.6%, 30.3% and 24.9%. The diagnostic yield of Xpert was significantly higher in previously treated patients (Table 2).
TABLE 2.
Valid test results and diagnostic yield of smear, Xpert and culture performed on gastric aspirate specimens in Rawalpindi Leprosy Hospital, Rawalpindi, Pakistan 2012–2015
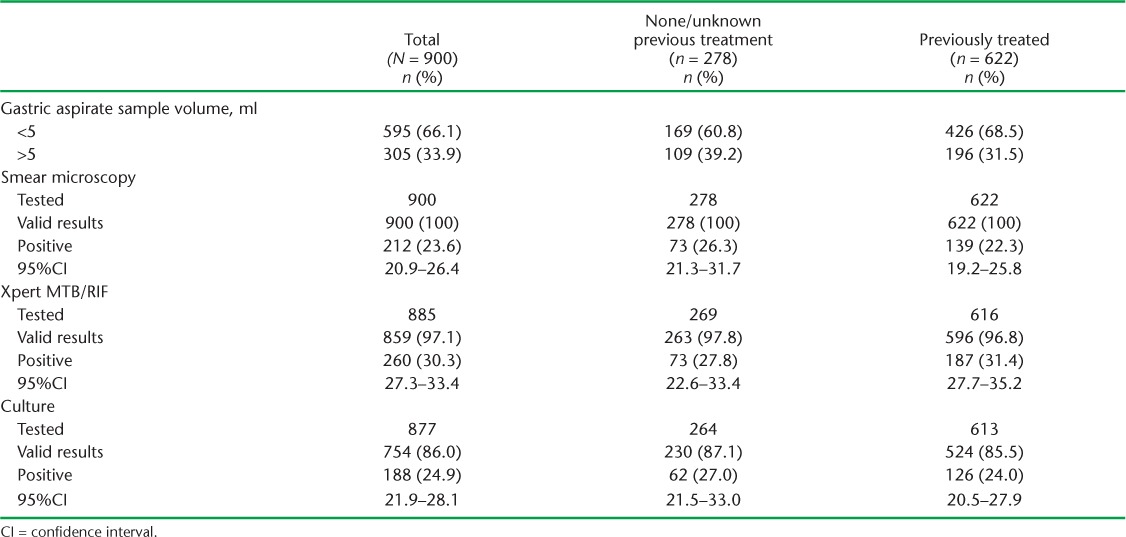
Of 900 samples tested, 212 were smear-positive, including 89 scanty positives. M. tuberculosis was detected on Xpert in 91.7% of smear-positive samples (excluding scanty), 88.2% of scanty positives and 11.4% with negative smears.
M. tuberculosis was grown on culture in 125 smear-positive cases. Culture yield, excluding contaminated specimens, was 76.9% (95% confidence interval [CI] 68.2–84.1) in smear-positive samples (excluding scanty), significantly higher than among scanty positive samples (54.5%, 95%CI 43.4–65.4) and smear-negative samples (9.4%, 95%CI 7.4–11.9; Table 3).
TABLE 3.
Smear, Xpert and culture results on gastric aspirations performed in Rawalpindi Leprosy Hospital, Rawalpindi, Pakistan, 2012–2015
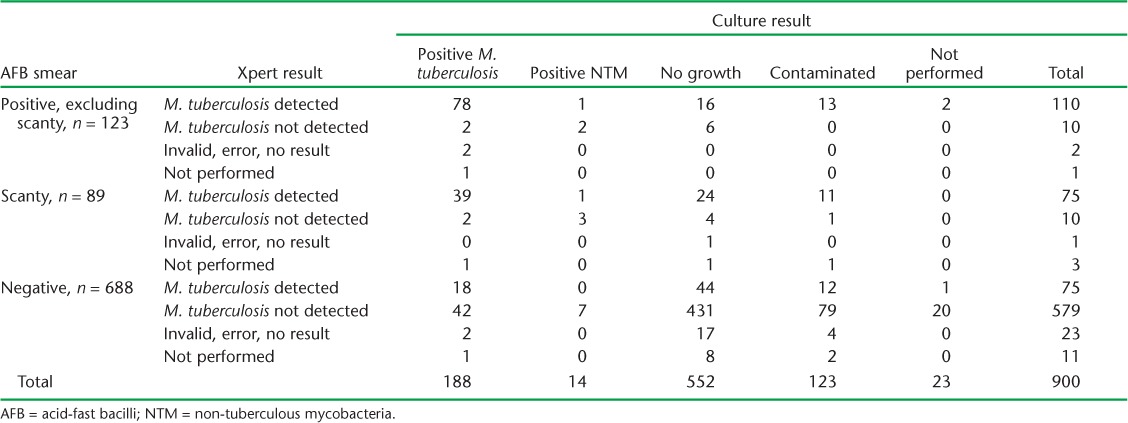
Fourteen per cent (123/877) of cultures were overgrown by contaminants. The contamination rate was significantly higher in samples >5 ml in volume (18.5%, 56/302, 95%CI 14.5–23.2) compared to samples ⩽5 ml in volume (11.7%, 67/575, 95%CI 9.2–14.4). NTM were grown on culture in 3.8% (7/184) of smear-positive samples and in 1.2% (7/570) of smear-negative samples (Table 3).
Using the composite reference standard, 313 cases were defined as definite TB and 14 as probable TB (Figure). There were 710 cases with valid results for all three tests; these included 267 definite TB cases. The sensitivity of AFB smear (163/267), Xpert (221/267) and culture (181/267) against the composite reference standard was respectively 61.0% (95%CI 54.9–66.9), 82.8% (95%CI 77.7–87.1) and 67.2% (95%CI 61.8–73.4). The sensitivity of Xpert was significantly higher than that of smear and culture.
FIGURE.
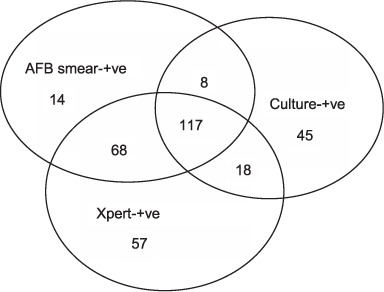
Diagnostic profile of 313 definite and 14 probable TB cases. AFB = acid-fast bacilli; +ve = positive; TB = tuberculosis.
DST results for RMP were available for 265/313 (85%) definite TB cases, 257 on Xpert and 53 on phenotypic DST, including 45 on both. Discordance was reported in only one case with RMP resistance detected on phenotypic DST. RMP-resistant TB was reported in 18.1% (38/209, 95%CI 13.4–23.8) of 705 patients at high risk for DR-TB and 8.9% (5/56, 95%CI 3.3–18.7) of patients at no or unknown risk for DR-TB (Table 1).
DISCUSSION
To the best of our knowledge, this is the first study to assess the usefulness of a single, non-neutralised GS in diagnosing bacteriologically confirmed TB and DR-TB in a large number of adult presumptive TB patients (900) with difficulties expectorating. GA was performed under routine programme conditions, and almost all GS were tested on smear, Xpert and culture. The yield was 24% for smear, 25% for culture and 30% for Xpert. There were 313 (34.8%) definite and 14 probable TB cases diagnosed. The sensitivity of Xpert (82.8%) was significantly higher than that of both smear (61.0%) and culture (67.8%).
As this was a retrospective study, the limitations included incomplete clinical data, including past history of TB, previous anti-tuberculosis treatment category and outcome of treatment and the interval between the current and previous TB episode. Information was missing on the extent and type of radiological abnormalities and on any fluid instilled before GA in individual cases. The study findings may not be representative of the general population, as it was based on the experience of only one hospital, the majority of the patients had been treated previously and all had abnormal X-ray findings. Finally, as culture had a low yield, it could not be used as the gold standard and, due to the use of a composite reference standard, the specificity of the Xpert results could not be determined.
The high diagnostic yield of GS (34.8%) in this study may be explained by all the presumptive PTB patients having CXR findings suggestive of TB and the simultaneous use of three diagnostic methods. Xpert had a significantly higher proportion of valid results and sensitivity than culture, as, unlike culture, its performance was not affected by the acidity of the GS, delays in processing or overgrowth of contaminating bacteria or NTM.
The diagnostic yield of Xpert (31.4%) was significantly higher than that of culture (24.0%) only in previously treated cases. Although all patients known to be on treatment were excluded from the study, due to missing information some patients treated for TB in the recent past may have been included. This may have to some extent contributed to further lowering the yield of culture compared to Xpert. As the excretion of residual persistent DNA from dead organisms has been suggested as a reason for positive Xpert but negative smear and/or culture results on samples taken during and at the end of treatment,18 a positive Xpert result in recently treated TB patients should be correlated with the clinical condition of the patient, and should ideally be confirmed by culture, although in GS the culture yield is known to be low.
We found no difference between the diagnostic yields of smear (23.6%) and culture (24.9%). The high yield of smear may be explained by the highly selective patients, the use of concentrated smears and examination by skilled staff at the NRL. The low yield of culture was most likely due to long delays (⩾24 h) in the processing of specimens, which were not neutralised on collection, resulting in loss of viability of a substantial proportion of TB bacilli due to the strong acidity of the gastric fluid, and false-negative cultures as a consequence. This also explains the significant difference in culture recovery in samples with a higher number of bacilli compared to those with scanty bacilli.
Brown et al. found a yield of 12% with smear and 32% with culture in gastric washings from new adult migrants with minimal changes on chest radiography.19 The wide difference in yield between smear and culture, in contrast to our study, was probably due to patients having early disease with minimal changes on CXR and specimens being processed within 2–4 h. Although the acidity of gastric fluid reduces the yield of culture, Parashar et al. reported a significantly higher contamination rate and lower yield of M. tuberculosis on MGIT 960 in neutralised samples (16.3% [38/232]) from paediatric patients than in non-neutralised samples (3.9% [9/232]).20 In our study, although the samples were not neutralised, the contamination rate was significantly higher in specimens with >5 ml volume, presumably because of the fluid instilled before aspiration.
Variations in the yield of GS for the diagnosis of TB have been reported in different studies conducted both in children5,9–13 and in adults.19,21–26 Maciel et al. studied variations in procedures in a systematic review and highlighted the need for a standardised protocol for GA in children.27 Variations observed in the yield of GS in adults are probably also due to non-standardisation of the protocol used for GA and the processing of GS.
Singh et al. used Xpert for testing archived GS and reported a sensitivity of 95% in smear-positive, culture-positive samples compared to 65% in smear-negative, culture-positive samples and 11.7% in bacteriologically negative samples.28 We reported an Xpert sensitivity of 83% using positive Xpert and/or culture as a reference standard, compared to a pooled sensitivity in GS of 78% using culture as the gold standard reported in a systematic review by Maynard et al.,29 and a sensitivity of 90.4% for all types of pulmonary specimens reported by Chang et al.30
The majority of the patients tested (78%) had a risk of DR-TB, and Xpert testing made rapid DST results available for RMP in 257/313 TB cases.
This study has a number of public health implications: GA can be performed in adults in out-patient clinics under routine conditions. GS that is collected without fluid instillation can be processed directly for smear and Xpert testing, with high yield. If facilities are available, the specimens should be processed for culture without delay for a better yield. While Xpert is the preferred test, in previously treated patients a positive result needs to be interpreted with caution in the light of the patient's clinical and treatment history and should ideally be confirmed by culture. The use of a standardised protocol for GA and the processing of GS can help optimise the yield of culture.
CONCLUSION
GS obtained from TB patients with difficulty expectorating can substantially improve the diagnosis of confirmed TB and RMP-resistant TB. Xpert has the advantages of a quick reporting time, a high proportion of interpretable results and high sensitivity. Positive Xpert results need to be interpreted with caution, however, in previously treated patients. The yield of culture should be improved by rapid processing and the use of standardised protocols.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médicins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the National Tuberculosis Control Program (Islamabad, Pakistan) through the support of the Global Fund (Geneva, Switzerland), WHO-TDR, the University of Bergen (Bergen, Norway), The Union and The Union South-East Asia Office (New Delhi, India). The authors would also like to acknowledge the contribution of AMV Kumar in the data analysis and of SG Hinderaker and A Yaqoob for their contributions and continuous support.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2015.22 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Khan M S, Dar O, Tahseen S, Godfrey-Faussett P.. Judging respiratory specimen acceptability for AFB microscopy: visual vs. microscopic screening. Trop Med Int Health 2009; 14: 571– 575. [DOI] [PubMed] [Google Scholar]
- 3. Steingart K R, Ng V, Henry M, . et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006; 6: 664– 674. [DOI] [PubMed] [Google Scholar]
- 4. Hepple P, Nguele P, Greig J, Bonnet M, Sizaire V.. Direct microscopy versus sputum cytology analysis and bleach sedimentation for diagnosis of tuberculosis: a prospective diagnostic study. BMC Infect Dis 2010; 10: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conde M B, Soares S L M, Mello F C Q, . et al. Comparison of sputum induction with fiberoptic bronchoscopy in the diagnosis of tuberculosis. Experience at an acquired immune deficiency syndrome reference center in Rio de Janeiro, Brazil. Am J Respir Crit Care Med 2000; 162: 2238– 2240. [DOI] [PubMed] [Google Scholar]
- 6. Ruiz Jiménez M, Guillén Martín S, Prieto Tato L M, . et al. Induced sputum versus gastric lavage for the diagnosis of pulmonary tuberculosis in children. BMC Infect Dis 2013; 13: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ugarte-Gil C, Elkington P T, Gotuzzo E, Friedland J S, Moore D A J.. Induced sputum is safe and well-tolerated for TB diagnosis in a resource-poor primary healthcare setting. Am J Trop Med Hyg 2015; 92: 633– 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larson J, Ridzon R, Hannan M.. Sputum induction versus fiberoptic bronchoscopy in the diagnosis of tuberculosis. Am J Respir Crit Care Med 2001; 163: 1279– 1280. [DOI] [PubMed] [Google Scholar]
- 9. Cruz A T, Revell P A, Starke J R.. Gastric aspirate yield for children with suspected pulmonary tuberculosis. J Pediatric Infect Dis Soc 2013; 2: 171– 174. [DOI] [PubMed] [Google Scholar]
- 10. Lobato M N, Loeffler A M, Furst K, Cole B, Hopewell P C.. Detection of Mycobacterium tuberculosis in gastric aspirates collected from children: hospitalization is not necessary. Pediatrics 1998; 102: e40. [DOI] [PubMed] [Google Scholar]
- 11. Abadco D, Steiner P.. Gastric lavage is better than bronchoalveolar lavage for isolation of Mycobacterium tuberculosis in childhood pulmonary tuberculosis. Pediatr Infect Dis J 1992; 11: 735– 738. [DOI] [PubMed] [Google Scholar]
- 12. Norrman E, Keistinen T, Uddenfeldt M, Rydström P, Lundgren R.. Bronchoalveolar lavage is better than gastric lavage in the diagnosis of pulmonary tuberculosis. Scand J Infect Dis 1988; 20: 77– 80. [DOI] [PubMed] [Google Scholar]
- 13. Kalu E I, Ojide C K, Ugochukwu N V.. Gastric aspirate smear microscopy as a diagnostic tool for childhood pulmonary tuberculosis. Ann Trop Med Public Health 2013; 6: 608– 613. [Google Scholar]
- 14. Qadeer E, Fatima R, Yaqoob A, . et al. Population-based national tuberculosis prevalence survey among adults (>15 years) in Pakistan, 2010–2011. PLOS ONE 2016; 11: e0148293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tahseen S, Qadeer E, Khanzada FM, . et al. Use of Xpert® MTB/RIF assay in the first national anti-tuberculosis drug resistance survey in Pakistan. Int J Tuberc Lung Dis 2016; 20: 448– 455. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. . Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO/HTM/TB/2011.4 Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 17. National Tuberculosis Control Program. . National guidelines for the control of tuberculosis in Pakistan. Islamabad, Pakistan: Ministry of National Health Services Regulations & Coordination, 2015. [Google Scholar]
- 18. Friedrich S O, Rachow A, Saathoff E, . et al. Pan African Consortium for the Evaluation of Anti-tuberculosis Antibiotics (PanACEA). Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum bio-marker of response to tuberculosis treatment. Lancet Respir Med 2013; 1: 462– 470. [DOI] [PubMed] [Google Scholar]
- 19. Brown M, Varia H, Bassett P, Davidson R N, Wall R, Pasvol G.. Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis 2007; 44: 1415– 1420. [DOI] [PubMed] [Google Scholar]
- 20. Parashar D, Kabra S K, Lodha R, . et al. Does neutralization of gastric aspirates from children with suspected intrathoracic tuberculosis affect mycobacterial yields on MGIT culture? J Clin Microbiol 2013; 51: 1753– 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heym B, Beauchet A, Ngo M T, Gaillard J L, Chinet T.. Sputum induction versus gastric washing for the diagnosis of pulmonary mycobacterial disease. Euro Respir J 2010; 36: 448– 450. [DOI] [PubMed] [Google Scholar]
- 22. Bonnave P E, Raoult D, Drancourt M.. Gastric aspiration is not necessary for the diagnosis of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 2013; 32: 569– 571. [DOI] [PubMed] [Google Scholar]
- 23. Rizvi N, Rao N A, Hussain M.. Yield of gastric lavage and bronchial wash in pulmonary tuberculosis. Int J Tuberc Lung Dis 2000; 4: 147– 151. [PubMed] [Google Scholar]
- 24. Okutan O, Kartaloglu Z, Kilic E, Bozkanat E, Ilvan A.. Diagnostic contribution of gastric and bronchial lavage examination in cases suggestive of pulmonary tuberculosis. Yonsei Med J 2003; 44: 242– 248. [DOI] [PubMed] [Google Scholar]
- 25. Saka D, Çalõúõr H C, Mihriban Ö.. The diagnostic role of gastric aspiration in cases without sputum and in smear-negative patients with suspected pulmonary tuberculosis. Turkish Respir J 2006; 7: 124– 127. [Google Scholar]
- 26. Baghaei P, Tabarsi P, Farnia P, Radaei A H, Kazempour M, Faghani Y A.. Utility of gastric lavage for diagnosis of tuberculosis in patients who are unable to expectorate sputum. J Glob Infect Dis 2011; 3: 339– 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maciel E L, Brotto L D, Sales C M, Zandonade E, Sant'anna C C.. Gastric lavage in the diagnosis of pulmonary tuberculosis in children: a systematic review. Rev Saúde Pública 2010; 44: 735– 742. [DOI] [PubMed] [Google Scholar]
- 28. Singh S, Singh A, Prajapati S, . et al. Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiol 2015; 15: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maynard-Smith L, Larke N, Peters J A, . et al. Diagnostic accuracy of the Xpert MTB/RIF assay for extra pulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis 2014; 14: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang K, Lu W, Wang J, . et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect 2012; 64: 580– 588. [DOI] [PubMed] [Google Scholar]


