Abstract
Setting: The tuberculosis (TB) clinics of five health facilities in western Kenya.
Objective: To assess the prevalence and associated determinants of diabetes mellitus (DM) and pre-diabetes hyperglycaemia among adult TB patients using point-of-care DCA Vantage glycated haemoglobin (HbA1c) devices.
Design: This was a cross-sectional study.
Results: Of 454 patients, 272 (60%) were males, the median age was 34 years, 175 (39%) were co-infected with the human immunodeficiency virus (HIV), and the median duration of anti-tuberculosis treatment was 8 weeks; 180 (40%) patients reported at least one classical symptom suggestive of DM. The prevalence of DM (HbA1c ⩾6.5%) was 5.1% (95%CI 3.2–7.5), while that of pre-diabetes (HbA1c 5.7–6.4%) was 37.5% (95%CI 33.1–42.2). The number needed to screen (NNS) was 19.6 for DM and 2.7 for pre-diabetes. Combined, 42.6% (95%CI 38.0–47.3) of the patients had either pre-diabetes or DM (NNS 2.3). Seven of the 23 patients with DM knew their prior DM status. Higher rates of DM were associated with age ⩾40 years and a family history of DM, but not obesity, type of TB, HIV status or suggestive symptoms.
Conclusions: High rates of pre-diabetes and DM were found in adult TB patients. This study supports the need for routine screening of all patients with TB for DM in Kenya.
Keywords: HbA1c, DCA Vantage, prevalence, operational research
Abstract
Contexte: Centres tuberculose (TB) de cinq structures de santé dans l'Ouest du Kenya.
Objectif: Evaluer la prévalence et les déterminants associés du diabète (DM) et de l'hyperglycémie pré-diabète chez des patients adultes atteints de TB grâce aux appareils DCA Vantage de dosage d'hémoglobine glyquée (HbA1c) utilisés sur place.
Schéma: Une étude transversale.
Résultats: Sur 454 patients, 272 (60%) ont été des hommes, leur âge médian a été de 34 ans et 175 (39%) ont été co-infectés par le virus de l'immunodéficience humaine (VIH) ; la durée médiane du traitement de la TB a été de 8 semaines et 180 (40%) patients ont fait état d'au moins un symptôme classique suggérant un DM. La prévalence du DM (HbA1c ⩾ 6,5%) a été de 5,1% (IC95% 3,2–7,5) tandis que celle du pré-diabète (HbA1c 5,7–6,4%) a été de 37,5% (IC95% 33,1–422). Le nombre de dépistages requis (NNS) a été de 19,6 pour diagnostiquer un DM et de 2,7 pour un pré-diabète. En combinant les deux, 42,6% (IC95% 38,0–47,3) des patients avaient soit un pré-diabète soit un DM (NNS 2,3). Sept des 23 patients atteints de DM étaient au courant de leur statut. Des taux de DM plus élevés ont été associés avec un âge ⩾ 40 ans et des antécédents de DM dans la famille, mais pas avec l'obésité, le type de TB, le statut du VIH ou des symptômes suggestifs du VIH.
Conclusions: Des taux élevés de pré-diabète et de DM ont été découverts chez des patients TB adultes. Cette étude est en faveur du dépistage de routine du DM chez tous les patients atteints de TB au Kenya.
Abstract
Marco de referencia: Los consultorios de atención de la tuberculosis (TB) en cinco establecimientos de salud en el occidente de Kenya.
Objetivo: Evaluar la prevalencia de diabetes (DM) e hiperglucemia prediabética y los factores determinantes asociados en los pacientes adultos con diagnóstico de TB, mediante la utilización de dispositivos de diagnóstico (DCA Vantage) que determinan la glucohemoglobina (HbA1c) en el lugar de atención.
Método: Un estudio transversal.
Resultados: De los 454 pacientes, 272 fueron de sexo masculino (60%), la mediana de la edad fue 34 años, 175 (39%) sufrían coinfección por el virus de la inmunodeficiencia humana (VIH), la mediana de la duración del tratamiento antituberculoso fue 8 semanas y 180 pacientes notificaron por lo menos un síntoma patognomónico de DM (40%). La prevalencia de DM (HbA1c ⩾ 6,5%) fue 5,1% (IC 95% 3,2–7,5) y la prevalencia de pre-diabetes (HbA1c 5,7–6,4%) fue 37,5% (IC95% 33,1–42,2). El número de pacientes que es necesario cribar (NNC) para detectar un caso de DM fue 19,6 y 2,7 para un caso de prediabetes. Combinados, el 42,6% de los pacientes presentaba ya sea prediabetes o DM (NNC 2,3; IC95% 38,0–47,3). Siete de los 23 pacientes con DM conocían ya su diagnóstico. Las tasas más altas de DM se asociaron con una edad de 40 años o más y el antecedente familiar de DM, pero no con la obesidad, el tipo de TB, la situación frente al VIH ni la presencia de síntomas indicativos.
Conclusión: Se encontraron altas tasas de prediabetes y DM en los pacientes adultos con diagnóstico de TB. El presente estudio respalda la práctica de la detección sistemática de la DM en los pacientes con TB en Kenya.
The global burden of diabetes mellitus (DM) is increasing exponentially, with the highest increases occurring in low- and middle-income countries (LMICs).1,2 In 2015, 415 million (8.8%) of the world's population had DM, with two thirds of those in the sub-Saharan Africa region remaining undiagnosed.3 This pandemic resulted in 5 million DM-associated deaths globally. The global prevalence of DM is expected to reach 10.4% by 2040. Tuberculosis (TB) remains a major cause of morbidity and mortality worldwide, with an estimated 10.4 million people developing the disease and 1.8 million dying from it in 2015.4 Most of the TB burden occurs in the same LMICs as DM.
DM and TB interact, and in so doing they worsen the prevalence, presentation, control and treatment outcomes of both diseases.5–12 DM increases the risk of TB disease by at least two to three fold,5–7 while impaired glucose tolerance (IGT) and DM have been found to be higher among TB patients.8–10 A systematic review on bi-directional screening of TB and DM demonstrated high TB prevalence among patients with DM, ranging from 1.7% to 36%, which increased with rising TB prevalence in the population, as did DM severity.11 Screening patients with TB for DM has yielded equally high DM prevalence, ranging from 1.9% to 35%.11
Another systematic review of the impact of DM on anti-tuberculosis treatment outcomes found an increased risk of death of at least 89% during anti-tuberculosis treatment and a 3.9 times increased risk for TB relapse after successful completion of treatment.12 Likewise, TB may lead to transient hyperglycaemia and, as with other intercurrent infections, contributes to the suboptimal control of DM in LMICs.8,13 Some TB medications, such as isoniazid, may also have a hyperglycaemic effect.14
Coupled with the slower-than-expected decline in TB rates worldwide and the increase in DM rates, the convergence of these two epidemics may lead to a resurgence of TB disease, particularly in LMICs.6,12,15,16 Undiagnosed, inadequately treated and poorly controlled DM appears to be a much greater threat to TB prevention and control than has been realised previously.17
Despite guidance from the World Health Organization (WHO),18 most facilities in sub-Saharan Africa are still not screening TB patients for DM, partly due to cost, perceived complexities19,20 and lack of a treatment infrastructure for those who screen positive. Transient hyperglycaemia caused by TB disease or medication, and similarities in symptom presentation, such as weight loss, further complicate the process. The standard diagnostic methods present their own challenges. Use of fasting blood glucose (FBG), the oral glucose tolerance test (OGTT) and random blood glucose (RBG) either require patients to fast for several hours before blood testing or have specific time-bound measurements and/or interpretations affected by transient hyperglycaemia, necessitating multiple measurements.19,20 Use of glycated haemoglobin (HbA1c) does not require the patient to fast, is equally sensitive and, if offered at point of care (POC), minimises the time to diagnosis.21,22 The devices are more expensive, however, and readings can be affected by anaemia and different haemoglobin variants.19,21,22
Kenya is heavily burdened by disease, with the principal challenges coming from communicable diseases such as malaria, human immunodeficiency virus (HIV) and TB, which accounted for 64% of deaths in 2014.23,24 Non-communicable diseases were responsible for 27% of all deaths in the same year, of which DM accounted for at least 1%,24 although this rate is likely an underestimate due to the many undiagnosed cases in the country.25 A recent national household survey found a DM prevalence rate of 1.9% in individuals aged 18–69 years in the country,26 while the International Diabetes Federation (Brussels, Belgium) estimated the prevalence to be 2.2% for those aged 20–79 years.3
In Kenya, there is a paucity of data on disease frequency and interactions between DM and TB. The aim of the present study was thus 1) to assess the prevalence of DM and pre-diabetes hyperglycaemia using POC DCA Vantage® HbA1c devices (Siemens Healthcare GmbH, Erlangen, Germany); and 2) to determine the association between sociodemographic and clinical characteristics and the occurrence of DM and pre-diabetes among adult patients with TB in selected high-volume clinics in western Kenya.
METHODS
Study design
This was a cross-sectional study.
Setting
General setting
Kenya is a lower-middle-income country with a population of 46 million, of whom 75% reside in rural areas.27 The gross domestic product stands at USD 60.9 billion and the economy is largely service-based and agricultural,27 with 46% of the population living in absolute poverty.28 The crude death rate is 8/1000 population and national life expectancy is 63 years.23,29 TB and HIV are responsible for respectively 6.3% and 29.3% of all deaths.23,28 Noncommunicable diseases account for approximately 50–70% of all hospital admissions.28 Overweight (pre-obese and obese), a significant risk factor for DM, is prevalent in 28% of all persons in Kenya.26
Tuberculosis and diabetes control
Kenya is divided into 47 administrative counties, with health care largely the responsibility of the devolved counties. In both public and private facilities, there is little systematic effort to coordinate the screening and care of patients with TB and DM. The current study was carried out in the TB clinics of five major health facilities in four counties in the western part of Kenya: Moi Teaching and Referral Hospital and Turbo Health Centre in Uasin Gishu county; Kitale County Referral Hospital (CRH) in Trans Nzoia county; Webuye County Hospital in Bungoma county; and Busia CRH in Busia county. A total of 284 facilities were offering TB treatment services in these four counties, and many fewer were offering DM care services. The five selected facilities notified 21% of the total 2952 TB cases in these counties in the first half of 2015. These facilities also offer DM care, mostly as a result of an aggressive expansion programme for DM and hypertension with the support of the Academic Model Providing Access to Healthcare (AMPATH) programme (Eldoret, Kenya).
Diagnosis of DM in these counties is largely based on RBG and FBG, with HbA1c being used for monitoring glycaemic control. For those not on care for DM, however, a value of HbA1c of ⩾6.5% is regarded as diagnostic for DM, while a value of 5.7–6.4% is diagnostic for pre-diabetes.21,30 HbA1c measurements are performed in laboratories or by the use of POC instruments. In this study, we used DCA Vantage POC devices to measure HbA1c levels among TB patients. DCA Vantage has been certified as a POC HbA1c instrument that has good correlation with existing methods of testing and has been approved for waiver under the Clinical Laboratory Improvement Amendments criteria.22
Study population, variables and sources
We recruited consenting adults aged ⩾18 years who were registered for treatment for drug-susceptible TB in the five health facilities between January and June 2016. Patients were consecutively recruited in each of the selected facilities until the overall sample size was attained. Some data were sourced from the facility TB registers (i.e., age, sex, body mass index [BMI] at registration, type and category of TB, TB treatment start date, HIV status), while other data were obtained through interviewer-administered questionnaires (i.e., DM history and symptoms, occupation and education level). Single blood pressure and HbA1c levels were measured using a standard automatic blood pressure machine (Omron Healthcare Inc, Lake Forest, IL, USA) and the fingerprick test, respectively.
Study procedures, data collection and management
The research assistant and the officers of the respective TB clinics were trained in the study procedures, study ethics and standardised methods of carrying out the related measurements. Potential subjects identified from the clinic TB registers were approached for informed consent during the usual TB clinic days. The data were extracted and recorded in the study case report forms (CRFs) and then double-entered into an EpiData database v. 3.1 (EpiData Association, Odense, Denmark); the data entries were compared, and any inconsistencies in the electronic database were resolved by cross-checking with the CRFs.
Analysis and statistics
The data were analysed using Stata v.13 (StataCorp, College Station, TX, USA). Descriptive statistics, such as frequencies and the corresponding percentages, were used to summarise categorical variables, while medians and the corresponding interquartile ranges (IQR) were used to summarise continuous variables. Associations between the outcomes (pre-diabetes and DM) and the categorical risk factors were assessed using Pearson's χ2 or Fisher's exact tests and the Mann-Whitney U test for continuous variables. The number needed to screen (NNS) was calculated as the reciprocal of the point prevalence for the given parameter. Multivariable logistic regression was used to identify factors independently associated with the outcomes. The classical symptoms suggestive of DM, and all variables at P < 0.2 at the bivariate level were included in the models. Income was excluded from the multivariable models due to sparse data. The level of significance was set at P < 0.05.
Ethics
Formal ethics approval for the study was obtained from the Moi University/Moi Teaching and Referral Hospital Institutional Research Ethics Committee (0001520), Eldoret, Kenya. Signed informed consent was sought from each of the subjects before enrolment into the study. Patients newly diagnosed with DM were referred to their respective DM clinics.
RESULTS
Characteristics of the participants
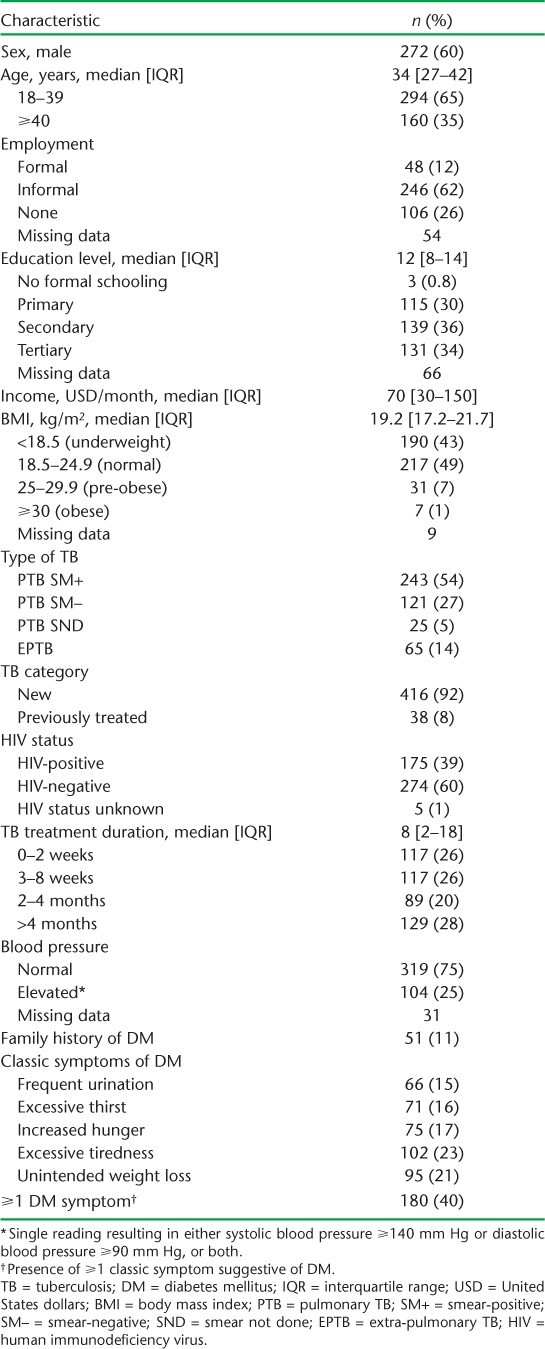
Of 454 adult TB patients in the study, HbA1c readings could not be obtained for three despite repeated attempts; two of these, however, were known DM cases. The characteristics of the patients are presented in Table 1. There were 272 (60%) males; the median age was 34 years, 389 (86%) had pulmonary TB, 175 (39%) were HIV co-infected, the median duration for anti-tuberculosis treatment was 8 weeks and 104 (25%) had elevated blood pressure (⩾140/90 mm Hg). At least one of the classic symptoms suggestive of DM (see Table 1) was reported by 180 (40%) patients.
TABLE 1.
Characteristics of adult patients with TB assessed for DM in five health facilities in western Kenya, 2015–2016
Prevalence of pre-diabetes and diabetes
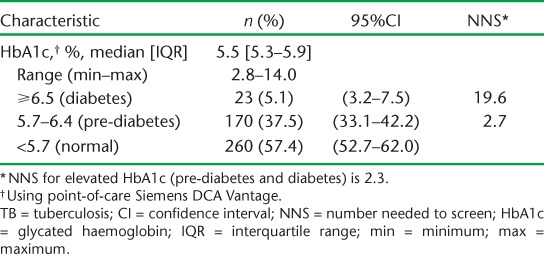
The median HbA1c level was 5.5% (Table 2). The prevalence of DM (HbA1c ⩾6.5%) was 5.1% (95% confidence interval [CI] 3.2–7.5), while that of pre-diabetes (HbA1c 5.7–6.4%) was 37.5% (95%CI 33.1–42.2). The NNS of adult TB patients to detect one case was 19.6 for DM and 2.7 for pre-diabetes. Combined, 42.6% (95%CI 38.0–47.3) of the patients had either pre-diabetes or DM, resulting in an NNS of 2.3. Of the 23 patients with DM, seven (30%) knew their status prior to assessment, of whom five were already on medication. All the known DM cases had HbA1c ⩾ 7.0%.
TABLE 2.
Prevalence of hyperglycaemia among adult patients with TB in five health facilities in western Kenya, 2015–2016
Factors associated with pre-diabetes and diabetes
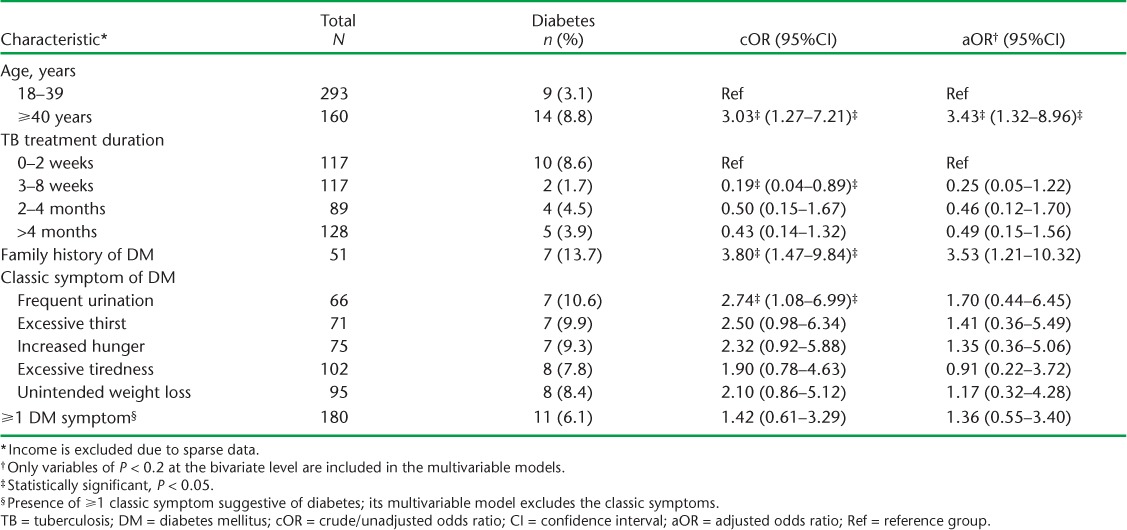
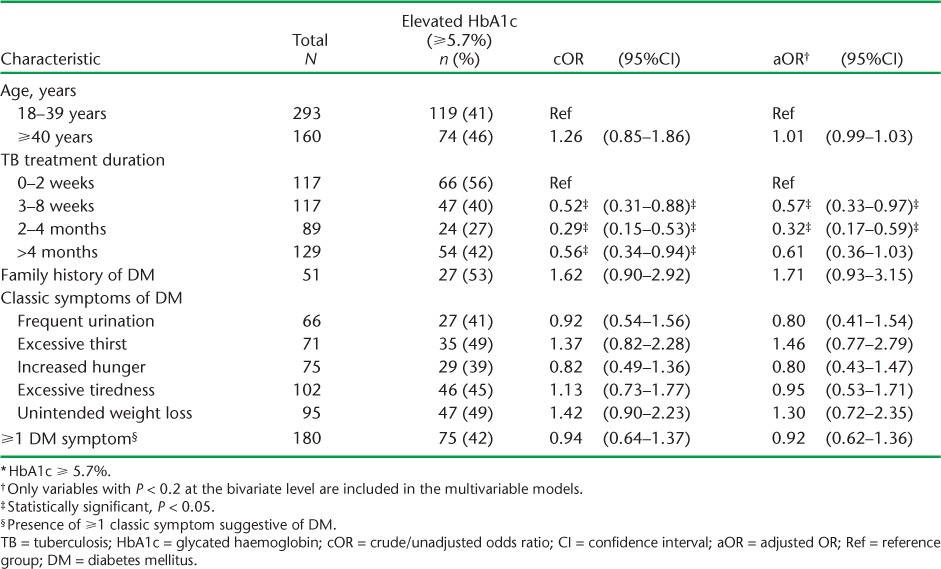
Table 3A and B shows the factors associated with DM. Sex, BMI, type and classification of TB and HIV status were not associated with DM at both the bivariate and multivariable levels. However, age ⩾40 years was associated with at least three times the odds of having DM (adjusted odds ratio [aOR] 3.43, 95%CI 1.32–8.96, P = 0.01). A family history of DM was also associated with 3.53 times the odds of having DM after adjusting for confounders (95%CI 1.21–10.32, P = 0.02). Those who had been on anti-tuberculosis treatment for between 3 and 8 weeks appeared to have reduced odds of DM compared to those on treatment for ⩽2 weeks, although these effects eroded upon further adjustments for confounders. Among the classical symptoms suggestive of DM, only frequent urination appeared to be associated with DM, the effects of which eroded upon adjustment.
TABLE 3A.
Bivariate comparison of characteristics of TB patients with and without DM in five health facilities in western Kenya, 2015–2016
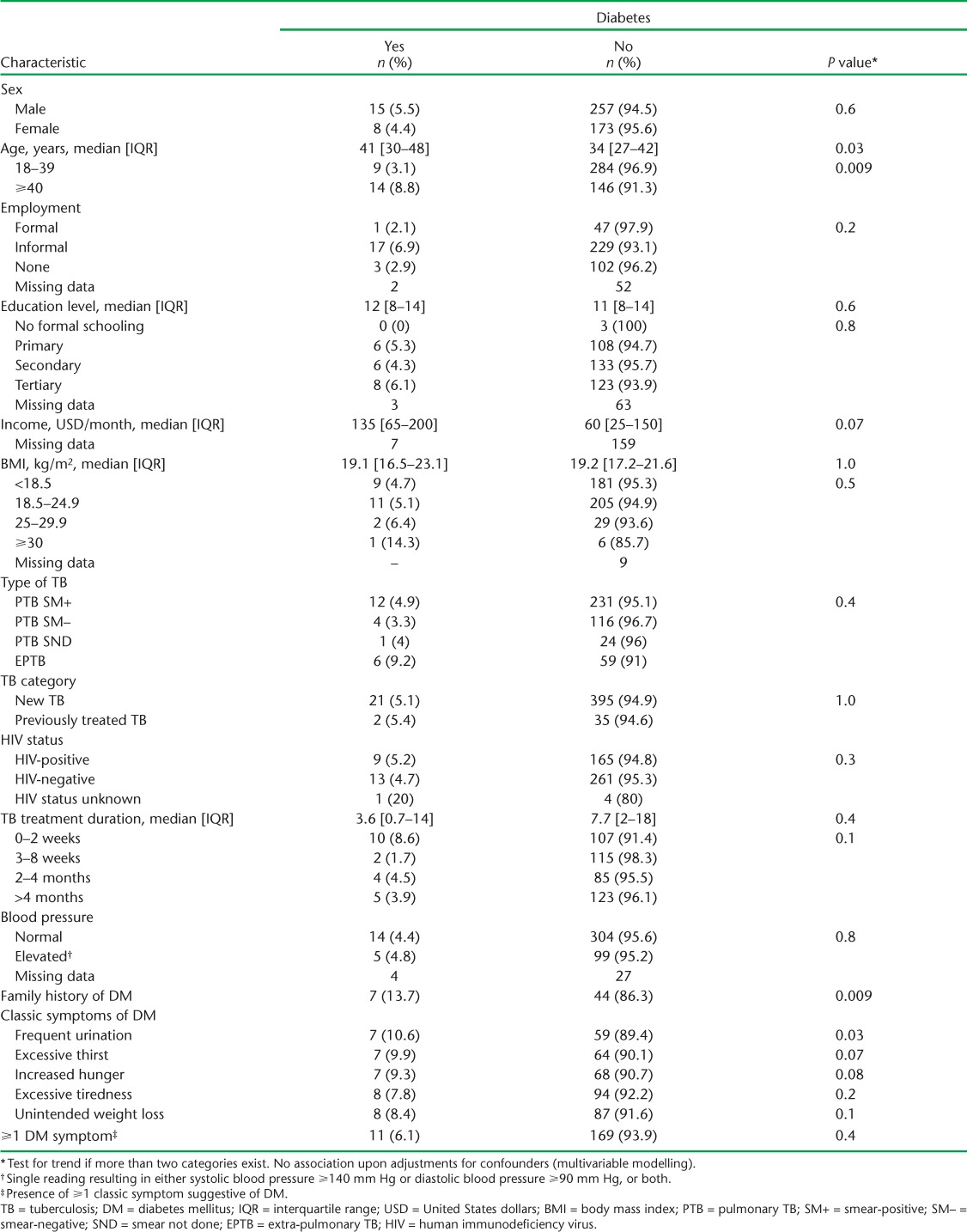
TABLE 3B.
Multivariable comparison of characteristics of TB patients with and without DM in five health facilities in western Kenya, 2015–2016
Duration of anti-tuberculosis treatment was the only variable associated with elevated HbA1c (⩾5.7%) (Table 4A and B). Treatment duration >2 weeks was generally associated with ⩾40% lower odds of elevated HbA1c. The presence of any or ⩾1 of the classical symptoms suggestive of DM was not associated with elevated HbA1c levels.
TABLE 4A.
Bivariate comparison of characteristics of TB patients with and without elevated HbA1c * in five health facilities in western Kenya, 2015–2016
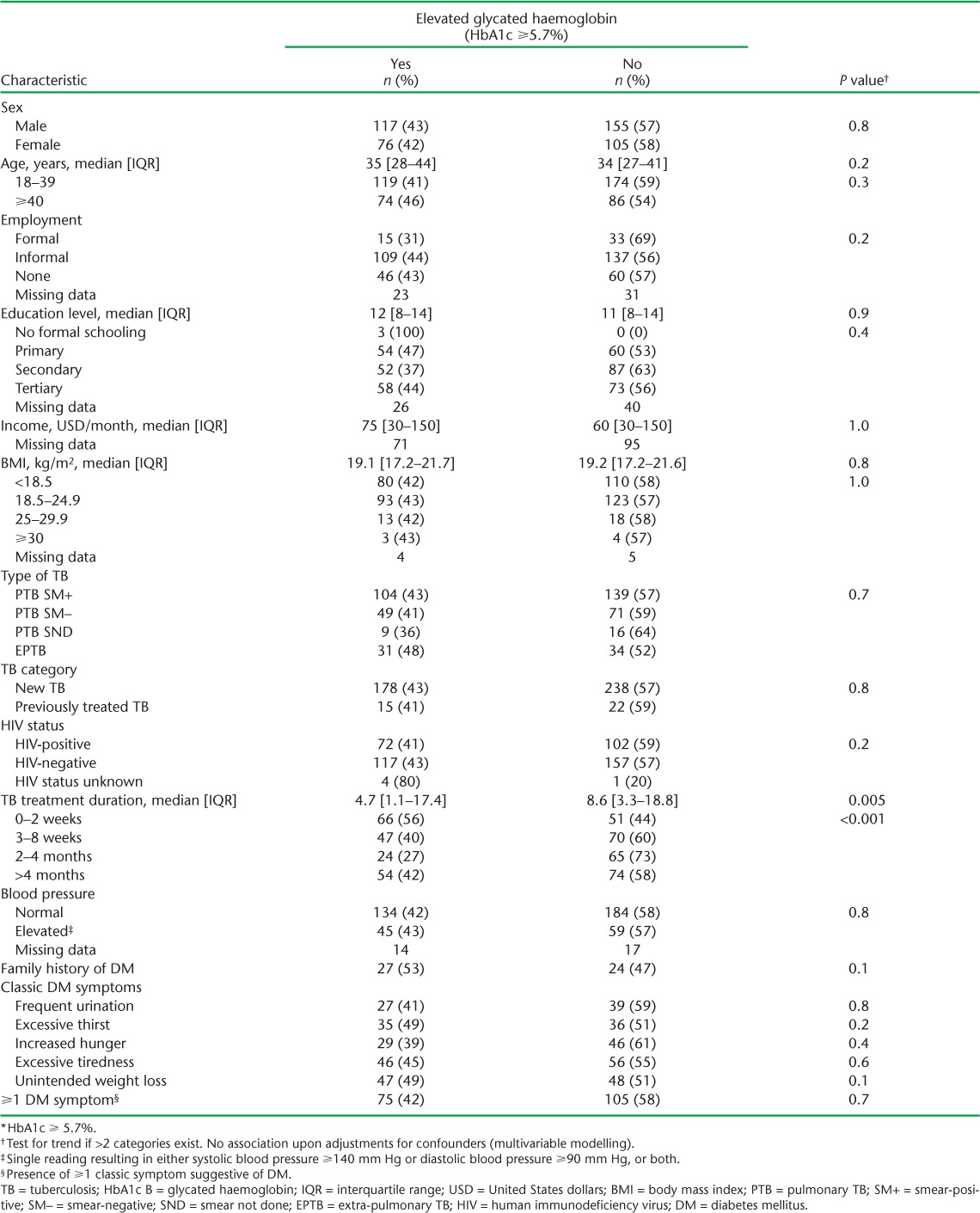
TABLE 4B.
Multivariable comparison of characteristics of TB patients with and without elevated HbA1c * in five health facilities in western Kenya, 2015–2016
DISCUSSION
This is the first study to assess DM and pre-diabetes levels in TB patients by a POC HbA1c method in a high TB-HIV co-infection setting. We found that 1/20 adult TB patients had DM, which is more than double the Kenyan population average of 1.9%.26 Almost half of the patients had elevated HbA1c levels (pre-diabetes or DM), and more than two thirds of those with DM were not aware of their prior status. Older age (⩾40 years) and a family history of DM were associated with at least a three times higher chance of having DM. There were no independent associations between DM and duration of anti-tuberculosis treatment, BMI, type of TB or even the classical symptoms suggestive of DM.
This study had several strengths. Patients were recruited consecutively, with high enrolment rates and varying lengths of anti-tuberculosis treatment, thus offering insights into DM rates across TB treatment. The POC HbA1c instruments used are internationally certified.22 The data were collected in a standardised manner and double-entered, and checks for consistency were applied. The conduct and reporting of the study adhered to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.31
One limitation of the study is that DCA Vantage has not been specifically validated amongst Kenyans. Due to limited funding, we did not perform tests for haemoglobin that might have affected the HbA1c readings. We did, however, select DCA Vantage as our preferred HbA1c test to minimise inaccuracies, as it has consistently been shown to be one of the most reliable POC HbA1c tests;22 for example, the DCA Vantage assay is affected only by the foetal haemoglobin (HbF) variant, and not the other variants HbC, HbD, HbE and HbS.22
Previous studies in sub-Saharan Africa have found rates of DM among TB patients varying from 1.9% to 6.7%,9,10,32,33 and these are much higher in the Indian subcontinent.11 Although none of these African studies used HbA1c, and the timing of screening for DM post-TB treatment initiation was at times unclear, the rates of DM were still higher than in the comparison groups or the general population, indicating a positive relationship between DM and TB.
There is thus a need to enhance routine programmatic screening for DM among TB patients in Kenya, as advised by the WHO.18 Although the technology used in the study may initially be unavailable in many programme settings due to cost (an average of USD $2100–3600 for the base station and USD $7.5 for one single-use test kit),22 other, cheaper certified POC HbA1c alternatives could be considered. HbA1c has several advantages over OGTT or FBG: it avoids the problems of day-to-day variability of glucose values, the need to fast or undergo dietary preparations, while retaining diagnostic accuracy and correlations with risk of DM-defining complications.21,34 As HbA1c is less affected by transient hyperglycaemia, it also reduces the need for multiple repeat tests.20 In its absence, however, cheaper alternatives could be used. In India and China, two large operational studies showed that TB patients could be screened for DM at the time of registration by first asking about the presence or absence of known DM and, in those denying any known disease, using RBG measurements to identify patients at risk, followed by FBG measurements in those needing further screening.17 Other programmatic management issues, such as recording and reporting, also need to be implemented.20
Of concern also are the high pre-diabetes rates in this study, with almost 4/10 adult TB patients affected. Similar findings have been reported by studies using OGTT, although these high rates may decline upon repeat measurements.9,10 As HbA1c reflects average plasma glucose over the previous 8–12 weeks,21 it is the least affected by transient hyperglycaemia. Such high rates could therefore be partly a reflection of high pre-diabetes rates in this population. Those with pre-diabetes have an increased risk of type 2 diabetes and its complications,17,35 and should be considered for DM prevention interventions.17,21,34,35 TB clinics could act as entry points for pre-diabetes and DM care.
Several studies have described increased rates of DM among older age groups, obese persons, pulmonary TB patients and those with a family history of DM, but no relationship with HIV status and sex.32,36–39 Our study did not find associations with BMI and type of TB. Although older age was associated with an increased chance of DM, the rate in the younger age group was still above that in the general population. Coupled with the lack of association with classical symptoms suggestive of DM, no clinical criteria (except known DM) should be used for pre-screening such patients.
In conclusion, this study has demonstrated higher rates of DM and pre-diabetes hyperglycaemia among adult TB patients in western Kenya. Higher rates of DM were associated with older age and a family history of DM. These findings support the need for routine screening of TB patients for DM in Kenya.
Acknowledgments
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health (NIH, Washington, DC, USA, award number D43 TW 009105). The content is solely the responsibility of the authors, and does not necessarily represent the official views of the NIH. The authors also thank the Department for International Development (London, UK) for funding this open access publication. J Mudogo, the able research assistant, ensured the project was carried to completion, while the staff of the respective TB clinics helped in the running of the project. The authors are very grateful to T Inui, the lead Kenya/CITE D43 principal investigator, and his team for their constant guidance in the project.
Footnotes
Conflicts of interest: none declared.
References
- 1. International Diabetes Federation. . IDF Diabetes Atlas. 6th ed Brussels, Belgium: IDF, 2013. http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf Accessed Nov 2016. [Google Scholar]
- 2. Wild S, Roglic G, Green A, Sicree R, King H.. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047– 1053. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation. . IDF Diabetes Atlas. 7th ed Brussels, Belgium: IDF, 2017. http://www.diabetesatlas.org/ Accessed May 2017. [Google Scholar]
- 4. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 5. Jeon C Y, Murray M B.. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLOS Med 2008; 5: 1091– 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevenson C R, Forouhi N G, Roglic G, . et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health 2007; 7: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung C C, Lam T H, Chan W M, . et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol 2008; 167: 1486– 1494. [DOI] [PubMed] [Google Scholar]
- 8. Young F, Critchley J A, Johnstone L K, Unwin N C.. A review of comorbidity between infectious and chronic disease in sub-Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Global Health 2009; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oluboyo P O, Erasmus R T.. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle 1990; 71: 135– 138. [DOI] [PubMed] [Google Scholar]
- 10. Mugusi F, Swai A B M, Alberti K G M M, McLarty D G.. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle 1990; 71: 271– 276. [DOI] [PubMed] [Google Scholar]
- 11. Jeon C Y, Harries A D, Baker M A, . et al. Bi-directional screening for tuberculosis and diabetes: A systematic review. Trop Med Int Health 2010; 15: 1300– 1314. [DOI] [PubMed] [Google Scholar]
- 12. Baker M A, Harries A D, Jeon C Y, . et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickup J C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004; 27: 813– 823. [DOI] [PubMed] [Google Scholar]
- 14. British National Formulary. . BNF 56. London, UK: British Medical Association and the Royal Pharmaceutical Society, 2008. [Google Scholar]
- 15. Lönnroth K, Raviglione M.. Global epidemiology of tuberculosis: prospects for control. Semin Respir Crit Care Med 2008; 29: 481– 491. [DOI] [PubMed] [Google Scholar]
- 16. Restrepo B I. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis 2007; 45: 436– 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harries A D, Satyanarayana S, Kumar A M V, . et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review. Public Health Action 2013; 3 Suppl 1: S3– S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization and International Union Against Tuberculosis and Lung Disease. . Collaborative framework for care and control of tuberculosis and diabetes. WHO/HTM/TB/2011.15 Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 19. Adepoyibi T, Weigl B, Greb H, Neogi T, McGuire H.. New screening technologies for type 2 diabetes mellitus appropriate for use in tuberculosis patients. Public Health Action 2013; 3 Suppl 1: S10– S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harries A D, Kumar A M V, Satyanarayana S, Lin Y, Zachariah R, Kapur A.. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tuberc Lung Dis 2015; 19: 879– 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. WHO/NMH/CHP/CPM/11.1 Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 22. Whitley H P, Yong E V, Rasinen C.. Selecting an A1C point-of-care instrument. Diabetes Spectr 2015; 28: 201– 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. . Country cooperation strategy at a glance: Kenya. WHO/CCU/16.03/Kenya Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 24. World Health Organization. . Noncommunicable diseases country profiles, Kenya, 2014. Geneva, Switzerland: WHO, 2014. http://www.who.int/nmh/countries/ken_en.pdf Accessed May 2017. [Google Scholar]
- 25. World Health Organization. . Kenya faces rising burden of diabetes. Geneva, Switzerland: WHO, 2014. http://www.who.int/features/2014/kenya-rising-diabetes/en/ Accessed May 2017. [Google Scholar]
- 26. Ministry of Health. . Kenya STEPwise survey for noncommunicable diseases risk factors, 2015 report. Nairobi, Kenya: MoH, 2015. [Google Scholar]
- 27. World Bank. . Kenya Population 2015. Washington, DC, USA, World Bank, 2017. http://data.worldbank.org/country/kenya Accessed May 2017. [Google Scholar]
- 28. Ministry of Health. . Kenya Health Policy 2012. Nairobi, Kenya: MoH, 2012. [Google Scholar]
- 29. World Health Organization. . World Health Statistics, 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 30. American Diabetes Association. . Standards of medical care in diabetes. Diabetes Care 2012; 35 Suppl 1: S11– S63. http://care.diabetesjournals.org/content/diacare/35/Supplement_1/S11.full.pdf Accessed May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 32. Baldé N M, Camara A, Camara L M, Diallo M M, Kaké A.. Associated tuberculosis and diabetes in Conakry, Guinea: prevalence and clinical characteristics. Int J Tuberc Lung Dis 2006; 10: 1036– 1040. [PubMed] [Google Scholar]
- 33. Ade S, Affolabi D, Agodokpessi G, . et al. Low prevalence of diabetes mellitus in patients with tuberculosis in Cotonou, Benin. Public Health Action 2015; 5: 147– 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gillett M J. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327– 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization. . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva, Switzerland: WHO, 2006. [Google Scholar]
- 36. Viswanathan V, Kumpatla S, Aravindalochanan V, . et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLOS ONE 2012; 7: e41367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ponce De Leon A, Garcia-Garcia M D L, Garcia-Sancho M C, . et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care 2004; 27: 1584– 1590. [DOI] [PubMed] [Google Scholar]
- 38. Naik B, Kumar A M V, Satyanarayana S, . et al. Is screening for diabetes among tuberculosis patients feasible at field level? Public Health Action 2013; 3 Suppl 1: S34– S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nair S, Kumari AK, Subramonianpillai J, . et al. High prevalence of undiagnosed diabetes among tuberculosis patients in peripheral health facilities in Kerala. Public Health Action 2013; 3 Suppl 1: S38– S42. [DOI] [PMC free article] [PubMed] [Google Scholar]


